Abstract
NKX2.2 is a new immunohistochemical marker that has been reported to be sensitive and specific for Ewing sarcoma (ES). It has not, however, been investigated specifically in the sinonasal small round blue cell tumor (SRBCT) differential diagnosis which includes many tumors specific to that site. It has also not been investigated in the newly recognized “adamantinoma-like” variant of ES. Immunohistochemistry for NKX2.2 was performed on 170 poorly differentiated sinonasal neoplasms: 73 squamous cell carcinomas (67 poorly differentiated, non-keratinizing, or basaloid types and 6 nasopharyngeal carcinomas), 46 olfactory neuroblastomas, 8 sinonasal undifferentiated carcinomas (SNUCs), 6 melanomas, 7 Ewing sarcomas, 6 SMARCB1-deficient carcinomas, 6 teratocarcinosarcomas, 5 alveolar rhabdomyosarcomas, 4 solid adenoid cystic carcinomas, 4 NK/T cell lymphomas, 3 NUT carcinomas, and 2 small cell carcinomas. NKX2.2 was positive in 7 of 7 (100%) Ewing sarcomas, including 3 adamantinoma-like variant (all diffuse, 5 strong and 2 weak). It was also positive in 5 of 6 (83%) teratocarcinosarcomas (strong, but focal), 12 of 46 (26%) olfactory neuroblastomas (diffuse, 2 strong and 10 weak), 4 of 6 melanomas (2 diffuse, 2 focal, all weak), and 1 of 2 small cell carcinomas (diffuse and strong). All squamous cell carcinomas, NUT carcinomas, SMARCB1-deficient carcinomas, SNUCs, solid adenoid cystic carcinomas, NK/T cell lymphomas, and alveolar rhabdomyosarcomas were negative. In the sinonasal SRBCT differential diagnosis, NKX2.2 is a useful and very sensitive marker for Ewing sarcoma, including the treacherous adamantinoma-like variant. At the same time, it is not entirely specific, as it will be positive in a subset of other neuroendocrine/neuroectodermal tumors. As a result, NKX2.2 must be utilized as part of an immunohistochemical panel with other markers, especially cytokeratins, melanoma markers, and CD99.
Keywords: NKX2.2, CD99, Ewing sarcoma, Primitive neuroectodermal tumor, EWSR1-FLI1
Introduction
Ewing sarcoma (ES) is a highly aggressive neoplasm that most commonly affects the deep soft tissues of the extremities of adolescents and young adults, and is characterized by translocations fusing EWSR1 with one of the ETS family of transcription factors, most frequently t(11;22)(q24;q12) resulting in EWSR1-FLI1 fusion (in approximately 90% of cases) [1]. Recently, NKX2.2—a homeodomain transcription factor that plays a crucial role in central nervous system development, oligodendrocyte differentiation, and neuroendocrine differentiation in the central nervous system, gastrointestinal tract, and pancreas [2–5]—has emerged as a new marker for ES that is reportedly both sensitive and specific [6, 7]. NKX2.2 has also been reported to be a downstream target of EWSR1-FLI1 that is necessary for oncogenic transformation via transcription repression, resulting in the block of mesenchymal features in ES [8–10]. Furthermore, the combination of CD99 and NKX2.2 has been reported to be highly specific for Ewing sarcoma [11].
NKX2.2 has not yet been investigated specifically in the sinonasal small round blue cell tumor (SRBCT) differential diagnosis which includes many tumors specific to that site. Indeed, the differential diagnosis for sinonasal SRBCTs is broad and includes epithelial (squamous cell carcinoma and variants, NUT carcinoma, lymphoepithelial carcinoma, small cell neuroendocrine carcinoma, teratocarcinosarcoma, salivary gland carcinomas, sinonasal undifferentiated carcinoma), neuroectodermal (olfactory neuroblastoma, malignant mucosal melanoma, Ewing sarcoma), soft tissue (rhabdomyosarcoma, desmoplastic small round cell tumor, synovial sarcoma), hematopoietic (NK/T cell lymphoma, diffuse large B cell lymphoma), and secondary neoplasms (either by direct extension such as nasopharyngeal carcinoma, or by metastasis) [12, 13]. Furthermore, NKX2.2 has not been investigated in the newly-recognized “adamantinoma-like” variant of Ewing sarcoma, a particularly challenging entity with significant morphologic and immunohistochemical overlap with other tumors in the differential diagnosis [14–16]. This study aimed to evaluate the utility of NKX2.2 as an immunohistochemical marker for Ewing sarcoma versus other small round blue cell tumors of the sinonasal tract.
Methods
We performed NKX2.2 on formalin fixed and paraffin embedded tissue blocks from 170 poorly differentiated sinonasal neoplasms were retrieved from the Surgical Pathology archives of The Johns Hopkins Hospital. The cases included 73 SCCs (67 poorly differentiated, non-keratinizing, or basaloid types and 6 nasopharyngeal carcinomas), 46 olfactory neuroblastomas, 8 SNUCs, 6 melanomas, 7 Ewing sarcomas, 6 SMARCB1-deficient carcinomas, 6 teratocarcinosarcomas, 5 alveolar rhabdomyosarcomas, 4 solid adenoid cystic carcinomas, 4 NK/T cell lymphomas, 3 NUT midline carcinomas, and 2 small cell carcinomas. 35 of the cases were tested on whole slides, while the remaining 133 tumors were present on previously constructed tissue microarrays [17–19]. Three of the four sinonasal adamantinoma-like Ewing sarcomas have been previously published [15, 20]. Immunohistochemistry for NKX2.2 (74.5A5 monoclonal antibody, BD Biosciences, San Diego, CA, 1:100 dilution) was performed on five-micron sections utilizing standard protocols on a Ventana Benchmark XT autostainer (Ventana Medical Systems, Inc. Tucson, AZ). The distribution and intensity of staining was noted. Nuclear staining in <50% of tumor cells was regarded as ‘‘focal’’ while positivity in ≥50% was regarded as “diffuse”.
Results
The results are summarized in Table 1. NKX2.2 was positive in 7 of 7 (100%) Ewing sarcomas (Fig. 1) (all diffuse, 5 strong and 2 weak), including four adamantinoma-like variants (Fig. 2). Among tumors that were not ES, NKX2.2 was also positive in 5 of 6 (83%) teratocarcinosarcomas (always strong and focal), 12 of 46 (26%) olfactory neuroblastomas (all diffuse, 2 strong and 10 weak), 4 of 6 melanomas (2 diffuse, 2 focal, all weak), and 1 of 2 small cell carcinomas (diffuse and strong) (Fig. 3). It was negative in 73 SCCs (67 poorly differentiated, non-keratinizing, or basaloid types and 6 nasopharyngeal carcinomas), 3 NUT carcinomas, 6 SMARCB1-deficient carcinomas, 8 SNUC, 4 solid adenoid cystic carcinomas, 4 NK/T cell lymphomas, and 5 alveolar rhabdomyosarcomas.
Table 1.
NKX2.2 immunohistochemistry in sinonasal neoplasms
| Diagnosis | Positivity | Extent | Intensity |
|---|---|---|---|
| Ewing sarcoma | 7/7 (100) | F0, D7 | W2, S5 |
| Teratocarcinosarcoma | 5/6 (83) | F5, D0 | W0, S5 |
| Melanoma | 4/6 (67) | F2, D2 | W4, S0 |
| Small cell carcinoma | 1/2 (50) | F0, D1 | W0, S1 |
| Olfactory neuroblastoma | 12/46 (26) | F0, D12 | W10, S2 |
| Squamous cell carcinoma | 0/73 (0) | – | – |
| Sinonasal undifferentiated carcinoma (SNUC) | 0/8 (0) | – | – |
| SMARCB1-deficient carcinoma | 0/6 (0) | – | – |
| Alveolar rhabdomyosarcoma | 0/5 (0) | – | – |
| Solid adenoid cystic carcinoma | 0/4 (0) | – | – |
| NK/T cell lymphoma | 0/4 (0) | – | – |
| NUT midline carcinoma | 0/3 (0) | – | – |
F focal, D diffuse, W weak, S strong
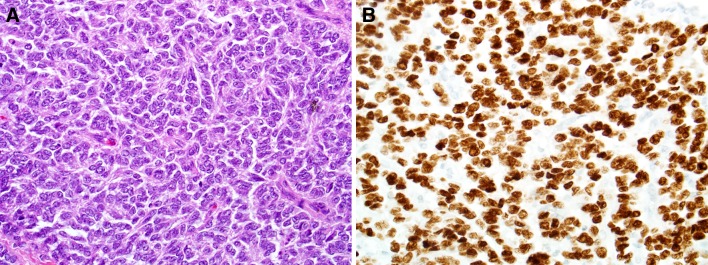
Fig. 1.
Ewing sarcoma demonstrating typical morphology (a, H&E stain, X400). All cases of Ewing sarcoma were diffusely positive for NKX2.2 by immunohistochemistry (b, X400)
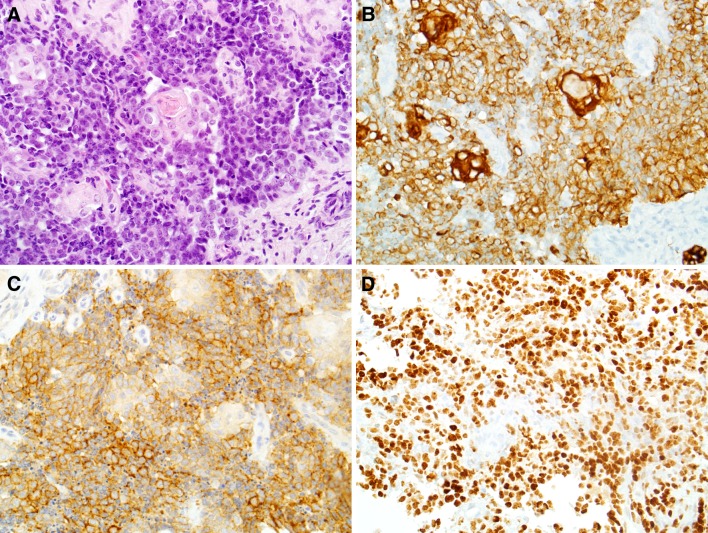
Fig. 2.
Adamantinoma-like Ewing sarcoma often exhibits squamous differentiation on routine histology (a, X400) and is diffusely positive for pan-cytokeratin (b, X400). However, adamantinoma-like Ewing sarcoma is also consistently positive for CD99 (c, X400) and NKX2.2 (d, X400)
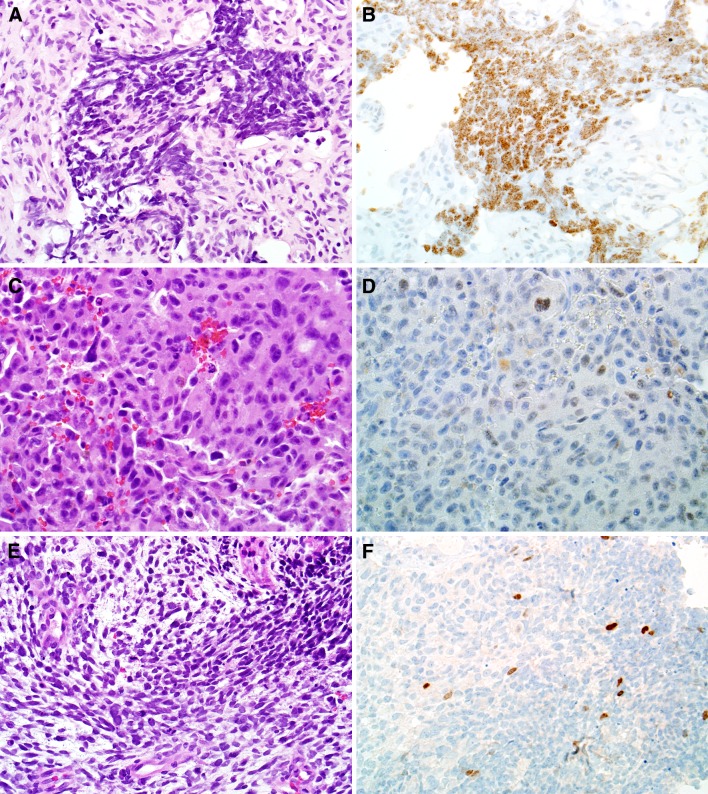
Fig. 3.
Some sinonasal tumors that were not Ewing sarcoma were also positive for NKX2.2, including olfactory neuroblastoma (a, b, X400), malignant melanoma (c, d, X400), and teratocarcinosarcoma (e, f, X200)
Discussion
Diagnostic pathology of the sinonasal tract poses a particular challenge due to the diverse number of neoplasms that can occur at this site, many of which have overlapping morphological features (so-called “small round blue cell tumors”), and because of their rarity, comprising <1% of all malignancies and only 3% of head and neck malignancies [12, 21]. Further complicating the differential diagnosis is the complex anatomy of the sinonasal tract which often results in suboptimal biopsies that are small, fragmented, crushed, and/or predominantly blood clot, and secondary extension of tumors from adjacent nearby locations such as the nasopharynx [12, 22]. As such, ancillary tests such as immunohistochemistry and molecular techniques play a crucial role in reaching the correct diagnosis [23]. Moreover, there are a number of recently described tumor types that many pathologists may not yet be familiar with, including NUT carcinoma [19], SMARCB1-deficient sinonasal carcinoma [24], HPV-related carcinomas [17], and adamantinoma-like Ewing sarcoma [15, 20].
ES is particularly challenging to diagnose in the sinonasal tract given its morphologic and immunohistochemical heterogeneity, which often overlaps with other tumors in the differential diagnosis [13, 21, 25, 26]. Despite the challenges in diagnosing ES in the sinonasal tract, accurate tumor classification is critical for both prognosis and treatment, as ES is typically treated with specific chemotherapy protocols [27, 28]. The gold standard for diagnosing ES involves demonstrating EWSR1 gene fusions by either reverse-transcription PCR (RT-PCR) or break-apart fluorescence in situ hybridization (FISH) [1, 29]. However, given the higher cost and increased turnaround time of molecular testing, immunohistochemistry has become an appealing alternative. When diffuse and membranous, CD99 (O13) is a highly sensitive marker staining almost all Ewing sarcomas, but it is unfortunately non-specific and stains a significant number of other small round blue cell tumors [1, 30]. Additionally, the FLI1 antibody has been used for diagnosing ES, but it too suffers from poor specificity and in fact appears to be less sensitive than CD99 [16, 30, 31]. The adamantinoma-like variant of ES poses an especially difficult challenge in the sinonasal tract as it demonstrates overt epithelial differentiation morphologically (i.e., squamous pearls, intracellular bridges) and/or immunophenotypically (i.e., diffuse p40 and/or high-molecular weight cytokeratin), thus mimicking squamous cell carcinoma, a much more common malignancy in the sinonasal tract. Nevertheless, adamantinoma-like ES cases harbor the classic EWSR1-FLI1 fusions of ES [14–16]. Consequently, an additional marker of ES would be of value in the work-up of small round blue cell tumors, particularly in the sinonasal tract.
Examining 170 poorly differentiated sinonasal neoplasms with a SRBCT appearance, we demonstrated that NKX2.2 is a highly sensitive marker for ES including the adamantinoma-like variant (100% of cases). However, much like CD99, NKX2.2 is not entirely specific for ES. Perhaps not surprisingly given that NKX2.2 is involved in central nervous system development and neuroendocrine differentiation [2–4], NKX2.2 was positive in a subset of olfactory neuroblastomas (12 of 46), small cell carcinomas (1 of 2), and teratocarcinosarcomas (5 of 6). Low-grade forms of olfactory neuroblastoma may closely resemble ES, but immunostains for synaptophysin and chromogranin—typically focal or negative in ES—are generally strongly positive in olfactory neuroblastoma. Moreover, olfactory neuroblastoma lacks CD99 staining and often exhibits a S100-positive sustentacular tumor cell population. Small cell carcinoma is typically cytokeratin-positive, but often shows a dot-like distribution not seen in adamantinoma-like ES. In addition, small cell carcinoma is negative for CD99 and, unlike adamantinoma-like ES, is focal or negative for p40. Teratocarcinosarcomas—a rare and unusual neoplasm of the sinonasal tract that demonstrates a heterologous morphology with varying proportions of benign and malignant epithelial, mesenchymal, and neuroepithelial elements [32–34]—often show an overt, though often focal, neuroepithelial component, which corresponds to the focal, strong NKX2.2 staining seen in our study. Recognizing the various elements of teratocarcinosarcoma is crucial in separating it from ES and other tumor types, and therefore proper tissue sampling is of utmost importance. Finally, NKX2.2 was positive in 4 of 6 cases of malignant mucosal melanoma, another neuroectodermal neoplasm that has a notoriously wide range of histomorphologic appearances. As a result, if melanoma is a consideration, melanocytic markers such as S100, SOX10, HMB45, and Melan-A must be included in the immunohistochemical panel.
In summary, NKX2.2 is a very sensitive marker for Ewing sarcoma including the adamantinoma-like variant, and is thus useful in the small round blue cell differential diagnosis. At the same time, this marker is not entirely specific, as it will be positive in a subset of other neuroendocrine/neuroectodermal tumors. As a result, it must be used in a panel with other markers, especially cytokeratins, melanocytic markers, and CD99.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Weiss SW, Goldblum JR. Enzinger and Weiss’s soft tissue tumors. 5. Philadelphia: Mosby Elsevier; 2008. [Google Scholar]
- 2.Qi Y, Cai J, Wu Y, Wu R, Lee J, Fu H, et al. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development. 2001;128:2723–2733. doi: 10.1242/dev.128.14.2723. [DOI] [PubMed] [Google Scholar]
- 3.Briscoe J, Sussel L, Serup P, Hartigan-O’Connor D, Jessell TM, Rubenstein JLR, et al. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398:622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y-C, Gallego-Arteche E, Iezza G, Yuan X, Matli MR, Choo S-P, et al. Homeodomain transcription factor NKX2.2 functions in immature cells to control enteroendocrine differentiation and is expressed in gastrointestinal neuroendocrine tumors. Endocr Relat Cancer. 2009;16:267–279. doi: 10.1677/ERC-08-0127. [DOI] [PubMed] [Google Scholar]
- 5.Sussel L, Kalamaras J, Hartigan-O’Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, et al. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 1998;125:2213–2221. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- 6.Hung YP, Fletcher CDM, Hornick JL. Evaluation of NKX2-2 expression in round cell sarcomas and other tumors with EWSR1 rearrangement: imperfect specificity for Ewing sarcoma. Mod Pathol. 2016;29:370–380. doi: 10.1038/modpathol.2016.31. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida A, Sekine S, Tsuta K, Fukayama M, Furuta K, Tsuda H. NKX2.2 is a Useful Immunohistochemical Marker for Ewing Sarcoma. Am J Surg Pathol. 2012;36:993–999. doi: 10.1097/PAS.0b013e31824ee43c. [DOI] [PubMed] [Google Scholar]
- 8.Fadul J, Bell R, Hoffman LM, Beckerle MC, Engel ME, Lessnick SL. EWS/FLI utilizes NKX2-2 to repress mesenchymal features of Ewing sarcoma. Genes Cancer. 2015;6:129–143. doi: 10.18632/genesandcancer.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith R, Owen LA, Trem DJ, Wong JS, Whangbo JS, Golub TR, et al. Expression profiling of EWS/FLI identifies NKX2.2 as a critical target gene in Ewing’s sarcoma. Cancer Cell. 2006;9:405–416. doi: 10.1016/j.ccr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Owen LA, Kowalewski AA, Lessnick SL. EWS/FLI mediates transcriptional repression via NKX2.2 during oncogenic transformation in Ewing’s sarcoma. PLoS ONE. 2008;3:e1965. doi: 10.1371/journal.pone.0001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shibuya R, Matsuyama A, Nakamoto M, Shiba E, Kasai T, Hisaoka M. The combination of CD99 and NKX2.2, a transcriptional target of EWSR1-FLI1, is highly specific for the diagnosis of Ewing sarcoma. Virchows Arch. 2014;465:599–605. doi: 10.1007/s00428-014-1627-1. [DOI] [PubMed] [Google Scholar]
- 12.Simons SA, Bridge JA, Leon ME. Sinonasal small round blue cell tumors: an approach to diagnosis. Semin Diagn Pathol. 2016;33:91–103. doi: 10.1053/j.semdp.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Bridge JA, Bowen JM, Smith RB. The small round blue cell tumors of the sinonasal area. Head Neck Pathol. 2010;4:84–93. doi: 10.1007/s12105-009-0158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bishop JA. Recently described neoplasms of the sinonasal tract. Semin Diagn Pathol. 2016;33:62–70. doi: 10.1053/j.semdp.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Bishop JA, Alaggio R, Zhang L, Seethala RR, Antonescu CR. Adamantinoma-like Ewing family tumors of the head and neck: a pitfall in the differential diagnosis of basaloid and myoepithelial carcinomas. Am J Surg Pathol. 2015;39:1267–1274. doi: 10.1097/PAS.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folpe AL, Goldblum JR, Rubin BP, Shehata BM, Liu W, Dei Tos AP, et al. Morphologic and immunophenotypic diversity in Ewing family tumors: a study of 66 genetically confirmed cases. Am J Surg Pathol. 2005;29:1025–1033. [PubMed] [Google Scholar]
- 17.Bishop JA, Guo TW, Smith DF, Wang H, Ogawa T, Pai SI, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37:185–192. doi: 10.1097/PAS.0b013e3182698673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilson MP, Gallia GL, Bishop JA. Among sinonasal tumors, CDX-2 immunoexpression is not restricted to intestinal-type adenocarcinomas. Head Neck Pathol. 2013;8:59–65. doi: 10.1007/s12105-013-0475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bishop JA, Westra WH. NUT midline carcinomas of the sinonasal tract. Am J Surg Pathol. 2012;36:1216–1221. doi: 10.1097/PAS.0b013e318254ce54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexiev BA, Tumer Y, Bishop JA. Sinonasal adamantinoma-like Ewing sarcoma: a case report. Pathol Res Pract. 2017;213:422–426. doi: 10.1016/j.prp.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Iezzoni JC, Mills SE. “Undifferentiated” small round cell tumors of the sinonasal tract: differential diagnosis update. Am J Clin Pathol. 2005;124(Suppl):S110–S121. doi: 10.1309/59RBT2RK6LQE4YHB. [DOI] [PubMed] [Google Scholar]
- 22.Tilson MP, Bishop JA. Utility of p40 in the differential diagnosis of small round blue cell tumors of the sinonasal tract. Head Neck Pathol. 2013;8:141–145. doi: 10.1007/s12105-013-0496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chapman-Fredricks J, Jorda M, Gomez-Fernandez C. A limited immunohistochemical panel helps differentiate small cell epithelial malignancies of the sinonasal cavity and nasopharynx. Appl Immunohistochem Mol Morphol AIMM. 2009;17:207–210. doi: 10.1097/PAI.0b013e31818fc85c. [DOI] [PubMed] [Google Scholar]
- 24.Bishop JA, Antonescu CR, Westra WH. SMARCB1 (INI-1)-deficient carcinomas of the sinonasal tract. Am J Surg Pathol. 2014;38:1282–1289. doi: 10.1097/PAS.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hafezi S, Seethala RR, Stelow EB, Mills SE, Leong IT, MacDuff E, et al. Ewing’s family of tumors of the sinonasal tract and maxillary bone. Head Neck Pathol. 2011;5:8–16. doi: 10.1007/s12105-010-0227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaccani JP, Forte V, de Jong AL, Taylor G. Ewing’s sarcoma of the head and neck in children. Int J Pediatr Otorhinolaryngol. 1999;48:209–216. doi: 10.1016/S0165-5876(99)00030-0. [DOI] [PubMed] [Google Scholar]
- 27.Huang M, Lucas K. Current therapeutic approaches in metastatic and recurrent Ewing sarcoma. Sarcoma (internet) (2011). http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2995926/. Accessed 5 June 2016. [DOI] [PMC free article] [PubMed]
- 28.Rodriguez-Galindo C, Spunt SL, Pappo AS. Treatment of Ewing sarcoma family of tumors: current status and outlook for the future. Med Pediatr Oncol. 2003;40:276–287. doi: 10.1002/mpo.10240. [DOI] [PubMed] [Google Scholar]
- 29.Bridge RS, Rajaram V, Dehner LP, Pfeifer JD, Perry A. Molecular diagnosis of Ewing sarcoma/primitive neuroectodermal tumor in routinely processed tissue: a comparison of two FISH strategies and RT-PCR in malignant round cell tumors. Mod Pathol. 2006;19:1–8. doi: 10.1038/modpathol.3800486. [DOI] [PubMed] [Google Scholar]
- 30.Weidner N, Tjoe J. Immunohistochemical profile of monoclonal antibody O13: antibody that recognizes glycoprotein p30/32MIC2 and is useful in diagnosing Ewing’s sarcoma and peripheral neuroepithelioma. Am J Surg Pathol. 1994;18:486–494. doi: 10.1097/00000478-199405000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Llombart-Bosch A, Machado I, Navarro S, Bertoni F, Bacchini P, Alberghini M, et al. Histological heterogeneity of Ewing’s sarcoma/PNET: an immunohistochemical analysis of 415 genetically confirmed cases with clinical support. Virchows Arch. 2009;455:397–411. doi: 10.1007/s00428-009-0842-7. [DOI] [PubMed] [Google Scholar]
- 32.Fatima SS, Minhas K, Din NU, Fatima S, Ahmed A, Ahmad Z. Sinonasal teratocarcinosarcoma: a clinicopathologic and immunohistochemical study of 6 cases. Ann Diagn Pathol. 2013;17:313–318. doi: 10.1016/j.anndiagpath.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Pai SA, Naresh KN, Masih K, Ramarao C, Borges AM. Teratocarcinosarcoma of the paranasal sinuses: a clinicopathologic and immunohistochemical study. Hum Pathol. 1998;29:718–722. doi: 10.1016/S0046-8177(98)90281-7. [DOI] [PubMed] [Google Scholar]
- 34.Yang S, Sun R, Liang J, Zhou Z, Zhou J, Rui J. Sinonasal teratocarcinosarcoma: a clinical and pathological analysis. Int J Surg Pathol. 2013;21:37–43. doi: 10.1177/1066896912457202. [DOI] [PubMed] [Google Scholar]