Abstract
A 70-year-old male presented with a slow growing, dome shaped and painless mass of the hard palate. The mass was excised. Histopathological examination confirmed the diagnosis of a angioleiomyoma (vascular leiomyoma). A leiomyoma is an uncommon benign tumor of smooth muscle differentiation. True leiomyomas of the oral cavity are rare and most oral tumors are derived from the smooth muscle of walls of blood vessels. Therefore, they are called vascular leiomyomas or angioleiomyomas. Clinically, they may resemble a myriad other conditions both benign and malignant. A definitive diagnosis depends upon histopathological examination of the biopsied tissue in correlation with the tumor cell immunohistochemistry. Tumors are excised and recurrence is rare. The histopathological findings and differential diagnosis of a case of a palatal angioleiomyoma are discussed.
Keywords: Oral, Vascular, Leiomyoma, Angioleiomyoma, Tumor, SMA
History
A 70-year-old male presented with a three-year history of a slow growing, painless mass of the hard palate. He had a 30-pack year history of smoking tobacco. His medical history was otherwise insignificant and he was not taking any medications.
Clinical Findings
Extraoral examination did not show any areas of concern. Intraoral examination showed a tobacco stained dentition and routine dental restorations. The lesion in question was a dome shaped, smooth surfaced mass of the hard palate just left of the midline. Anterioposteriorly, it lay along the plane of the premolar teeth. The color was dusky red to purple. Scattered superficial linear and small irregular areas of erosion were noted on the surface. The polypoid growth measured 2.0 cm × 1.5 cm approximately (Fig. 1), was non-tender, soft in consistency, and did not blanch on pressure. There was no discharge or exudate. Radiographs showed no bone involvement and none of the teeth showed evidence of pulpal or periapical pathology. The patient elected to have the lesion excised.
Fig. 1.

A smooth surfaced, dome-shaped, polypoid mass of the left hard palate
Diagnosis
Routinely processed, hematoxylin and eosin stained sections of the excised mass showed a well-circumscribed, densely cellular benign tumor of the superficial connective tissue. The tumor appeared separated from the surface epithelium by a variably thick Grenz zone of fibrofatty connective tissue. The deep surgical margin was synonymous with the tumor’s deep margin and apparently having coincided with the tumor’s natural plane of separation. Therefore, the mass was considered to be excised (Fig. 2). Higher magnification showed that the tumor consisted of brightly eosinophilic, spindle shaped cells with copious amounts of cytoplasm but indistinct cytoplasmic membranes. Nuclei were small, round to elongated with blunt ends. No cellular or nuclear atypia were seen. No mitotic activity was noted. The tumor cells were arranged in concentric rings around endothelium-lined vascular channels that looked compressed. Interstitial areas between such concentric whorls of cells were occupied by loose fascicles of similarly bright eosinophilic spindle cells (Fig. 3). The tumor cells were diffusely and uniformly, strongly reactive to alpha smooth muscle actin (SMA) (Fig. 4). The strong cytoplasmic SMA reactivity extended to both; the cells arranged in whorls around the vascular channels and to those arranged in short fascicles (Fig. 5). Based on the histopathological and immunohistochemical findings, a diagnosis of an angioleiomyoma (vascular leiomyoma) was given.
Fig. 2.
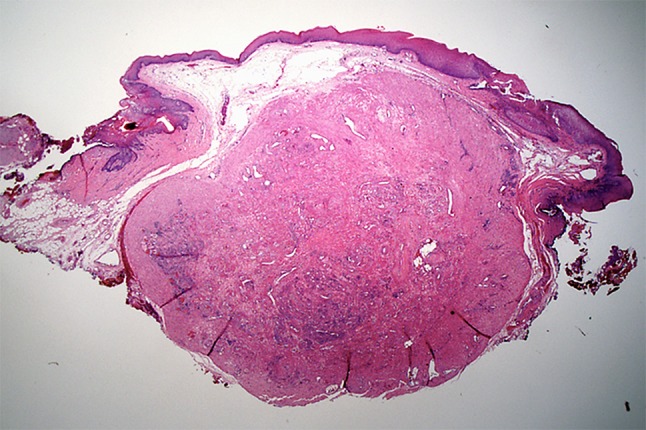
A well-circumscribed tumor occupying the superficial connective tissue. The tumor is separated from the surface epithelium by a Grenz zone of fibro-fatty connective tissue. (H & E, 4X)
Fig. 3.
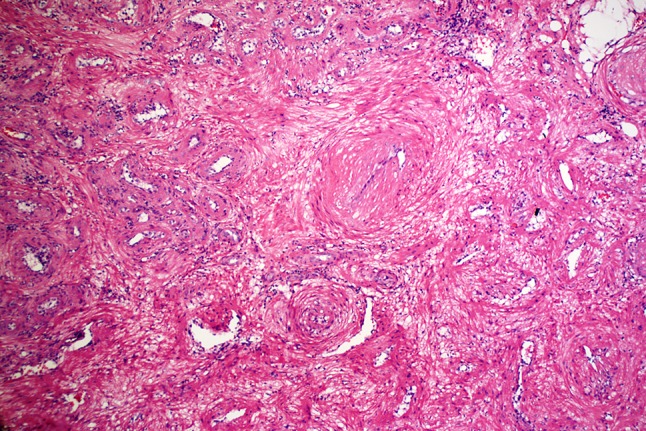
Closely packed whorls and short fascicles of brightly eosinophilic spindle cells with indistinct cytoplasmic membranes. The whorls surround compressed endothelium-lined vascular channels. (H & E, 20X)
Fig. 4.
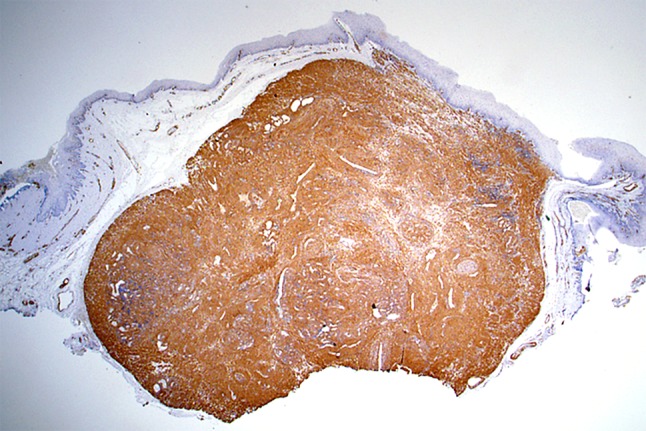
The tumor cells express diffuse and strong immunohistochemical reactivity to Smooth muscle actin (SMA) (IHC, 4X)
Fig. 5.
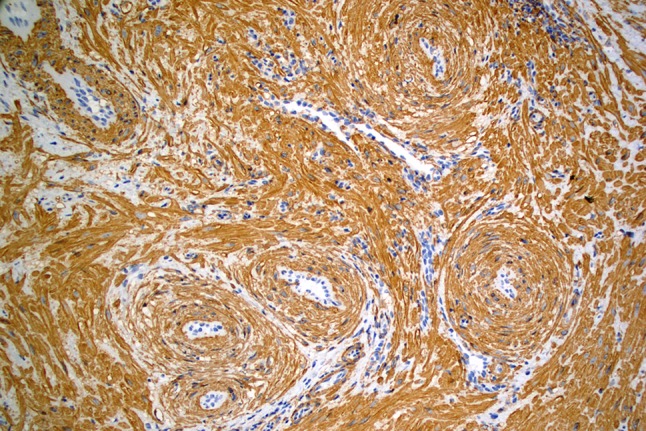
Alpha smooth muscle actin immunohistochemical reactivity of lesional cells highlights concentric whorls surrounding the compressed, endothelium-lined vascular channels. SMA positive cells are also arranged in short fascicles between the perivascular whorls (IHC, 20X)
Discussion
Leiomyomas are benign spindle cell tumors that are common in anatomical sites that are innately rich in smooth muscles such as the myometrium of the uterus (uterine fibroids) and the gastrointestinal tract [1, 2]. This inherently makes oral leiomyomas a rare tumor. Oral leiomyomas arise from the tunica media of blood vessels and are therefore called vascular leiomyomas or angioleiomyomas [1, 3]. Oral angioleiomyomas have been reported on the lips, palate, buccal mucosa, tongue and gingiva in that order of decreasing frequency [2–5]. Rare intraosseous tumors have also been reported [2, 3, 6, 7].
This tumor is diagnosed across a wide age range from 14 to 79 years. However, majority of tumors are seen from the 4th to the 6th decades of life [3–5]. In a review of 109 cases of oral angioleiomyomas, a male to female ratio of 1.43:1 was noted [3]. Tumor duration has ranged from one month to 14 years [5]. The largest tumor has measured 5 cm × 8 cm in size [8]. Small tumors are asymptomatic. Large tumors produce mass effects such as difficulty in closing the mouth, chewing and swallowing. Tumors may occasionally get traumatized and ulcerated during oral function. This may result in discomfort and pain [3–5]. A tongue lesion reportedly also produced occasional numbness [3].
Angioleiomyomas of the oral cavity appear reddish blue in color and are soft and compressible on palpation. These tumors are circumscribed, round to oval and pedunculated or sessile masses [3]. Clinically, they are frequently diagnosed as benign tumors and tumor-like conditions including tumors of minor salivary gland origin [3]. Oral angioleiomyomas are surgically excised and recurrence has been reported in only two cases, most likely due to incomplete removal [9, 10].
The diagnosis of oral angioleiomyoma is established only upon histopathological examination of the biopsied tissue. These tumors are well-circumscribed, compact masses of multiple vascular spaces that are compressed into slit-like lumens by thick walls of circumferentially arranged spindle cells that express smooth muscle actin protein using immunohistochemistry [4, 5].
The clinical differential diagnosis must take into account the site affected. The differential diagnosis of this slow growing, smooth surfaced mass includes a palatal abscess and a number of tumor and tumor-like lesions such as the pyogenic granuloma, peripheral giant cell granuloma, peripheral ossifying fibroma, peripheral odontogenic tumors, schwannoma and salivary gland tumors.
A palatal abscess may occur secondary to a periapical infection of the palatal roots of maxillary teeth affected by pulpal inflammation. Carious, fractured or non-vital teeth are seen in the vicinity of the lesion. There is some degree of pain and radiographs would show bone loss associated with a periapical pathology. Clinically, a palatal abscess may also exhibit a patent sinus tract discharging pus or a serosanguinous fluid [11].
The pyogenic granuloma is a common benign tumor-like growth. While it may occur on any mucosal surface of the oral cavity, majority of lesions affect the gingiva. It presents as a painless, soft, pinkish-red, frequently ulcerated lobulated growth. On the gingiva, it results from chronic irritation by dental calculus, prosthetic appliance extensions or restoration margins. The pyogenic granuloma occurs more frequently in children and young adults and shows a female predilection. Hormonal changes associated with pregnancy leads to an increased incidence of pyogenic granulomas. In this setting, the pyogenic granuloma is referred to as a pregnancy tumor or granuloma gravidarum [11, 12].
The peripheral giant cell granuloma (PGCG) is an intraoral benign tumor-like growth that occurs exclusively over the facial/buccal or palatal/lingual gingiva. Clinically, the PGCG appears as a dark red to bluish purple nodular asymptomatic mass. It is non-tender and soft to touch. PGCGs are more common in adult females. A slight predilection for the mandibular gingiva is observed. The clinical appearance is attributed to the vascularity and deposition of hemosiderin pigment in the tissue [11, 12].
The peripheral ossifying fibroma (POF) is a tumor-like reactive mass. Cases are localized to the gingiva with a slight predilection for the maxillary gingiva. The POF is an asymptomatic, pale pink, circumscribed, nodule. Upon palpation, it is non-tender and feels firm to hard in consistency. The color and hardness are due to the dense collagen and presence of variable amounts of metaplastic osteoid within the lesion. The POF is seen more commonly in the second decade of life and is more common in females [11, 12].
The most common peripheral odontogenic tumors reported in literature include the peripheral odontogenic fibroma and the peripheral ameloblastoma. Peripheral odontogenic tumors present as slow growing, pink to red, sessile, firm, asymptomatic nodules. They may resemble the more common reactive lesions such as the POF and PGCG. An accurate diagnosis of these lesions is dependent on histopathological examination of the biopsied tissue [11, 13].
Schwannomas are benign tumors of peripheral nerve origin that affect adults. The head and neck and more specifically, the oral cavity is the commonest location for schwannomas. Common intraoral locations include the tongue and the palate [14]. Oral schwannomas are slow growing painless, circumscribed masses surrounded by a collagenous capsule and are enucleated with ease. Clinically, they are indistinguishable from other benign masses and the confirmatory diagnosis rests on microscopic examination of the biopsied tissue [14].
Approximately 15% of salivary gland neoplasms affect the minor glands of the oral cavity. The palate is the most common site of oral involvement (42.5%) followed by the upper lip (18.5%) and buccal mucosa (15%) [15]. Fifty-eight percent of palatal salivary tumors are benign and the pleomorphic adenoma accounts for slightly more than 50% of these benign tumors of the palate. The polymorphous adenocarcinoma accounts for 38% of the malignant salivary gland tumors of the palate while the mucoepidermoid carcinoma and the adenoid cystic carcinoma account for 25% each. The mucoepidermoid and adenoid cystic carcinomas tend to be low-grade neoplasms at this site and may show ulceration [15].
Soft tissue masses growing over the masticatory mucosa (attached gingiva and hard palate) may either show no radiographic changes or they may exert pressure over the cortical plate to produce a characteristic saucerization or cupping resorption of bone that may register as a unilocular radiolucency on a two dimensional film [11, 13].
Oral angioleiomyomas are well-circumscribed tumors. These benign tumors present as a slow growing, dome-shaped mass most commonly affecting the lips or the palate. These tumors are asymptomatic and may be present from months to years. Clinically, they may resemble many other common reactive conditions as well as benign and malignant tumors. A dusky red to bluish purple hue may suggest vascular content. Although a well-formed collagenous capsule is not seen, surgical excision is easily enabled and is the treatment of choice. Ease of access and small tumor size permits an excisional biopsy in most cases. Although these tumors have a significant vascular component, excessive bleeding during the excision is not encountered. Excision with clear margins is curative with a very low potential for recurrence. Prognosis is excellent.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest associated with this report.
Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Contributor Information
Swati Y. Rawal, Email: SwatiYeshwant@imu.edu.my
Yeshwant B. Rawal, Phone: 1 206 221 3960, Email: ybrawal@uw.edu
References
- 1.Ramesh P, Annapureddy SR, Khan F, Sutaria PD. Angioleiomyoma: a clinical, pathological and radiological review. Int J Clin Pract. 2004;58:587–591. doi: 10.1111/j.1368-5031.2004.00085.x. [DOI] [PubMed] [Google Scholar]
- 2.Hachisuga T, Hashimoto H, Enjoji M. Angioleiomyoma: a clinicopathologic reappraisal of 562 cases. Cancer. 1984;54:126–130. doi: 10.1002/1097-0142(19840701)54:1<126::AID-CNCR2820540125>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Brooks JK, Nikitakis NG, Goodman NJ, Levy BA. Clinicopathologic characterization of oral angioleiomyomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:221–227. doi: 10.1067/moe.2002.125276. [DOI] [PubMed] [Google Scholar]
- 4.Eley KA, Alroyayamina S, Golding SJ, et al. Angioleiomyoma of the hard palate: report of a case and review of the literature and magnetic resonance imaging findings of this rare entity. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:e45–e49. doi: 10.1016/j.oooo.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Tsuji T, Satoh K, Nakano H, Kogo M. Clinical characteristics of angioleiomyoma of the hard palate: report of a case and an analysis of the reported cases. J Oral Maxillofac Surg. 2014;72:920–926. doi: 10.1016/j.joms.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Goldblatt LI, Edesess RB. Central leiomyoma of the mandible. Oral Surg Oral Med Oral Pathol. 1977;43:591–597. doi: 10.1016/0030-4220(77)90114-1. [DOI] [PubMed] [Google Scholar]
- 7.White DK, Selinger LR, Miller AS, Behr MM, Damm DD. Primary angioleiomyoma of the mandible. J Oral Maxillofac Surg. 1985;43:640–644. doi: 10.1016/0278-2391(85)90140-5. [DOI] [PubMed] [Google Scholar]
- 8.Hemani DD, Gupta AK, Sharma KK, Sharma SD. Leiomyoma of the palate. J Laryngol Otol. 1983;97:471–477. doi: 10.1017/S0022215100094408. [DOI] [PubMed] [Google Scholar]
- 9.Svane TJ, Smith BR, Cosentino BJ, et al. Oral leiomyomas: review of the literature and report of a case of palatal angioleiomyoma. J Periodontol. 1986;57:433–435. doi: 10.1902/jop.1986.57.7.433. [DOI] [PubMed] [Google Scholar]
- 10.Natiella JR, Neiders ME, Greene GW. Oral leiomyoma. Report of six cases and a review of the literature. J Oral Pathol. 1982;11:353–365. doi: 10.1111/j.1600-0714.1982.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 11.Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and maxillofacial pathology. 3. St.Louis: Saunders Elsevier; 2009. [Google Scholar]
- 12.Esmeili T, Lozada-Nur F, Epstein J. Common benign oral soft tissue masses. Dent Clin North Am. 2005;49:223–240. doi: 10.1016/j.cden.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Curran AE. Peripheral odontogenic tumours. Oral Maxillofac Surg Clin North Am. 2004;16:399–408. doi: 10.1016/j.coms.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Butler RT, Patel RM, McHugh JB. Head and neck schwannomas: 20-year experience of a single institution excluding cutaneous and acoustic sites. Head Neck Pathol. 2016;10:286–291. doi: 10.1007/s12105-016-0680-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waldron CA, El-Mofty SK, Gnepp DR. Tumours of the intraoral minor salivary glands: a demographic and histologic study of 426 cases. Oral Surg Oral Med Oral Pathol. 1988;66:323–333. doi: 10.1016/0030-4220(88)90240-X. [DOI] [PubMed] [Google Scholar]


