Abstract
Nasopharyngeal angiofibroma is a benign but aggressive tumor of unknown etiology, typically occurring in adolescent males. It is described as a rare neoplasm; however, the prevalence seems to have geographic differences. All cases referred to our head and neck clinical and pathology service were reviewed. Most of the patients presented at an advanced stage. The clinical and radiographic features are presented and discussed. Histologically, the tumor shows a highly vascular fibrous proliferation with characteristic plump, angulated and stellate cells, categorized as fibroblasts. Immunohistochemistry was performed on 42 cases to further elucidate the nature of these cells. The stromal cells expressed vimentin and factor XIIIa, the latter expressed most commonly in the giant stellate cells. Inflammation was almost exclusively present in peripheral subepithelial areas. Mast cells were abundant, even in the absence of other inflammatory cells. Lymphatics were observed principally in peripheral regions. Proliferating cells (Ki-67 reactive) were restricted to endothelial cells.
Keywords: Nasopharyngeal angiofibroma, Sinonasal tumor, Vascular tumor, Immunohistochemistry, Imagenology, Adolescent, Males
Introduction
Nasopharyngeal angiofibroma (formerly juvenile nasopharyngeal angiofibroma) is a rare (<1% of head and neck tumors) benign mesenchymal neoplasm composed of a vascular proliferation within a cellular, densely collagenized stroma, typically originating in the nasopharynx, affecting adolescent males [1]. Most of the tumors arise in the superior part of the sphenopalatine foramen and affect the posterolateral nasal wall of the nasopharynx; however, the tumors often show extension into the surrounding tissues [2].
Microscopically, the vascular component consists of variable sized and shaped vessels, ranging from small rounded or slit-like to larger staghorn, branching or irregular vascular channels. Endothelial cells are mainly flat, but plump endothelial cells can also be observed [3]. Vessel walls vary from a thin pericyte layer around the vessel to a thick smooth muscle layer. Peripheral regions show abundant microvascular proliferation [4]. The stroma varies from highly cellular, collagenized areas to less cellular regions with a fibromyxoid background. Stromal cells vary from spindle, angular to plump stellate shaped fibroblasts [5, 6] with central round vesicular nuclei and small nucleoli. Stromal cells show intense positivity for vimentin [4, 7]. Mast cells are abundant, and areas of thrombosis and infarction may represent findings related to pre-operative embolization. Inflammation varies from acute to chronic [4] most often in a subepithelial location, and most likely not tumor-related. Recent studies have shown expression of stem-cell related proteins including c-kit and c-myc suggesting that both contribute to the development of NAF. Chromosomal imbalances have also been demonstrated in both endothelial and stromal cells [8, 9]. Furthermore, there is immunohistochemical expression of androgen, testosterone, dihydrotestosterone receptors and basic fibroblast growth factor, while estrogen and progesterone receptors are usually negative [10–12]. There is no evidence for Human Herpes Virus type VIII and Epstein-Barr virus as etiological factors [13]. The objective of this study is to describe the clinicopathological features of 42 cases of NAF, further highlighting stromal immunohistochemical findings.
Materials and Methods
Forty-two cases of NAF were retrieved from the Pathology Department at Centro Clínico de Cabeza y Cuello, in Guatemala City. Clinical information included sex, age, affected site, size, and clinical symptoms at presentation, along with management. Immunohistochemistry (Table 1) was performed using standard protocols, and immunoexpression were analyzed in the endothelial cells, vessel walls and stromal cells.
Table 1.
Antibodies used for immunohistochemical evaluation of 42 cases of nasopharyngeal angiofibroma from Guatemala
| Antibody | Source/clone | Dilution | Antigen retrieval |
|---|---|---|---|
| Vimentin | Dako/Vim3B4 | 1:400 | Citrate buffer (pH 6.0) |
| CD34 | Dako/QBEnd10 | 1:50 | Citrate buffer (pH 6.0) |
| CD31 | Dako/1C70A | 1:200 | EDTA/TRIS (pH 9.0) |
| FVIII (Von Willebrand Factor) | Dako/F8/86 | 1:100 | Citrate buffer (pH 6.0) |
| CD105 | Dako/SNGH | 1:30 | Proteinase K |
| Podoplanin | Dako/D2-40 | 1:100 | Citrate buffer (pH 6.0) |
| Desmin | Dako/D33 | 1:800 | Citrate buffer (pH 6.0) |
| Smooth muscle actin | Dako/1A4 | 1:400 | Citrate buffer (pH 6.0) |
| Muscle specific actin | Dako/HHF-35 | 1:800 | Citrate buffer (pH 6.0) |
| H-caldesmon | Dako/N-CD | 1:400 | Citrate buffer (pH 6.0) |
| Calponin | Dako/CALP | 1:600 | Citrate buffer (pH 6.0) |
| Mast cell tryptase | Dako/AA1 | 1:10,000 | Citrate buffer (pH 6.0) |
| CD1a | Dako/010 | 1:600 | Citrate buffer (pH 6.0) |
| S100 | Dako/polyclonal | 1:10,000 | Citrate buffer (pH 6.0) |
| Factor XIIIa | Novocastra/E980.1 | 1:100 | EDTA/TRIS (pH 9.0) |
| CD68 | Dako/PGM1 | 1:400 | Citrate buffer (pH 6.0) |
| CD163 | Novocastra/10D6 | 1:4000 | Citrate buffer (pH 6.0) |
| Ki-67 | Dako/Mib-1 | 1:100 | Citrate buffer (pH 6.0) |
Results
All 42 patients were males, with a mean age of 14.7 years, ranging from 8 to 27 years; age was unknown in 3 patients (Table 2). The tumors originated in the nasopharynx, frequently extending anteriorly into the nasal cavity and oropharynx, with 9 cases also involving the soft palate. All patients presented with nasal obstruction and 73% with epistaxis, often for a long duration (12–24 months) prior to seeking clinical evaluation. Facial deformity can be a characteristic in some cases (Fig. 1 ). Despite the tumor being well defined (Fig. 2 ), severe hemorrhage at the time of surgery occurred in all cases, even with prior placement of unilateral or bilateral coils in the internal maxillary artery. Tumors did not recur, after a follow period ranging from 6 months to 10 years, except for 3 cases in which complete resection was not achieved at other institutions, with consequent persistence during puberty. None of the NAF cases were associated with familial adenomatous polyposis.
Table 2.
Summary of clinical data of 42 cases of NAF
| Case | Age | Sex | Initial clinical manifestations |
|---|---|---|---|
| 1 | 10 | M | Unilateral facial deformity |
| 2 | 13 | M | Nasal and oropharyngeal mass and epistaxis |
| 3 | 15 | M | Nasopharyngeal mass |
| 4 | 17 | M | Nasopharyngeal mass and epistaxis |
| 5 | 22 | M | Nasopharyngeal mass and epistaxis |
| 6 | 17 | M | Nasopharyngeal mass and epistaxis |
| 7 | 13 | M | Nasopharyngeal mass and epistaxis |
| 8 | 15 | M | Pharyngeal mass and epistaxis |
| 9 | 17 | M | Recurrent lesion |
| 10 | 23 | M | Nasal mass and epistaxis |
| 11 | 27 | M | Rhinopharyngeal mass and epistaxis |
| 12 | unk | M | Retronasal mass and epistaxis |
| 13 | 15 | M | Nasopharyngeal mass and facial swelling |
| 14 | 11 | M | Nasopharyngeal mass and epistaxis |
| 15 | 15 | M | Epistaxis |
| 16 | 15 | M | Epistaxis |
| 17 | 14 | M | Nasal mass and epistaxis |
| 18 | 15 | M | Left nasal obstruction and epistaxis |
| 19 | 8 | M | Nasal mass and epistaxis |
| 20 | 16 | M | Nasopharyngeal mass and nasal obstruction |
| 21 | 15 | M | Nasopharyngeal mass, nasal obstruction and epistaxis |
| 22 | 12 | M | Rhinopharyngeal mass and nasal obstruction |
| 23 | 14 | M | Nasopharyngeal mass, nasal obstruction and epistaxis |
| 24 | 17 | M | Nasal mass and epistaxis |
| 25 | 11 | M | Nasal mass and epistaxis |
| 26 | unk | M | Nasal mass and obstruction |
| 27 | 11 | M | Large occupying tumor of left nasal fossa, ethmoid and maxillary sinus |
| 28 | 13 | M | Nasal mass and epistaxis |
| 29 | 14 | M | Nasal mass and epistaxis |
| 30 | 14 | M | Nasal mass and epistaxis |
| 31 | 17 | M | Massive tumor of nasopharynx with extension to nasal fossae, oropharynx and cheekbone |
| 32 | 13 | M | Nasopharyngeal mass |
| 33 | 18 | M | Unilateral nasal obstruction and epistaxis |
| 34 | unk | M | Nasopharyngeal mass and epistaxis |
| 35 | 13 | M | Nasopharyngeal mass |
| 36 | 11 | M | Epistaxis |
| 37 | 13 | M | Nasal obstruction and epistaxis |
| 38 | 16 | M | Nasal obstruction and epistaxis |
| 39 | 14 | M | Nasal obstruction and epistaxis |
| 40 | 10 | M | Nasopharyngeal mass and epistaxis |
| 41 | 15 | M | Nasopharyngeal mass and epistaxis |
| 42 | 16 | M | Nasal obstruction and epistaxis |
Mean age: 14.7
M male, unk unknown
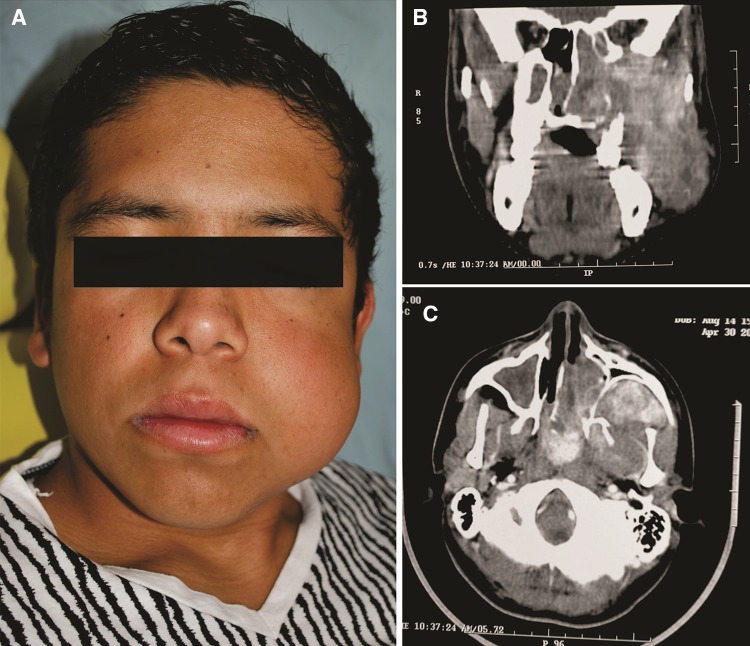
Fig. 1.
14-year-old male with a tumoral mass affecting the nasopharynx and extending into left cheek. a Clinically the patient shows facial left swelling and asymmetry. This clinical aspect was favored by a previously unsuccessful attempt of surgical removal. b In a coronal CT section, a large tumoral mass deviating the nasal septa and extending from sinonasal spaces to pterygopalatine fossa, infratemporal fossa and extents into soft tissues of the check. c Axial CT image of the same tumor shows anterior displacement of posterior wall of left maxillary sinus is evident. Contrast media highlights the prominent vascularity
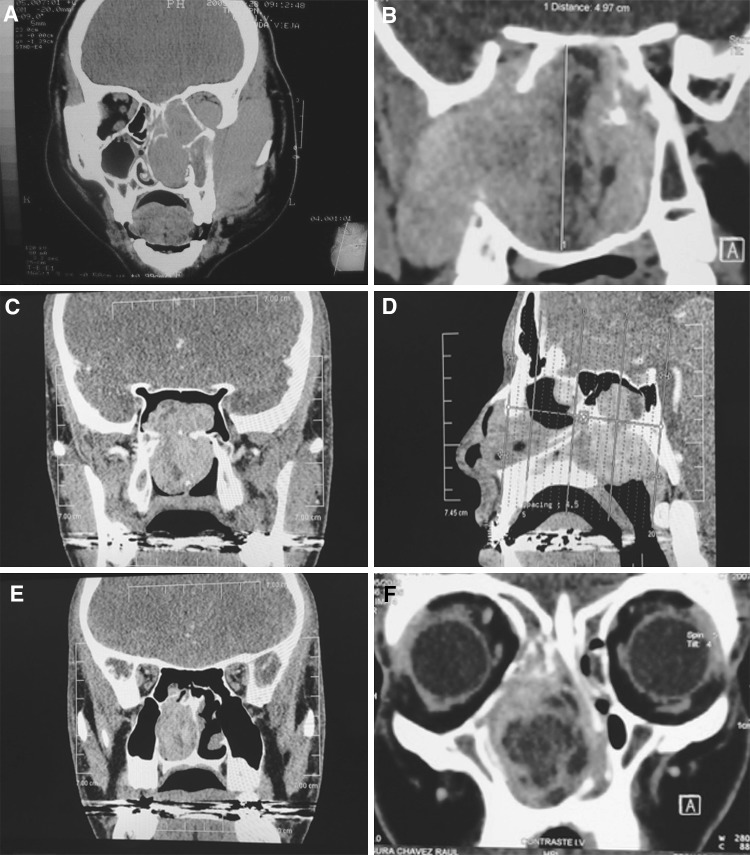
Fig. 2.
Imaging features of NAF. a Coronal CT image without contrast media of a 14 y.o.m. patient showing a large tumor occupying nasal cavity and extending into the orbit, infratemporal fossa and facial soft tissues. b MRI in T1 with enhancement of a 14 y.o.m. patient showing a tumor with complete occupation of sphenoidal sinus with extension into pterygopalatine fossa, and infratemporal fossa. c CT scan Coronal projection of a 16 y.o.m. shows hypodense tumor located in roof of right pterygoid plate (implantation area), secondary involvement of sphenoidal sinus and important occupation of posterior nasal cavity. d CT scan in a sagittal plane of same patient of figure c showing NAF located in posterior nasal cavity with extension to the sphenoidal sinus and nasopharynx. e Coronal CT scan showing complete unilateral occlusion of the right nasal cavity with partial involvement to sphenoidal sinus. f MRI in T1 with enhancement of a 14 y.o.m. patient, presenting complete occupation of anterior nasal cavity and extension to ethmoidal cavity causing nasal septal deviation. Tumor displays central hypodensity
Macroscopically, the tumors often conformed to the anatomic structures around, expanding into the adjacent anatomic structures (Fig. 3 ). The tissue was firm to elastic in consistency, varying from yellow–brown to deep red–black depending on the amount of intraoperative hemorrhage. Microscopically all cases presented typical features of NAF, showing endothelial lined vascular spaces, often supported by a prominent smooth muscle wall. The diameter of the vascular spaces was variable, ranging from small and slit-like to large, staghorn and patulous (Fig. 4a). In more cellular areas, the vessels appeared compressed and not readily identified (Fig. 4b). Near the surface, mainly in ulcerated areas or surrounding thrombosed vessels, focal areas of chronic inflammation were evident. The fibrous stroma varied from loose and myxoid below the surface respiratory epithelium to a more collagenized, highly cellular stroma in central areas. Stromal cells varied from plump and spindled to round, stellate, angulated cells with long cytoplasmic processes, with a myofibroblast-like appearance, showing round vesicular nuclei and prominent nucleoli. Occasional binucleation and mild pleomorphism were also observed (Fig. 4c). Mitotic figures were rarely present. Some vessels displayed fibrinous thrombus, focally or completely obstructing the lumen (Fig. 4d). Abundant mast cells were found in all of the cases, even in the absence of other inflammatory cells.
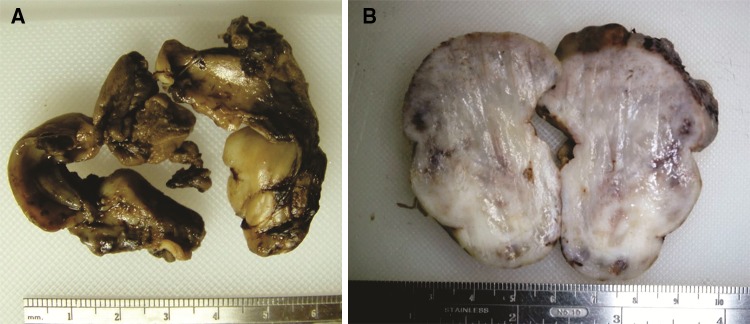
Fig. 3.
Gross features, a Gross specimen has a firm-elastic consistency, color may vary from yellowish-brown to blackish depending on the grade of intraoperative hemorrhage. Specimens show the shape of the normal anatomical cavities, as shown in the picture, as tumors in general, tend to fill anatomical spaces, before causing bone resorption by secondary pressure. b A cut section of a different specimen showing grayish color and inconspicuous vascular channels, which can eventually be difficult to visualize macroscopically
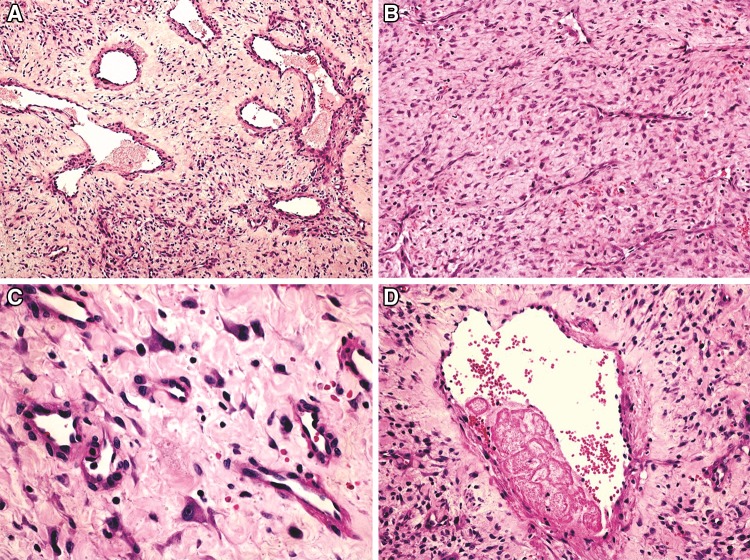
Fig. 4.
Microscopic features of nasopharyngeal angiofibroma. a Vessels of different caliber in a myxoid to densely arranged fibrous stroma; also, large vessels present thick muscular walls (HE, ×100). b More cellular areas tend to be associated with compressed small vessels (×200). c Stellate and angulated fibroblasts and some giant, binucleated cells are common (×400). d Partially thrombosed vessel (×200)
Immunohistochemical Findings
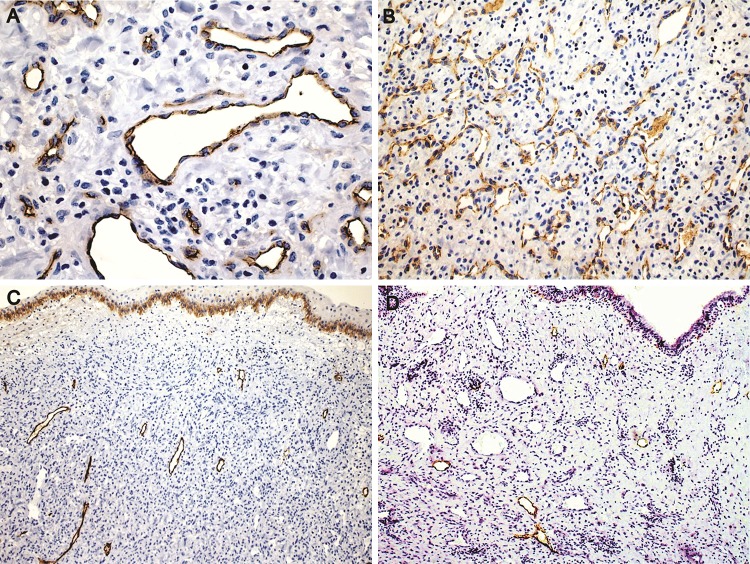
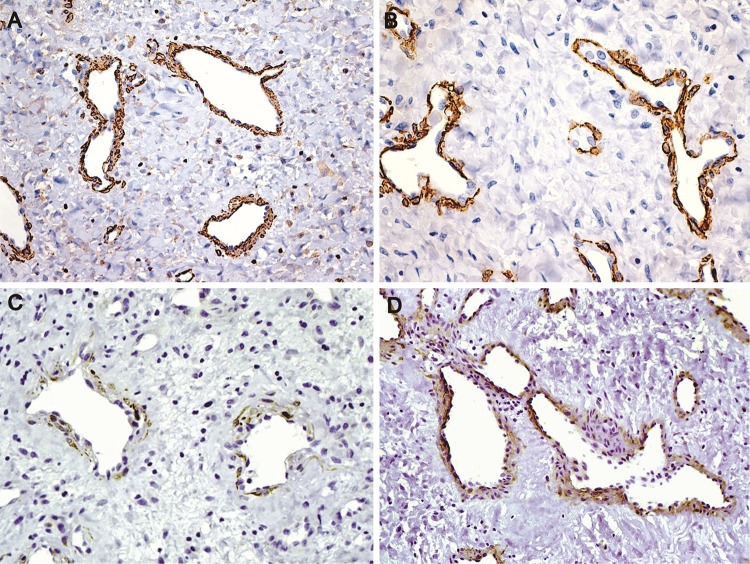
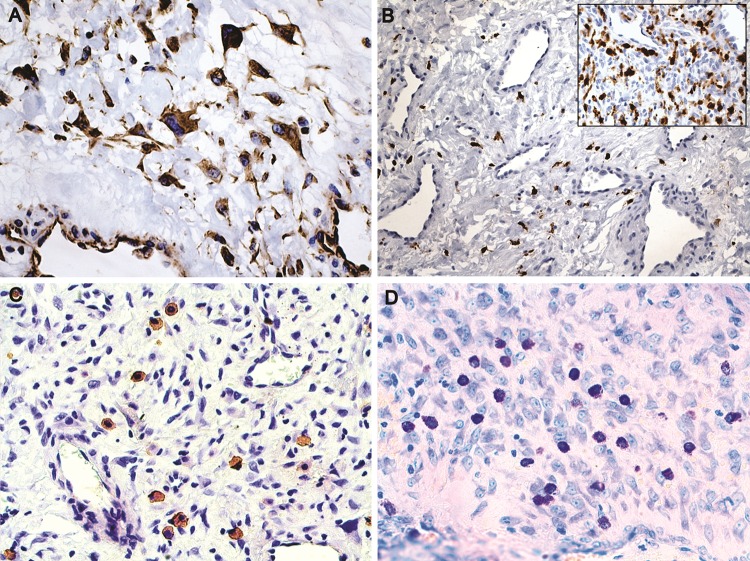
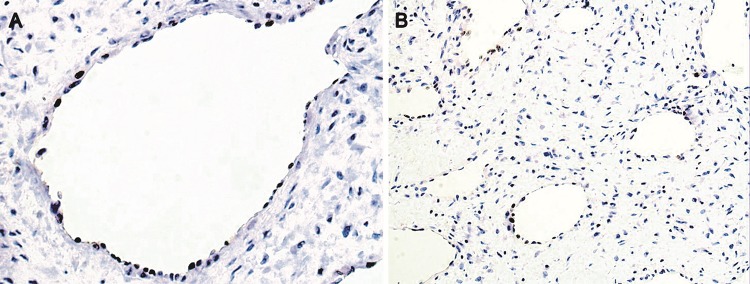
Endothelial cells were highlighted with CD34 and CD31 (Fig. 5a, b). Smaller and less common lymphatics were accentuated with D2-40 in the subepithelial peripheral regions (Fig. 5c, d). Smooth muscle actin (SMA) and specific muscle actin (HHF-35) highlighted the muscular wall of the vessels, sometimes limited to a very thin layer. H-caldesmon reactivity was restricted to the external layer of large vessels, and was weaker than SMA expression, while desmin was focally positive in the external wall of the vessels (Fig. 6a–d). SMA was negative in the stromal cells. Stromal cells were strongly positive for vimentin (Fig. 7a). Large stellate cells were common in the hypercellular areas and positive for factor XIIIa (Fig. 7b). CD68 (PGM1) and CD163 positive cells were located mainly in subepithelial regions, but negative in the stromal tumoral cells. Mast cells were abundant and highlighted by both anti-Mast Cell Tryptase and toluidine blue (Fig. 7c, d). Ki-67 was expressed almost exclusively in only endothelial cells (Fig. 8a, b).
Fig. 5.
Immunohistochemical features. a Varying size vessels showing flat endothelial cells (CD34, ×400). b CD31 expression in area rich in microvessels (×200). c, d Lymphatic vessels localized exclusively in regions near the surface epithelium. The number of lymphatic vessels is variable. Note the squamous metaplasia of the epithelium with basal epithelial cells positive for podoplanin serving as internal control in figure c (Podoplanin, ×200)
Fig. 6.
Immunohistochemical features of the larger vessels. a Muscular wall highlighted by muscle specific actin (HHF35, ×200). b Irregular vessels showing strong smooth muscle actin expression, and negativity in the stromal cells (SMA, ×400). c Focal positivity for desmin in the external muscular layer (×400). d H-Caldesmon positivity in the external smooth muscular layer (×200)
Fig. 7.
a Stellate and elongated stromal fibroblasts (vimentin, ×400). b Scattered cells expressing factor XIIIa (×200) in a hypocellular area and strong positivity in a hypercellular area (inset ×200). c, d Mast cells were numerous in the stroma as shown by IHC and toluidine blue. (Mast cell antibody and toluidine blue, ×400)
Fig. 8.
Immunohistochemical expression of Ki-67. a, b Proliferating cells expressing Ki-67 restricted to endothelium of blood vessels (Ki-67, ×400 and ×200). Note lack of Ki-67 expression in stromal cells (×200)
Discussion
In the literature, NAF has been described as a relatively rare neoplasm accounting for <1% of head and neck tumors [14]. Further, the association of NAF with familial adenomatous polyposis, in which the prevalence of NAF is 25 times more common than in age-matched controls [15], has not been confirmed in this case series.
We consider that NAF occurs exclusively in males, and previous reports in females probably do not represent this tumor, or at least, documentation is not convincing [16–19]. In 1965, Apostol and Frazell [20] reported 40 cases of NAF in male patients, and suggested that if this diagnosis is confirmed in a female, sex chromosome studies must be performed, to investigate for androgen insensitivity syndrome (formerly testicular feminization) of a phenotypic female but genetic male [18, 20].
As the disease presents around the onset of puberty, it may be related to increased circulating hormones, with the androgen, testosterone and dihydrotestosterone receptors in the tumor cells likely playing a role in pathogenesis. Patients treated with antiandrogenic medications, principally Flutamide, experienced significant side effects including gynecomastia and other feminization features [21], and given the usual association with puberty, clinicians are reluctant to employ hormone therapies. Recently, Thakar et al. [22] observed that prepubertal and postpubertal patients differ in their response to flutamide and demonstrated flutamide-induced partial regression of NAF only in postpubertal patients. These findings support the theory of an androgenic mechanism in the pathogenesis of the tumor.
The age of the patients in this series ranged from 8 to 27 years, with a mean of 14.7 years, slightly younger than the reported average (17 years) [1]. According to the literature, the diagnosis of NAF is uncommon over the age of 25, and we had only 1 patient 27 years old. Even though the term “juvenile” has been removed by the WHO classification, it is important to remember that this disorder does show a marked predilection for pediatric and adolescent males. The most common clinical complaints are epistaxis and persistent nasal obstruction, often starting as unilateral obstruction that progresses to complete nasal obstruction in more advanced cases. In contrast to cases from Europe and North America [23, 24], many of the cases in this clinical series showed a more rapid growth and clinical aggressiveness that resulted in facial deformity and asymmetry (Fig. 1 ). While epistaxis is a more likely symptom that results in seeking earlier clinical attention, the overall average symptoms for our patients and for those reported in the literature is similar. Thus, late diagnosis due to delayed clinical attention is not a likely scenario. Thus, with tumor growth, expansion into the adjacent structures will yield the clinical findings or a more advanced tumor. All tumors were non-encapsulated, characteristically filling the sinonasal and nasopharyngeal spaces, resulting in secondary bone resorption due to compression, leading to cortical bone destruction allowing the tumor to grow into surrounding soft tissues with pushing borders (Figs. 2, 3 ). Thus, by inference, tumors that reach such a large size clinically, are much more likely to be associated with significant morbidity.
In spite of the reported postpubertal regression or spontaneous involution, the severe symptoms of epistaxis and upper airway obstruction requires prompt and complete surgical treatment with very close clinical follow-up rather than delayed therapy or watchful waiting.
Malignant transformation is rare, usually linked to previous radiation therapy, representing post-irradiation sarcomas [25–27]; no malignant transformation was found in this series.
In Guatemala, it is interesting to note that this condition nearly exclusively affected male patients of low socioeconomic level, with only a single patient from a middle-class family. However, approximately 59% of Guatemalans live below the poverty line [28]. Chronic malnutrition is seen in approximately 50%, the highest in Latin America [29], and disproportionately affects indigenous people, where it reaches nearly 70%. The indigenous population represents about 50% of the overall Guatemalan population. This potential socioeconomic and ethnic relationship needs to be further investigated in other countries with a high percentage of their population living in poor socioeconomic conditions or with indigenous populations. In support, similar clinical aggressiveness has recently been reported in poor patients from India, supporting this observation [30, 31].
Preoperative diagnosis must be established on the basis of clinical symptoms and imaging studies (CT/MRI), including the use of contrast. Any biopsy is contraindicated as uncontrollable hemorrhage may occur. In our patients, severe intraoperative bleeding was the rule, leading to potential lethal complications. While presurgical embolization is recommended, optimal embolization was not employed due to economic constraints with Guatemala. Instead, fibered platinum coils are placed in the internal maxillary artery in order to mechanically decrease the blood flow and reduce bleeding at the time of surgery, although often to limited avail.
Some authors described tumor spindle cells co-expressing vimentin and SMA [4, 7], but in our cases, this feature was not observed. The stromal cells in all of our cases were positive for vimentin but negative for SMA, HHF-35 and calponin, thus suggesting a fibroblastic origin.
In our study, the proliferation marker Ki-67 was practically restricted to endothelial cells indicating that cell proliferation occurs predominantly in the vascular endothelial cells, a feature uncommon in other benign vascular tumors with normal-appearing endothelium (i.e., lacking pleomorphism; in angiosarcoma the endothelial cells are in proliferation but are highly atypical). This finding must be confirmed in NAF diagnosed in other geographic regions, as it may be related to the more aggressive clinical behavior in our patients. Other studies suggest that uncontrolled vascular proliferation is the hallmark for NAF [32], but the stromal cells also play a role [8, 9], while participation of mast cells also needs further understanding.
All cases showed factor XIIIa expression in the giant stellate fibroblast-like stromal cells. Entities composed of a fibrovascular proliferation with giant plump stellate cells including cutaneous angiofibroma, acquired digital fibrokeratoma and fibrotic lesions as oral fibrous hyperplasia also express factor XIIIa in these plump stellate stromal cells. This factor has been identified in macrophages and dermal dendritic cells suggesting a fibro-histiocytic lineage for this fibroblast-like cell. However, in this series, these cells were negative for CD68 and CD163. It has been hypothesized that its activity may be as a growth factor for fibroblasts in lesions with a prominent fibrotic component [33, 34].
Conclusions
Based on the 42 cases of NAF presented, it is postulated that there is a difference in biologic behavior of NAF probably due to geographic region and socioeconomic status of the affected patients. This observation needs to be further evaluated comparing this data with other large series of NAF from different geographic parts of the world. The role of mast cells in the pathogenesis of NAF needs to be better clarified¸ possibly related to vascular proliferation. Immunohistochemical studies suggest the stromal tumoral cells are not myofibroblasts, but are fibroblasts instead. The high Ki-67 expression in endothelial cells suggests that the vascular component may be responsible for the aggressive growth observed in our patients. Depending on the type of resection, persistence rather than recurrences is likely to account for disease presentation after initial surgery. It is recommended that even though age, grade, and site terminology is eschewed by the WHO, perhaps the unique characteristics of this tumor justify the use of the term nasopharyngeal juvenile angiofibroma.
Funding
This study was not funded.
Compliance with Ethical Standards
Conflict of interest
All authors declare they have no conflicts of interest.
Ethical Approval
All reviews performed in this study with human participants were done in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. As a retrospective review, informed consent was not required.
References
- 1.Prasad ML, Franchi A, Thompson LDR. Tumours of the nasopharynx: soft tissue tumours: nasopharyngeal angiofibroma. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. World health organization classification of head and neck tumours. 4. Lyon, France: IARC Press; 2017. pp. 74–75. [Google Scholar]
- 2.Martins MBB, de Lima FVF, Mendonça CA, et al. Nasopharyngeal angiofibroma: our experience and literature review. Int Arch Otorhinolaryngol. 2013;17(1):14–19. doi: 10.7162/S1809-97772013000100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu Y-S, Perzin KH. Non-epithelial tumors of the nasal cavity, paranasal sinuses, and nasopharynx: a clinicopathological study. I. General features and vascular tumors. Cancer. 1974;33:1275–1288. doi: 10.1002/1097-0142(197405)33:5<1275::AID-CNCR2820330513>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Pauli J, Gundelach R, Vanelli-Rees A, Rees G, Campbell C, Dubey S, et al. Juvenile nasopharyngeal angiofibroma: an immunohistochemical characterisation of the stromal cell. Pathology. 2008;40(4):396–400. doi: 10.1080/00313020802035857. [DOI] [PubMed] [Google Scholar]
- 5.Beham A, Fletcher CDM, Kainz J, Schmid C, Humer U. Nasopharyngeal angiofibroma: an immunohistochemical study of 32 cases. Virchows Archiv A Pathol Anat. 1993;423:281–285. doi: 10.1007/BF01606891. [DOI] [PubMed] [Google Scholar]
- 6.Beham A, Beham-Schmid C, Regauer S, Auböck L, Stammberger H. Nasopharyngeal angiofibroma: true neoplasm or vascular malformation? Adv Anat Pathol. 2000;7(1):36–46. doi: 10.1097/00125480-200007010-00006. [DOI] [PubMed] [Google Scholar]
- 7.Beham A, Kainz J, Stammberger H, Auböck L, Beham-Schmid C. Immunohistochemical and electron microscopical characterization of stromal cells in nasopharyngeal angiofibromas. Eur Arch Otorhinolaryngol. 1997;254:196–199. doi: 10.1007/BF00879273. [DOI] [PubMed] [Google Scholar]
- 8.Renkonen S, Häyry V, Heikkilä P, Leivo I, Haglund C, Mäkitie AA, et al. Stem-cell related proteins c-kit, c-myc and bmi-1 in juvenile nasopharyngeal angiofibroma-do they have a role? Virchows Arch. 2011;458:189–195. doi: 10.1007/s00428-010-1010-9. [DOI] [PubMed] [Google Scholar]
- 9.Silveira SM, Custódio Domingues MA, Butugan O, Brentani MM, Rogatto SR. Tumor microenvironmental genomic alterations in juvenile nasopharyngeal angiofibroma. Head Neck. 2012;34:485–492. doi: 10.1002/hed.21767. [DOI] [PubMed] [Google Scholar]
- 10.Farag MM, Ghanimah SE, Ragaie A, Saleem TH. Hormonal receptors in juvenile nasopharyngeal angiofibroma. Laryngoscope. 1987;97(2):208–211. doi: 10.1288/00005537-198702000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Hwang HC, Mills SE, Patterson K, Gown AM. Expression of androgen receptors in nasopharyngeal angiofibroma: an immunohistochemical study of 24 cases. Mod Pathol. 1998;11(11):1122–1126. [PubMed] [Google Scholar]
- 12.Saylam G, Yücel OT, Sungur A, Onerci M. Proliferation, angiogenesis and hormonal markers in juvenile nasopharyngeal angiofibroma. Int J Pediatr Otorhinolaryngol. 2006;70(2):227–234. doi: 10.1016/j.ijporl.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Carlos R, Thompson DRL, Netto AC, Santos Pimenta LGG, Correia-Silva JF, Cavaliéri Gomes C, et al. Epstein-Barr virus and human herpes virus-8 are not associated with juvenile nasopharyngeal angiofibroma. Head Neck Pathol. 2008;2:145–149. doi: 10.1007/s12105-008-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakshi SS, Bhattacharjee S. Juvenile nasopharyngeal angiofibroma. J Pediatr Hematol Oncol. 2016;38(6):491–492. doi: 10.1097/MPH.0000000000000568. [DOI] [PubMed] [Google Scholar]
- 15.Ponti G, Losi L, Pellacani G, Rossi GB, Presutti L, Mattioli F, et al. Wnt pathway, angiogenetic and hormonal markers in sporadic and familial adenomatous polyposis-associated juvenile nasopharyngeal angiofibromas (JNA) Appl Immunohistochem Mol Morphol. 2008;16(2):173–178. doi: 10.1097/PAI.0b013e31806bee12. [DOI] [PubMed] [Google Scholar]
- 16.Finerman WB. Juvenile nasopharyngeal angiofibroma in the female. AMA Arch Otolaryngol. 1951;54(6):620–623. doi: 10.1001/archotol.1951.03750120013002. [DOI] [PubMed] [Google Scholar]
- 17.Osborn DA, Sokolovski A. Juvenile nasopharyngeal angiofibroma in a female. Report of a case. Arch Otolaryngol. 1965;82(6):629–632. doi: 10.1001/archotol.1965.00760010631014. [DOI] [PubMed] [Google Scholar]
- 18.Patrocínio JA, Patrocínio LG, Borba BH, Bonatti Bde S, Guimarães AH. Nasopharyngeal angiofibroma in an elderly woman. Am J Otolaryngol. 2005;26(3):198–200. doi: 10.1016/j.amjoto.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Salimov A, Ozer S. A rare location of angiofibroma in the inferior turbinate in young woman. Int Arch Otorhinolaryngol. 2015;19(2):187–190. doi: 10.1055/s-0034-1398471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apostol JV, Frazell EL. Juvenile nasopharyngeal angiofibroma. A clinical study. Cancer. 1965;18:869–878. doi: 10.1002/1097-0142(196507)18:7<869::AID-CNCR2820180715>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 21.Labra A, Chavolla-Maga R, Lopez-Ugalde A, Alanis-Calderon J, Huerta-Delgado A. Flutamide as a preoperative treatment in juvenile angiofibroma (JA) with intracranial invasion: report of 7 cases. Otolaryngol Head Neck Surg. 2004;130(4):466–469. doi: 10.1016/j.otohns.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Thakar A, Gupta G, Bhalla AS, Jain V, Sharma SC, Sharma R, et al. Adjuvant therapy with flutamide for presurgical volume reduction in juvenile nasopharyngeal angiofibroma. Head Neck. 2011;33(12):1747–1753. doi: 10.1002/hed.21667. [DOI] [PubMed] [Google Scholar]
- 23.Kania RE, Sauvaget E, Guichard JP, Chapot R, Huy PT, Herman P. Early postoperative CT scanning for juvenile nasopharyngeal angiofibroma: detection of residual disease. AJNR Am J Neuroradiol. 2005;26(1):82–88. [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers DJ, Bevans SE, Harsha WJ. Endoscopic resection of juvenile nasopharyngeal angiofibroma. Adv Otorhinolaryngol. 2012;73:132–136. doi: 10.1159/000334470. [DOI] [PubMed] [Google Scholar]
- 25.Gisselsson L, Lindgren M, Stenram U. Sarcomatous transformation of a juvenile, nasopharyngeal angiofibroma. Acta Pathol Microbiol Scand. 1958;42(4):305–312. doi: 10.1111/j.1699-0463.1958.tb01744.x. [DOI] [PubMed] [Google Scholar]
- 26.Donald PJ. Sarcomatous degeneration in a nasopharyngeal angiofibroma. Otolaryngol Head Neck Surg. 1979;87(1):42–46. doi: 10.1177/019459987908700112. [DOI] [PubMed] [Google Scholar]
- 27.Spagnolo DV, Papadimitriou JM, Archer M. Postirradiation malignant fibrous histiocytoma arising in juvenile nasopharyngeal angiofibroma and producing alpha-1-antitrypsin. Histopathology. 1984;8(2):339–352. doi: 10.1111/j.1365-2559.1984.tb02346.x. [DOI] [PubMed] [Google Scholar]
- 28.The World Bank. Data. [Internet]. Washington, DC. Available from http://data.worldbank.org/country/guatemala28. Accessed Mar 2017.
- 29.World Food Program. Guatemala. [Internet]. Rome. Available from http://www1.wfp.org/countries/guatemala. Accessed Mar 2017.
- 30.Mishra S, Praveena NM, Panigrahi RG, Gupta YM. Imaging in the diagnosis of juvenile nasopharyngeal angiofibroma. J Clin Imaging Sci. 2013;3(Suppl 1):1. doi: 10.4103/2156-7514.109469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pardhe N, Chhibber N, Agarwal D, Jain M, Vijay P. Juvenile nasopharyngeal angiofibroma extending into the oral cavity: a rare entity. J Clin Diagn Res. 2015;9(6):ZD31-3. doi: 10.7860/JCDR/2015/12935.6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang M, Sun X, Yu H, Hu L, Wang D. Biological distinctions between juvenile nasopharyngeal angiofibroma and vascular malformation: an immunohistochemical study. Acta Histochem. 2011;113:626–630. doi: 10.1016/j.acthis.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Nemeth AJ, Penneys NS. Factor XIIa is expressed by fibroblasts in fibrovascular tumors. J Cutan Pathol. 1989;16:266–271. doi: 10.1111/j.1600-0560.1989.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 34.Cerio R, Griffiths CEM, Cooper KD, Nickoloff BJ, Headington JT. Characterization of factor XIIIa positive dermal dendritic cells in normal and inflamed skin. Br J Dermatol. 1989;121:421–431. doi: 10.1111/j.1365-2133.1989.tb15509.x. [DOI] [PubMed] [Google Scholar]