ABSTRACT
Previous studies suggested that degradation of contractile tissue requires cleavage of the costamere, a structural protein complex that holds sarcomeres in place. This study examined if costamere turnover is affected by a rotator cuff tear in a previously established ovine model. We found the activity of focal adhesion kinase (FAK), a main regulator of costamere turnover, was unchanged at 2 weeks but decreased by 27% 16 weeks after surgical release of the infraspinatus tendon. This was accompanied by cleavage of the costamere protein talin into a 190 kDa fragment while full length talin remained unchanged. At 2 weeks after tendon release, muscle volume decreased by 17 cm3 from an initial 185 cm3, the fatty tissue volume was halved, and the contractile tissue volume remained unchanged. After 16 weeks, the muscle volume decreased by 36 cm3, contractile tissue was quantitatively lost, and the fat content increased by 184%. Nandrolone administration mitigated the loss of contractile tissue by 26% and prevented fat accumulation, alterations in FAK activity, and talin cleavage. Taken together, these findings imply that muscle remodeling after tendon release occurs in two stages. The early decrease of muscle volume is associated with reduction of fat; while, the second stage is characterized by substantial loss of contractile tissue accompanied by massive fat accumulation. Regulation of costamere turnover is associated with the loss of contractile tissue and seems to be impacted by nandrolone treatment. Clinically, the costamere may represent a potential intervention target to mitigate muscle loss after a rotator cuff tear. © 2017 The Authors. Journal of Orthopaedic Research® published by Wiley Periodicals, Inc. on behalf of the Orthopaedic Research Society. J Orthop Res 36:272–281, 2018.
Keywords: rotator cuff tear, fatty infiltration, atrophy, costamere, tenotomy
Tears of rotator cuff (RC) tendons are frequent and affect a large portion of the elderly population.1, 2 These tears lead to retraction, fat accumulation, and atrophy of the musculotendinous unit.3 To reestablish normal shoulder function, RC tendon tears are often surgically repaired, and the degree of muscle atrophy and fat accumulation correlates with the probability of repair failure.4, 5 Until recently, these tendon tear‐induced changes were considered irreversible.3, 6 Thus, it is important to characterize these alterations to find solutions for their prevention and treatment and allow a successful recovery from a RC tear.
Tendon release induces a broad range of adaptations in the muscle, with reduced muscle length most frequently described.7 Regulation of muscle length occurs by adding (longitudinal hypertrophy) or removing (longitudinal atrophy) contractile material (i.e., sarcomeres) at the ends of myofibrils8 and it has been shown that tendon release leads to a reduced number of sarcomeres in series.9 Sarcomeres are tightly incorporated in a myofibril and cannot simply be removed and/or degraded,10 as they are held in place by the costamere, a structural protein complex that links the Z‐line to the extracellular matrix.11 Therefore, longitudinal atrophy first requires disruption of the structural anchors (i.e., the costameres) by specific Ca2+‐activated proteases12 to release the respective sarcomeres. In fact, Z‐line streaming is observed after tendon release in the rat13 and after spontaneous tendon tears in various human muscles.14
The main regulator of costamere turnover is focal adhesion kinase (FAK), an integrin‐associated phosphotransfer kinase that is activated by phosphorylation on its tyrosine 397 site (pY397). It is mechanosensitive and reacts to altered loading of the muscle.15 Changes in protein concentrations of FAK and FAK‐pY397 after reduced muscle loading, for example, unloading of human m. vastus lateralis 16 or tendon release of the rat m. soleus 17 are well described. However, it is currently unknown whether FAK, FAK‐pY397, and concomitant alterations in costamere turnover are affected by RC tears.
Apart from early surgical repair, another treatment option, which has been described in sheep6 and rabbits,18 is weekly administration of the anabolic steroid nandrolone decanoate, which prevented fat accumulation when treatment was started with the onset of the tear. To our knowledge, the action of anabolic steroids on the costamere is not yet described after tendon release. Nevertheless, nandrolone decreased cytosolic Ca2+ in the mouse m. soleus after hindlimb unloading.19 This may result in lower Ca2+‐activated protease activity and thereby decreased costamere cleavage, which potentially explains protection from muscle loss.10, 12, 20 In the ovine model of RC tear, the reduction of total muscle volume after tendon release did not differ with or without nandrolone administration; although, fat accumulation was prevented by nandrolone.6 This model has been used to replicate chronic tears (e.g., at 16–40 weeks)6, 21, 22 with high intramuscular fat content (around 50%; Goutallier stage 323). However, early alterations of muscle volume and composition (i.e., representing an acute tear after 2 weeks) and the impact of nandrolone on the costamere complex have not been investigated yet.
Therefore, the aim of the current study was (i) to define RC muscle volume and composition after tendon release in sheep and (ii) to describe alterations of costamere turnover with and without weekly doses of nandrolone. We specifically hypothesized that muscle remodeling starts early after a RC tear and is already detectable after 2 weeks. Additionally, we hypothesized that changes in muscle volume and composition are accompanied by decreased FAK phosphorylation and increased costamere turnover, assessed by the associated protein levels of FAK, talin, and vinculin. Furthermore, we hypothesized that nandrolone administration not only prevents fat accumulation, but also reduces the loss of contractile tissue and mitigates costamere cleavage.
METHODS
Experimental Design
This experiment was performed according to the Swiss law of animal welfare (TSchG455) and approved by the Veterinary Office of the Canton of Zurich (No. 72/2013). The tendon of the m. infraspinatus was surgically released in 18 female Swiss Alpine sheep to simulate a RC tendon tear. Follow‐up measurements were performed either 2 weeks (TR2 group; n = 6; [mean ± SD] age: 16.6 ± 0.0 months; weight: 59.7 ± 2.5 kg) or 16 weeks after tendon release (TR16 group; n = 6; age: 23.2 ± 1.2 months; weight: 45.3 ± 4.8 kg). In a third group, sheep underwent the same procedure as in the TR16 group, but also received weekly doses of 150 μg nandrolone decanoate in the m. gluteus maximus (TR16 + NAN group; n = 6, age: 23.8 ± 1.2 months; weight: 46.7 ± 2.4 kg). Prior to tendon release (PRE) and 2 (2 W) or 16 weeks (16 W) after tendon release, alterations in muscle volume were assessed by magnetic resonance imaging (MRI). Furthermore, at 16 W, the tendon was repaired in the TR16 and TR16 + NAN groups and measurements were performed again 6 weeks later (REP + 6 W) to describe the effects of repair. Biopsy samples were collected from the m. infraspinatus to determine the tissue composition and costamere protein levels at the time points: PRE, 2 W, 16 W, and REP + 6 W. The contralateral shoulder served as the control (CC). Sheep were killed after harvesting the last biopsy, which was at 2 W for the TR2 group and at REP + 6 W for the TR16 and TR16 + NAN groups.
Surgical Tendon Release
Surgery was performed as described previously.6 In brief, the tendon of the m. infraspinatus was released via osteotomy of the greater tuberosity using an oscillating saw. The tendon and bone chip were wrapped in a silicone tube to prevent spontaneous reattachment. After surgery, sheep were allowed to move freely in the stable.
Single Step Repair
The details of this procedure, which involves removal of the silicon wrap and reattachment of the greater tuberosity to its original site, have been previously described.6
Sampling of Muscle Tissue
Approximately 40 mg of tissue (0.02% of average PRE muscle volume) was collected intraoperatively using a Bergstroem needle with a 5 mm diameter (Dixons Surgical Instruments LTD, Wickford, UK). If necessary, samples were rapidly cleaned of blood and immediately frozen in liquid nitrogen cooled isopentane and stored in 2 ml cryotubes at −80°C.
Assessment of Tissue Composition on Biopsy Cross‐Section
To assess the tissue composition of the biopsy specimens, consecutive sections (average section area: 6.54 mm2) were either stained with oil red O to detect fatty tissue (Fig. 1, right), or using the Goldner trichrome technique24 to stain muscle fibers and connective tissue (Fig. 1, left). For the oil red O staining, sections were fixed for 10 min in 4% paraformaldehyde. After washing, sections were incubated for 5 min in 60% isopropanol and then stained for 10 min in the oil red O working solution (40% of 5 g/L oil red O [#0684‐100G, VWR, Radnor, PA] in isopropanol; 60% ddH2O). Then, sections were again incubated in 60% isopropanol, washed, counterstained in hematoxylin, and then rinsed in ddH2O before coverslips were mounted.
Figure 1.
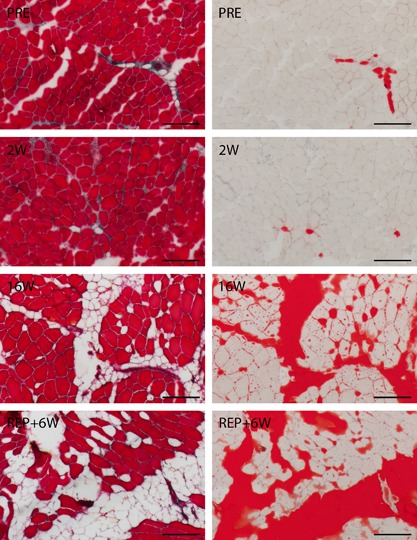
Histologically illustrated temporal changes in tissue composition using representative images of Goldner (left) and oil red O (right) staining at the PRE, 2 W, 16 W, and REP + 6 W time points of the groups TR2 and TR16. On Goldner images, contractile tissue is stained red, the membranes of adipocytes are stained pink, and connective tissue is stained pink and green. On oil red O images, fatty tissue is stained red, while contractile and other types of tissue are unstained. Scale bar denotes 200 μm.
The total area was determined on the oil red O stained section with ImageJ (v1.48v, National Institutes of Health, USA) using the “analyze particles” tool. Afterward, red stained areas (i.e., fatty tissue) were visually isolated, quantified with the same tool, and related to the total area. The area corresponding to contractile tissue was determined from the red‐colored muscle fibers on the Goldner stained sections. Then, fatty tissue and contractile tissue were related to the total area. The total area (=100%) − (fatty tissue + contractile tissue) was named “other types of tissue” and covered all extramyocellular tissue apart from fat.
Assessment of Muscle Volume and Composition
To determine muscle volume, MRI scans of both shoulders were performed immediately after surgery with the sheep still under general anesthesia. Scanning and readout analysis were performed as described previously.6 The approximate volumes of the different tissue types in the total muscle were calculated by splitting the total volume according to the percentages of fatty, contractile, and other tissue types that were assessed histologically in the biopsy specimen.
Immunoprecipitation of FAK‐pY397
Muscle homogenates were prepared from cryosections of muscle biopsies as previously described.25 The protein homogenate (250 μg) was brought to a total volume of 750 μl with RIPA buffer. This was mixed for 30 min at 4°C under steady rotation (20 rpm) and then centrifuged. Without touching the pellet, 700 μl of the homogenate was moved to a new tube and the centrifugation step was repeated. Then, 650 μl of the homogenate was moved to a new tube and incubated over night with 200 μg protein A‐sepharose (#P9424, Sigma–Aldrich, St. Louis, MO) and a combination of anti‐pFAK antibodies (1 μl each, #44‐624G, Thermo Fisher Scientific, Waltham, MA; #sc‐11765‐R, Santa Cruz Biotechnology, TX), again under steady rotation at 4°C. The next day, the protein A‐sepharose was washed twice with RIPA buffer to remove non‐bound proteins. The FAK‐pY397 was resuspended in 2× Laemmli buffer, separated from the immune complex, and stored at −30°C until detection via Western blot.
Assessment of Costamere Protein Levels
Biopsy homogenates were denatured in 2× Laemmli sample buffer and separated on 7.5% SDS–PAGE gels (Bio‐Rad Mini‐protean TGX stain‐free), with 10 μg of protein loaded per lane. To detect FAK‐pY397, 10 μl of precipitate was loaded. Proteins were blotted on a nitrocellulose membrane using the Bio‐Rad Trans‐Blot Turbo System (Bio‐Rad, Cressier, Switzerland). Equal loading and transfer quality were verified using Ponceau S staining. After blocking in 5% milk/1% BSA in TBST, the following antibodies were used to detect the target proteins: FAK‐pY397 and total FAK were detected using a home‐made polyclonal rabbit α‐FAK serum (1:1,000) described previously17; gamma‐ and meta‐vinculin were detected using a mouse α‐vinculin serum (gift of Dr. M. A. Glukhova, Paris, France; 1:100); and talin and integrin‐beta6 were detected using the monoclonal mouse α‐talin antibody #ab95034 (Abcam, Cambridge, UK; 1:100) and the polyclonal mouse α‐ITGB6 antibody #ab169271 (Abcam; 1:500), respectively. Then, the following secondary antibodies (1:20000) were applied on the respective membranes: Goat α‐mouse antibody #A9917 (Sigma–Aldrich) or mouse α‐rabbit antibody #55676 (MP Biomedicals, OH). The signals were recorded using the PXi System (Syngene, Labgene Scientific SA, Chatel St Denis, Switzerland) and quantified using the rectangular mode in Quantity One (Bio‐Rad) according to the user manual. To remove inter‐membrane variability, signals were first divided by a standard loaded on every gel and after that no statistically significant group differences were detected at baseline, values were scaled per group to set the mean of the PRE‐values to 1 to display relative changes compared with baseline.
Localization of Costamere Proteins
To localize FAK, myosin heavy chain and FAK were co‐stained on tissue sections following the oil red O staining (Fig. 2, right). On another microscopic slide, talin and FAK were co‐stained (Fig. 2, left). In brief, sections were fixed for 10 min in 4% paraformaldehyde and blocked in a humid chamber in 5% normal goat serum in phosphate buffer saline and 0.2% triton (PBS‐triton) for 1 h at room temperature. Primary antibody incubation was performed either in a mix of mouse α‐myosin heavy chain antibodies (α‐slow muscle myosin #MAB1628, Millipore Corp., Temecula, CA; α‐fast skeletal myosin #M4276, Sigma–Aldrich; both 1:100) and rabbit anti‐FAK #sc‐557 (Santa Cruz Biotechnology, TX; 1:50), or in rabbit anti‐FAK and mouse anti‐talin (#T3287, Sigma–Aldrich; 1:50). After 1 h, incubation was stopped and slides were washed in PBS‐triton. Then, sections were incubated in a mix of the secondary antibodies, anti‐mouse Alexa Fluor® 488 #A11017 and anti‐rabbit Alexa Fluor® 555 #A21428 (Thermo Fisher Scientific) diluted 1:200 in PBS‐triton, for 45 min. After washing with PBS, nuclei were stained with Hoechst #62249 (Thermo Fisher Scientific; 1:2,000) in PBS for 1 min before coverslips were mounted.
Figure 2.
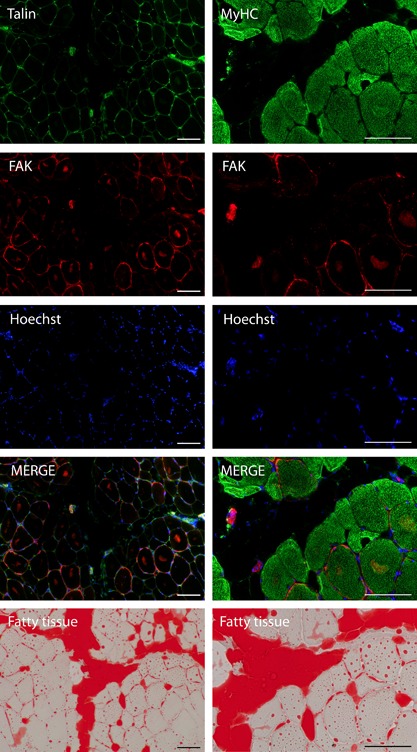
Immunofluorescence images co‐stained with talin and FAK (left column) and myosin heavy chain (MyHC) and FAK (right column) followed by consecutive oil red O images (bottom row) of a representative TR16 sheep at 16 W. Scale bar denotes 100 μm.
Statistical Analysis
Data are presented as the mean ± SD. To detect differences between time points and groups, a general linear model with repeated measures was applied with “time point” as the within‐subjects variable (repeated) and “group” as the between‐subjects factor (split‐plot ANOVA). A bivariate, two‐tailed Pearson's test revealed correlations between protein levels and muscle composition. For the statistical analysis, SPSS Statistics v22.0 (IBM, Chicago, IL) was used. Statistical significance was defined as p < 0.05.
RESULTS
Tendon Release Leads to Reduction of Muscle Volume and Changes in Tissue Composition
The initial muscle volume and composition did not differ between groups (Fig. 3). In TR2 and TR16, tendon release led to a decrease in muscle volume, which was already detectable at 2 W (−17.0 ± 5.0 cm3, p < 0.001 compared with PRE) and was more pronounced at 16 W (−35.8 ± 12.1 cm3, p < 0.001 compared with PRE). At REP + 6W, muscle volume was further decreased (−51.2 ± 15.0 cm3, p < 0.001 compared with PRE; −15.3 ± 12.8 cm3, p = 0.004 compared with 16 W; Fig. 3). Microscopy images representing the relative distribution of tissue types are shown in Figure 1 and the percent areas from histological analysis that were used to calculate the volumes of contractile, fatty, and remaining tissue are presented in Figure 4.
Figure 3.
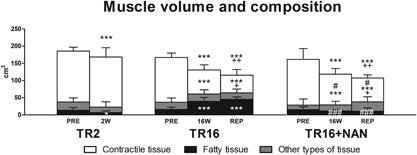
Muscle volume and composition. Indications above columns refer to alterations in total volume, indications inside columns refer to alterations in the volume of the specific tissue type. The volume of other types of tissue was calculated from the total volume (contractile tissue + fatty tissue). *p < 0.05, ***p < 0.001 for time effect compared with PRE within the same group; + p < 0.05, ++ p < 0.01 for time effect compared with 16 W within the same group; # p < 0.05, ### p < 0.001 for group effect compared with TR16 at the same time point. Values are means + SD.
Figure 4.
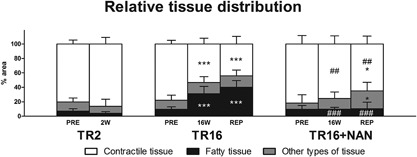
Relative tissue distribution assessed on biopsy cross‐sections. Indications inside columns refer to alterations in the % area of the specific tissue type. The % of other types of tissue was defined as 100% (contractile tissue + fatty tissue). *p < 0.05, ***p < 0.001 for time effect compared with PRE within the same group; ## p < 0.01, ### p < 0.001 for group effect compared with TR16 at the same time point. Values are means + SD.
In TR2 and TR16, the volume of contractile tissue remained unchanged at 2 W (−2.0 ± 25.6 cm3, p = 0.859 compared with PRE), but was significantly decreased at 16 W (−59.4 ± 10.2 cm3, p < 0.001 compared with PRE). Contractile tissue was further reduced at REP + 6W (−77.9 ± 17.9 cm3, p < 0.001 compared with PRE; −18.6 ± 16.7 cm3, p = 0.028 compared with 16 W; Fig. 3). The volume of fatty tissue was reduced at 2 W (−6.4 ± 5.9 cm3, p = 0.045 compared with PRE) but then increased at 16W (+23.6 ± 12.8 cm3, p < 0.001 compared with PRE) in TR2 and TR16, respectively. At REP + 6W (+29.4 ± 11.6 cm3, p < 0.001 compared with PRE), it was similar to 16W (+5.8 ± 11.1 cm3, p = 0.186; Fig. 3). Fatty tissue measured in the biopsy sample correlated quantitatively with the fat portion (r = 0.756, p < 0.001, N = 66) assessed using the Dixon method (described by Gerber et al.)6 on the MRI scans (data not shown). Tendon release did not influence the volume of other tissues (i.e., non‐myocellular and non‐fatty tissue; Fig. 3).
Nandrolone Does Not Influence Total Muscle Volume, but Reduces the Loss of Contractile Tissue and Prevents Fat Accumulation
In TR16 + NAN, the total muscle volume was decreased at 16 W (−43.5 ± 18.3 cm3, p < 0.001 compared with PRE). At REP + 6 W, muscle volume was further decreased (−54.7 ± 19.0 cm3, p < 0.001 compared with PRE; −11.2 ± 5.8 cm3, p = 0.020 compared with 16 W; Fig. 3). In the TR16 + NAN group, the volume of contractile tissue was reduced at 16 W (−43.7 ± 22.1 cm3, p < 0.001 compared with PRE) and at REP + 6 W (−64.3 ± 35.0 cm3, p < 0.001 compared with PRE; −20.5 ± 18.7 cm3, p = 0.018 compared with 16W). Compared with TR16, the volume of contractile tissue in TR16 + NAN was significantly larger at 16 W (+20.7 cm3, p = 0.046) and REP + 6 W (+18.8 cm3, p = 0.036; Fig. 3). The volume of fatty tissue remained unchanged in the nandrolone‐treated sheep at both 16 W (−4.0 ± 7.0 cm3, p = 0.368) and REP + 6 W (−4.6 ± 8.0 cm3, p = 0.283) compared with PRE. This corresponds to a significantly lower volume of fatty tissue compared with TR16 at both 16 W (−27.8 cm3, p < 0.001) and REP + 6 W (−34.3 cm3, p < 0.001; Fig. 3). Nandrolone did not influence the volumes of other types of tissue (Fig. 3).
Tendon Release Leads to a Relative Deactivation of FAK on the Y397 Site, Which Is Prevented by Nandrolone Administration
Representative Western blot images are shown in Figure 5. The majority of FAK protein is localized around muscle fibers and not fatty tissue (Fig. 2). Its relative activity, expressed as the ratio of Y397‐phosphorylated FAK per total FAK, remained unchanged after 2 weeks of tendon release (−8.2 ± 41.0%, p = 0.545 compared with PRE), but was significantly reduced at 16 W (−27.1 ± 20.6%, p = 0.039 compared with PRE). At REP + 6 W, the initial level of relative FAK activity was not reestablished (−34.2 ± 19.5%, p = 0.010 compared with PRE; Fig. 6C). The total amount of FAK protein was unchanged at 2 W but tended to increase after 16 weeks of tendon release (+44.6 ± 33.5%, p = 0.062 compared with PRE), and was only significantly increased after repair compared with baseline (+94.6 ± 61.5%, p = 0.012; Fig. 6A). Nandrolone prevented the deactivation of FAK on the Y397 site, after 16 weeks of tendon release, and after the subsequent 6 weeks of repair (Fig. 6C), as the total amount of FAK (Fig. 6A) remained unchanged.
Figure 5.
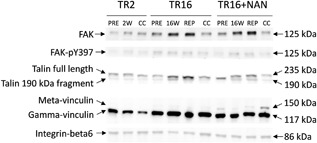
Representative Western blot images of focal adhesion kinase (FAK), its phosphorylated form FAK‐pY397, full length talin and its 190 kDa fragment, gamma‐ and meta‐vinculin, and integrin‐beta6. Relative protein amounts were assessed at baseline (PRE), 2 weeks (2 W), or 16 weeks (16 W) after tendon release and 6 weeks after repair (REP + 6 W). The measurements in the contralateral control (CC) took place at the end of the intervention.
Figure 6.
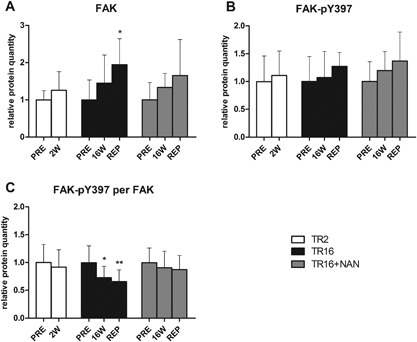
Relative protein quantities of (A) total FAK, (B) FAK‐pY397, and (C) the amount of FAK‐pY397 per total FAK. *p < 0.05, **p < 0.01 for time effect compared with PRE within the same group. Values are means + SD.
Tendon Release and Subsequent Repair Leads to Increased Costamere Turnover
Representative Western blot images are shown in Figure 5. Tendon release did not exert an effect on talin quantity, but 6 weeks of repair significantly increased it (+241.4 ± 160.4%, p < 0.001 compared with PRE; Fig. 7A). Instead, the 190 kDa fragment of talin was increased already at 16 W (+122.4 ± 60.3%, p = 0.003) and remained elevated at REP + 6 W (+161.1 ± 84.4%, p = 0.026 compared with PRE; Fig. 7B). Correlation analysis revealed that the abundance of the 190 kDa fragment of talin is tightly associated with the levels of FAK (r = 0.801, p < 0.001; Fig. 8). Cleavage of talin was also associated with the loss of contractile tissue (r = −0.407, p < 0.001; Fig. 8). Similar to full length talin, gamma‐vinculin was not affected by tendon release, but was increased with subsequent repair (+105.9 ± 111.6%, p = 0.017 compared with PRE; Fig. 7C). Meta‐vinculin remained unaffected by tendon release and repair (Fig. 7D). The integrin‐beta6 protein level dropped after 2 weeks of tendon release (−44.6 ± 36.0%, p = 0.029) and the resting level was reestablished at 16 W. Repair did not influence integrin‐beta6 protein expression (Fig. 7E).
Figure 7.
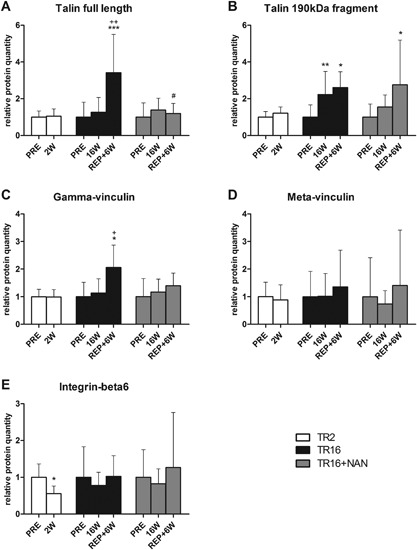
Relative quantities of the costamere proteins. (A) Full length talin, (B) the proteolytic 190 kDa fragment of talin, (C) gamma‐vinculin, (D) meta‐vinculin, and (E) integrin‐beta6. *p < 0.05, **p < 0.01, ***p < 0.001 for time effect compared with PRE within the same group; + p < 0.05, ++ p < 0.01 for time effect compared with 16 W within the same group; # p < 0.05 for group effect compared with TR16 at the same time point. Values are means + SD.
Figure 8.
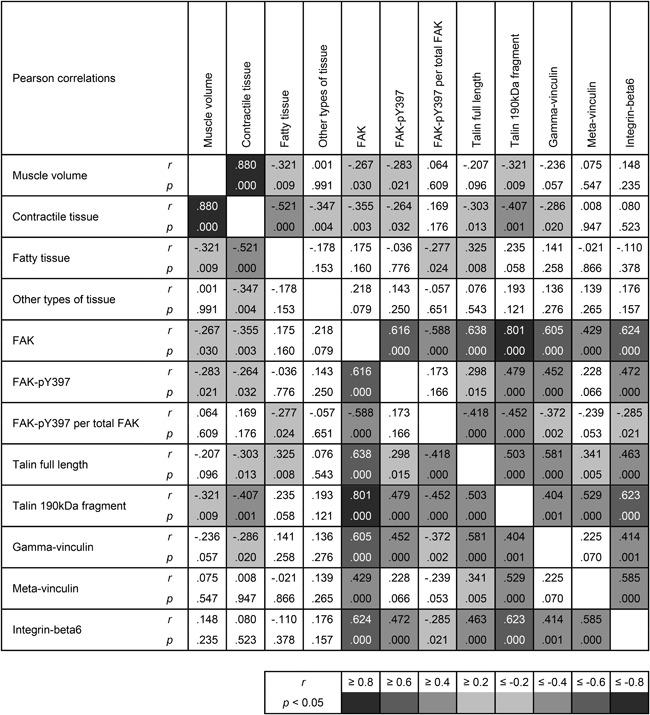
Pearson correlations for all groups (TR2, TR16, TR16 + NAN) and all time points (PRE, 2 W, 16 W, REP + 6 W, CC). Results are presented as the correlation coefficient r and p‐value; N = 66.
Nandrolone Prevents Changes in Costamere Turnover After Tendon Release
In the TR16 + NAN group, tendon release had no effect on the relative quantities of talin, its 190 kDa fragment, gamma‐ and meta‐vinculin, and integrin‐beta6 (Fig. 7A–E). Instead, 6 weeks after repair, the amount of full length talin protein was significantly decreased compared with TR16 (−65.0%, p = 0.031; Fig. 7A). Concomitantly, the 190 kDa fragment of talin was increased at REP + 6 W compared with baseline (+175.7 ± 195.9%, p = 0.017; Fig. 7B). Compared with TR16, nandrolone kept the gamma‐vinculin protein level similar to baseline after repair (Fig. 7C). Furthermore, after repair, meta‐vinculin and integrin‐beta6 quantities were not distinguishable from baseline or the respective level in the TR16 group (Fig. 7D and E).
DISCUSSION
The purpose of this study was to define the RC muscle composition after tendon release in sheep and to describe costamere turnover with and without weekly doses of nandrolone. In this model, fat accumulated and costamere turnover increased at 16 weeks, but not 2 weeks after tendon release and these effects were prevented by nandrolone. Furthermore, nandrolone mitigated the loss of contractile tissue.
Assessments of total muscle volumes via MRI revealed that muscle remodeling starts immediately after tendon release, as the total muscle volume was already reduced at 2 weeks (Fig. 3). This loss in volume continued until 16 weeks and was not stopped by repair (Fig. 3). The relative loss of muscle volume corresponds well to the magnitude and time course of atrophy observed in humans during bed rest, unilateral limb suspension, immobilization, and spaceflight studies,26 indicating that the ovine model might also be suitable for replication of human muscle unloading situations other than the RC tear. Interestingly, the reduction in muscle volume after 2 weeks is, in part, explained by a decreased volume of fat but not contractile tissue (Fig. 3). Liu et al.27 showed previously, in a rat model, that fat accumulation is not detectable at 2 weeks after RC tendon release; while, it is present after 6 weeks. Also in sheep, fat accumulation was detectable on MRI scans after 6 weeks,6 indicating the turning point for fat accumulation is between 2 and 6 weeks after tendon release. As blood flow, and thereby delivery of substrates, is contraction dependent,28 we hypothesize that the unloaded muscle has to live on its local energy stores (i.e., intramuscular fatty tissue), leading to decreased fat content until pathological fat accumulation occurs. The findings that the contractile tissue volume remained unchanged after 2 weeks and was significantly decreased after 16 weeks (Fig. 3) supprt the conclusion that pathological muscle loss does not start before 2 weeks after the injury.
The present study revealed that nandrolone protects contractile tissue. The mitigated loss of contractile tissue is the reason for similar total muscle volumes with/without nandrolone despite significantly lower fat content in the nandrolone‐treated sheep (Fig. 3). It was shown previously that nandrolone reduces unloading‐induced loss of muscle protein content in mice.19 The present study confirmed this also occurs with RC tendon release in sheep. Furthermore, exclusive assessment of the relative tissue distribution does not sufficiently describe shifts in tissue types if the total volume, which the distribution is related to, varies throughout the intervention, for example, contractile tissue in the TR16 + NAN group at 16 W was lost without a relative change in tissue distribution (compare Fig. 3 with Fig. 4). Conversely, the assessment of total muscle volume alone does not allow conclusions to be drawn about its composition (Fig. 3).
The breakdown of contractile tissue has to be coupled to the disassembly of its structural anchors.10 The costamere is such an anchor and the mechanosensor, FAK, its main regulator, is very sensible to alterations of muscle loading.15 The relative decrease of FAK‐pY397 (Fig. 6) reflects reduced loading, which was previously shown after unloading of human m. vastus lateralis.16 Similar to our results, total FAK protein tended to increase in a human model with unloading by limb suspension16 and bedrest.29 Apart from FAK, one of the main structural costamere components, the protein talin, was also affected by tendon release. The increase of the 190 kDa talin fragment at 16 W (Fig. 7) may be indicative of increased costamere turnover, as the rise in the fragment did not decrease the amount of full length protein; thus, the full length talin seems to be replaced immediately. This 190 kDa fragment of talin originates from cleavage of the full length protein by the Ca2+‐activated protease calpain.30 The calpain system is responsible for initial cleavage of structural proteins like the costamere to release sarcomeres and make them accessible for quantitative degradation by the proteasome system.10 Indeed, the increased cleavage of talin was associated with loss of contractile tissue (Fig. 8). There were no changes in costamere turnover (Figs. 6C and 7A and B) or loss of contractile tissue (Fig. 3) at 2 weeks; while, both were present at 16 weeks after tendon release. Nandrolone treatment prevented both the decrease of relative FAK activation and talin cleavage by calpain, probably by lowering Ca2+‐levels19 and thereby decreasing calpain activity.10, 12 In general, costamere protein levels seem to be predictive of contractile tissue volume (and vice versa; Fig. 8), but further study is needed to determine whether there is a causal connection between preservation of normal costamere turnover and mitigated loss of contractile tissue. Interestingly, repair increased the relative amounts of FAK, talin, and gamma‐vinculin in the TR16 group, indicating that the number of costameres increased, as talin and gamma‐vinculin are some of the main structural components of the costamere11 and FAK acts as a main regulator.17
This study has several limitations. First, the muscle biopsy was harvested from one site only and does not necessarily represent the entire muscle; although, fatty tissue measured in the biopsy correlated quantitatively with the fat portion measured on MRI scans. Furthermore, the sample was harvested from the distal third of the lateral portion of the muscle, which is close to the myotendinous junction. This area was previously described as having the highest degree of fat accumulation in rat31 and rabbit32, 33 models of RC tears. Due to potential regional differences, the calculated volumes of contractile, fatty, and other types of tissue should be considered as approximate values. Furthermore, the nandrolone‐untreated sheep had to be divided into two groups, which was necessary because local authorities declared the surgical interventions as too numerous/frequent to be ethical for one group alone. Nevertheless, statistical analysis did not identify group differences in any PRE muscle characteristics.
CONCLUSIONS
In the ovine model of RC tears, muscle atrophy starts with the tendon tear and takes place in two stages. In the first 2 weeks, atrophy is associated with reduced fat content and preservation of contractile tissue. Thereafter, there is substantial fat accumulation and loss of contractile tissue. Degradation of contractile tissue is tightly associated with increased costamere turnover and can be mitigated by the administration of nandrolone decanoate.
OUTLOOK
Future studies should address whether the repair of acute tears is possible and successful if started within 2 weeks after the tear, as contractile tissue is not quantitatively lost by this time point, at least in this animal model. The finding that intramuscular fat decreases before it accumulates leads to questions about the timing of this turning point and what causes it. Moreover, the functional relevance of costamere protein cleavage on the degradation of contractile tissue needs to be determined. In addition, the clinical benefit of early administration of nandrolone decanoate needs to be further elaborated.
AUTHORS’ CONTRIBUTIONS
Conception and design of research: MF, CG; performed animal experiments: KW, DCM, BVR, MCB; performed muscle analysis: SR, CBM; analyzed data: SR, CBM, KW; interpreted the results of experiments: SR, MF, CG; funding: MF, CG; prepared figures: SR; drafted the manuscript: SR; edited and revised the manuscript: SR, MF, CG, BVR; and approved the final version of the manuscript: All authors.
ACKNOWLEDGMENTS
We thank Dr. Marina A. Glukhova for providing the α‐vinculin antibody. Furthermore, we thank MSc Céline Ferrié for performing the Goldner and oil red O staining and providing technical assistance. Also, we acknowledge the effort of Dr. Karina Klein, the anesthesia team, and Eric Parigoris. This work has been supported by RESORTHO and the Swiss National Science Foundation (grant #149786), Switzerland.
Conflicts of interest: The authors declare that they have no competing interests.
REFERENCES
- 1. Sher JS, Uribe JW, Posada A, et al. 1995. Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Joint Surg Am 77:10–15. [DOI] [PubMed] [Google Scholar]
- 2. Yamaguchi K, Ditsios K, Middleton WD, et al. 2006. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am 88:1699–1704. [DOI] [PubMed] [Google Scholar]
- 3. Gerber C, Fuchs B, Hodler J. 2000. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am 82:505–515. [DOI] [PubMed] [Google Scholar]
- 4. Chaudhury S, Gwilym SE, Moser J, et al. 2010. Surgical options for patients with shoulder pain. Nat Rev Rheumatol 6:217–226. [DOI] [PubMed] [Google Scholar]
- 5. Galatz LM, Ball CM, Teefey SA, et al. 2004. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am 86‐A:219–224. [DOI] [PubMed] [Google Scholar]
- 6. Gerber C, Meyer DC, Fluck M, et al. 2015. Anabolic steroids reduce muscle degeneration associated with rotator cuff tendon release in sheep. Am J Sports Med 43:2393–2400. [DOI] [PubMed] [Google Scholar]
- 7. Jamali AA, Afshar P, Abrams RA, et al. 2000. Skeletal muscle response to tenotomy. Muscle Nerve 23:851–862. [DOI] [PubMed] [Google Scholar]
- 8. Williams PE, Goldspink G. 1971. Longitudinal growth of striated muscle fibres. J Cell Sci 9:751–767. [DOI] [PubMed] [Google Scholar]
- 9. Van Dyke JM, Bain JL, Riley DA. 2012. Preserving sarcomere number after tenotomy requires stretch and contraction. Muscle Nerve 45:367–375. [DOI] [PubMed] [Google Scholar]
- 10. Goll DE, Neti G, Mares SW, et al. 2008. Myofibrillar protein turnover: the proteasome and the calpains. J Anim Sci 86:E19–E35. [DOI] [PubMed] [Google Scholar]
- 11. Peter AK, Cheng H, Ross RS, et al. 2011. The costamere bridges sarcomeres to the sarcolemma in striated muscle. Prog Pediatr Cardiol 31:83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tidball JG, Spencer MJ. 2002. Expression of a calpastatin transgene slows muscle wasting and obviates changes in myosin isoform expression during murine muscle disuse. J Physiol 545:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baker JH, Margolis RN. 1987. Calcium‐activated protease activity in tenotomized muscle. Muscle Nerve 10:34–40. [DOI] [PubMed] [Google Scholar]
- 14. Jozsa L, Balint JB, Demel S. 1978. Histochemical and ultrastructural study of human muscles after spontaneous rupture of the tendon. Acta Histochem 63:61–73. [DOI] [PubMed] [Google Scholar]
- 15. Graham ZA, Gallagher PM, Cardozo CP. 2015. Focal adhesion kinase and its role in skeletal muscle. J Muscle Res Cell Motil 36:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fluck M, Li R, Valdivieso P, et al. 2014. Early changes in costameric and mitochondrial protein expression with unloading are muscle specific. Biomed Res Int 2014:519310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klossner S, Li R, Ruoss S, et al. 2013. Quantitative changes in focal adhesion kinase and its inhibitor, FRNK, drive load‐dependent expression of costamere components. Am J Physiol Regul Integr Comp Physiol 305:R647–R657. [DOI] [PubMed] [Google Scholar]
- 18. Gerber C, Meyer DC, Nuss KM, et al. 2011. Anabolic steroids reduce muscle damage caused by rotator cuff tendon release in an experimental study in rabbits. J Bone Joint Surg Am 93:2189–2195. [DOI] [PubMed] [Google Scholar]
- 19. Camerino GM, Desaphy JF, De Bellis M, et al. 2015. Effects of nandrolone in the counteraction of skeletal muscle atrophy in a mouse model of muscle disuse: molecular biology and functional evaluation. PLoS ONE 10:e0129686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alderton JM, Steinhardt RA. 2000. Calcium influx through calcium leak channels is responsible for the elevated levels of calcium‐dependent proteolysis in dystrophic myotubes. J Biol Chem 275:9452–9460. [DOI] [PubMed] [Google Scholar]
- 21. Gerber C, Meyer DC, Schneeberger AG, et al. 2004. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am 86‐A:1973–1982. [DOI] [PubMed] [Google Scholar]
- 22. Gerber C, Meyer DC, Von Rechenberg B, et al. 2012. Rotator cuff muscles lose responsiveness to anabolic steroids after tendon tear and musculotendinous retraction: an experimental study in sheep. Am J Sports Med 40:2454–2461. [DOI] [PubMed] [Google Scholar]
- 23. Goutallier D, Postel JM, Bernageau J, et al. 1994. Fatty muscle degeneration in cuff ruptures. Pre‐ and postoperative evaluation by CT scan. Clin Orthop Relat Res 304:78–83. [PubMed] [Google Scholar]
- 24. Goldner J. 1938. A modification of the masson trichrome technique for routine laboratory purposes. Am J Pathol 14:237–243. [PMC free article] [PubMed] [Google Scholar]
- 25. Klossner S, Durieux AC, Freyssenet D, et al. 2009. Mechano‐transduction to muscle protein synthesis is modulated by FAK. Eur J Appl Physiol 106:389–398. [DOI] [PubMed] [Google Scholar]
- 26. Narici MV, de Boer MD. 2011. Disuse of the musculo‐skeletal system in space and on earth. Eur J Appl Physiol 111:403–420. [DOI] [PubMed] [Google Scholar]
- 27. Liu X, Manzano G, Kim HT, et al. 2011. A rat model of massive rotator cuff tears. J Orthop Res 29:588–595. [DOI] [PubMed] [Google Scholar]
- 28. Mackie BG, Terjung RL. 1983. Blood flow to different skeletal muscle fiber types during contraction. Am J Physiol 245:H265–H275. [DOI] [PubMed] [Google Scholar]
- 29. Salanova M, Gelfi C, Moriggi M, et al. 2014. Disuse deterioration of human skeletal muscle challenged by resistive exercise superimposed with vibration: evidence from structural and proteomic analysis. FASEB J 28:4748–4763. [DOI] [PubMed] [Google Scholar]
- 30. Beckerle MC, Burridge K, DeMartino GN, et al. 1987. Colocalization of calcium‐dependent protease II and one of its substrates at sites of cell adhesion. Cell 51:569–577. [DOI] [PubMed] [Google Scholar]
- 31. Itoigawa Y, Kishimoto KN, Sano H, et al. 2011. Molecular mechanism of fatty degeneration in rotator cuff muscle with tendon rupture. J Orthop Res 29:861–866. [DOI] [PubMed] [Google Scholar]
- 32. Rowshan K, Hadley S, Pham K, et al. 2010. Development of fatty atrophy after neurologic and rotator cuff injuries in an animal model of rotator cuff pathology. J Bone Joint Surg Am 92:2270–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rubino LJ, Stills HF, Jr , Sprott DC, et al. 2007. Fatty infiltration of the torn rotator cuff worsens over time in a rabbit model. Arthroscopy 23:717–722. [DOI] [PubMed] [Google Scholar]


