Abstract
With the advent of targeted therapies, expression of sex hormone receptors and HER-2 in salivary gland tumors (SGTs) is of clinical interest. Previous reports of estrogen (ER) and progesterone (PR) receptor expression have varied. Androgen receptor (AR) and HER-2 overexpression are frequently reported in salivary duct carcinoma (SDC), but have not been studied systematically in other SGTs. This study examines ER, PR, AR, and HER-2 expression in SGTs. Immunohistochemistry for ER, PR, AR, and HER-2 was performed on 254 SGTs (134 malignant). ER, PR, and AR expression was scored using Allred system. HER-2 expression was scored using Dako HercepTest guidelines. FISH for HER-2 amplification was performed on select cases with HER-2 overexpression (2–3+). No SGT demonstrated strong expression of ER or PR. Combined strong AR and HER-2 expression was seen in 22 carcinomas: 14/25 SDC, 3/16 poorly differentiated, two oncocytic, and one each carcinoma ex pleomorphic adenoma, squamous cell, and intraductal carcinoma. Eighteen additional high grade carcinomas had HER-2 overexpression with absent, weak, or moderate AR expression; eight high grade carcinomas had isolated strong AR expression with 0–1+ HER-2 staining. Of 15 tested cases, six demonstrated HER-2 amplification by FISH, all of which had 3+ immunoreactivity. Neither benign nor malignant SGTs had strong expression of ER or PR. None of the benign SGTs overexpressed AR or HER-2. Coexpression of AR and HER-2 should not define SDC, but immunostaining should be considered in high grade salivary carcinomas, as some show overexpression and may benefit from targeted therapy.
Keywords: Salivary gland tumors, Salivary duct carcinoma, Hormone receptors, Estrogen, Progesterone, Androgen, HER-2, FISH
Introduction
Most salivary gland tumors are benign, however, salivary gland carcinomas account for up to 11% of all head and neck cancers. Clinical interest in the expression of sex hormone receptors and human epidermal growth factor receptor 2 (HER-2) in salivary gland tumors (SGTs) has been growing due to potential for treatment with hormone antagonists and targeted HER-2 therapy, particularly in aggressive salivary duct carcinomas (SDC) [1–8]. Standard therapy for SDC is surgical resection with or without adjuvant radiotherapy. Still, approximately 60% of patients die within 3 years of diagnosis, typically of distant widespread metastases. Reports of estrogen (ER) and progesterone (PR) receptor expression by immunohistochemistry (IHC) have varied greatly in SGTs, likely due to variability in antibody clones, fixation processes, scoring systems, and observers [9–13]. Androgen receptor (AR) and HER-2 overexpression is frequently reported in SDC, but has not been well-studied in other SGTs [3, 4, 6, 7, 11, 12, 14–22]. This study examines ER, PR, AR, and HER-2 expression in 254 benign and malignant SGTs.
Materials and Methods
Tumor Collection
With institutional review board (IRB) approval, salivary gland tumor resections (1991–2017) were identified and retrieved from diagnostic surgical pathology archives. A total of 254 SGTs were included (120 benign and 134 malignant, including nine intermediate grade and 85 high grade/dedifferentiated): 91 pleomorphic adenomas (PA), 23 Warthin tumors, two basal cell adenomas, two “monomorphic adenomas,” two oncocytomas, 25 salivary duct carcinomas (SDC), 26 adenoid cystic carcinomas (AdCC), 16 acinic cell carcinomas (AcCC), 16 mucoepidermoid carcinomas (MEC), 16 poorly differentiated carcinomas not otherwise specified (NOS), ten carcinomas ex PA (CAexPA), six polymorphous adenocarcinomas (PAC), eight primary salivary squamous cell carcinomas (SCC, in patients without known skin or aerodigestive primaries), five mammary analogue secretory carcinomas (MASC), four oncocytic carcinomas (OnCA), one intraductal carcinoma, and one myoepithelial carcinoma.
Tissue Microarray (TMA) Construction
For 189 cases, TMA blocks were constructed by removing duplicate cores from formalin-fixed paraffin-embedded “donor” diagnostic blocks and re-embedding them into “recipient” TMA blocks according to TMA maps, using a Beecher Instruments Manual Tissue Arrayer (Beecher Instruments, Sun Prairie, WI). Both 1 and 2 mm core sizes were used for the TMA construction in this study. Unstained sections for IHC and FISH were taken after TMA construction was complete and H&E slides were provided to the pathologist for quality review. For the remaining 65 cases, stains were performed on whole tissue sections.
Immunohistochemistry (IHC)
Staining for ER (NCL-L-ER-6F11, mouse IgG1, clone 6F11, 1:60, Novocastra, Buffalo Grove, IL 60089), PR (mouse IgG1, clone16, 1:300, Novocastra, Buffalo Grove, IL 60089), and AR (M3562, mouse IgG, clone AR441, 1:500, DAKO, Denmark) was performed on the Leica Bond RX automatic stainer. Epitope retrieval solution I (Leica Biosystems, AR9961) was used for a 20-min treatment. Anti-ER, PR, and AR antibody was applied on tissue sections with 1-h incubation. The antigen–antibody binding was detected with Bond polymer refine detection (Leica Biosystems, DS9800). Expression of ER, PR, and AR was scored by consensus by two pathologists (NTC and NAC) using the Allred system for immunohistochemical analysis (Table 1) [23]. Tumors were considered to have absent expression if the total score was 0–1, weak expression if the total score was 2–3, moderate expression if the total score was 4–6, and strong expression if the total score was 7–8.
Table 1.
Allred scoring system for hormone receptor expression
| Proportion score (%) | Intensity score | Total score | |||
|---|---|---|---|---|---|
| 0 | 0 | 0 | Negative | 0 1 |
Negative |
| 1 | 1 | ||||
| 1 | Weak | 2 3 |
Weak | ||
| 2 | 10 | ||||
| 3 | 33 | 2 | Moderate | 4 5 6 |
Moderate |
| 4 | 66 | ||||
| 5 | 100 | 3 | Strong | 7 8 |
Strong |
Staining for HER-2 was performed according to the HercepTest (Carpinteria, California) kit protocol. Tissue sections were deparaffinized and rehydrated through xylenes and serial dilutions of alcohol to deionized water. Tissue sections were incubated in heated antigen retrieval buffer for water bath treatment for 40 min at 95–99 °C. Peroxidase blocking solution was applied on tissue sections for 5 min. Ready-to-use antibody or negative control reagent was applied on tissue sections for 30 min incubation at room temperature. HRP-labeled polymer was applied on the tissue sections for 30 min incubation. Tissue sections were treated with chromogen substrate for 10 min, and then Gill III hematoxylin was used for counterstaining. Expression of HER-2 was scored by consensus by two pathologists (NTC and NAC) using guidelines established by Dako (Carpinteria, California) for the HercepTest as follows:
0 Negative: No staining or membranous staining in <10% of tumor cells.
1 Negative: Faint, incomplete membranous staining in >10% of tumor cells.
2+ Weakly positive (equivocal): Weak to moderate complete membranous staining in >10% of tumor cells.
3+ Strongly positive: Strong, complete membranous staining in >10% of tumor cells.
Fluorescence in Situ Hybridization (FISH)
FISH was attempted on 16 carcinomas with 2+ or 3+ HER-2 IHC expression. Slides were deparaffinized with Citrisolve and 100% alcohol. Pepsin Protease solution was used for proteolytic pre-treatment of slides. The slides were fixed and then denatured. Spectrum Orange labeled HER2 probes were used together with Spectrum Green labeled centromere 17 reference probes (PathVysion; Vysis-Abbott, USA). After overnight hybridization at 37 °C in a humid chamber, slides were washed and counterstained with 2.5 mg/mL DAPI. The average copy number and ratio of HER-2 to chromosome 17 were estimated in each tumor based on analysis of 20 representative tumor cells. A tumor was considered amplified if the HER-2/CEP17 ratio was ≥2.0 with ≥4 HER-2 signals per cell or if the HER-2/CEP17 ratio was <2.0 with >6 HER-2 signals per cell.
Results
ER Immunohistochemistry (Table 2)
Table 2.
Summary of hormone receptor and HER-2 expression in salivary gland tumors
| BENIGN | |||||||
|---|---|---|---|---|---|---|---|
| Tumor type | Number of cases |
ER (n = 120) |
PR (n = 120) |
AR (n = 118)a |
HER-2 (n = 119)b |
||
| Pleomorphic adenoma | 91 | 28 (31%) 17 W, 11 M |
5 (5%) 3 W, 2 M |
12 (13%) 10 W, 2 M |
0 | ||
| Warthin tumor | 23 | 10 (43%) 7 W, 3 M |
0 | 1 (4%) W | 0 | ||
| Basal cell adenoma | 2 | 2 (100%) M | 0 | 0 | 0 | ||
| Monomorphic adenoma | 2 | 0 | 0 | 0 | 0 | ||
| Oncocytoma | 2 | 0 | 0 | 0 | 0 | ||
| Total | 120 | 40 (33%) 24 W, 16 M |
5 (4%) 3 W, 2 M |
13 (11%) 11 W, 2 M |
0 | ||
| MALIGNANT | |||||
|---|---|---|---|---|---|
| Tumor type | Number of cases |
ER (n = 134) |
PR (n = 134) |
AR (n = 132)a |
HER-2 (n = 133)b |
| Salivary duct carcinoma | 25 (All high grade) |
3 (12%) W | 1 (4%) W | 25 (100%) 2 W, 3 M, 20 S |
19 (76%) |
| Adenoid cystic carcinoma | 26 (Eight high grade/dedifferentiated) |
5 (19%) 3 W, 2 M |
3 (12%) 1 W, 2 M |
3 (12%) 2 W, 1 M |
1 (4%) (High grade) |
| Acinic cell carcinoma | 16 (Ten high grade/dedifferentiated) |
1 (6%) W | 0 | 3 (19%) 1 W, 1 M, 1 S (1 S = high grade) |
4 (25%) (All high grade) |
| Mucoepidermoid carcinoma | 16 (Five intermediate and six high grade) |
2 (13%) W | 1 (6%) W | 0 | 2 (13%) (Both high grade) |
| Poorly differentiated carcinoma, not otherwise specified | 16 (All high grade) |
2 (13%) M | 0 | 6 (38%) 1 W, 2 M, 3 S |
5 (31%) |
| Carcinoma ex pleomorphic adenoma | 10 (Eight invasive and high grade) |
3 (30%) 2 W, 1 M |
2 (20%) 1 W, 1 M |
7 (70%) 2 W, 3 M, 2 S (2 S = high grade) |
2 (20%) (Both high grade) |
| Polymorphous adenocarcinoma | 6 (One cribriform variant) |
4 (67%) 1 W, 3 M |
2 (33%) 1 W, 1 M |
2 (33%) 1 W, 1 M |
0 |
| Squamous cell carcinoma | 8 (All high grade) |
2 (25%) 1 W, 1 M |
0 | 1 (13%) S | 3 (38%) |
| Mammary analogue secretory carcinoma | 5 (Two high grade) |
2 (40%) 1 W, 1 M |
0 | 2 (40%) M | 0 |
| Oncocytic carcinoma | 4 (One intermediate and two high grade) |
2 (50%) 1 W, 1 M |
0 | 2 (50%) S (One intermediate and one high grade) | 3 (75%) (One intermediate and two high grade) |
| Intraductal carcinoma | 1 | 0 | 0 | 1 S (100%) | 1 (100%) |
| Myoepithelial carcinoma | 1 | 0 | 0 | 0 | 0 |
| Total | 134 | 26 (19%) 15 W, 11 M |
9 (7%) 5 W, 4 M |
52 (39%) 9 W, 13 M, 30 S |
40 (30%) |
| Total High Grade or Dedifferentiated | 85 | 15 (18%) 9 W, 6 M |
3 (4%) 2 W, 1 M |
42 (49%) 5 W, 9 M, 28 S |
38 (45%) |
ER estrogen receptor, PR progesterone receptor, AR androgen receptor, W weak, M moderate, S strong
aOne pleomorphic adenoma, one monomorphic adenoma, and two adenoid cystic carcinomas were missing from the AR TMA slides
bOne pleomorphic adenoma and one adenoid cystic carcinoma was missing from the HER-2 TMA slides
Tissue for ER interrogation was present in all 120 benign and 134 malignant SGTs. The majority of benign (n = 80, 67%) and malignant (n = 108, 81%) SGTs were negative for ER. Weak expression was seen in 24 (20%) benign and 15 (11%) malignant SGTs: 17 (19%) PA, 7 (30%) Warthin tumor, 3 (12%) SDC, 3 (12%) AdCC, 1 (6%) AcCC, 2 (13%) MEC, 2 (20%) CAexPA, 1 (17%) PAC, 1 (13%) SqCC, 1 (20%) MASC, and 1 (25%) oncocytic carcinoma. Moderate expression was seen in 16 (13%) benign and 11 (8%) malignant SGTs: 11 (12%) PA, 3 (13%) Warthin tumor, 2 (100%) basal cell adenoma, 2 (8%) AdCC, 2 (13%) NOS, 1 (10%) CAexPA, 3 (50%) PAC, 1 (13%) SqCC, 1 (20%) MASC, and 1 (25%) oncocytic carcinoma. Strong expression of ER was not seen in any benign or malignant SGT. Of the 85 high grade/dedifferentiated carcinomas, 15 (18%) were positive for ER, nine weak and six moderate.
PR Immunohistochemistry (Table 2)
Tissue for PR interrogation was present in all 120 benign and 134 malignant SGTs. The majority of benign (n = 115, 96%) and malignant (n = 125, 93%) SGTs were negative for PR. Weak expression was seen in 3 (3%) benign and 5 (4%) malignant SGTs: 3 (3%) PA, 1 (4%) SDC, 1 (4%) AdCC, 1 (6%) MEC, 1 (10%) CAexPA, and 1 (16%) PAC. Moderate expression was seen in 2 (2%) benign and 4 (3%) malignant SGTs: 2 (2%) PA, 2 (8%) AdCC, 1 (10%) CAexPA, and 1 (16%) PAC. Strong expression of PR was not seen in any benign or malignant SGT. Of the 85 high grade/dedifferentiated carcinomas, 3 (4%) were positive for PR, two weak and one moderate.
AR Immunohistochemistry (Table 2)
Tissue for AR interrogation was present in 118 benign and 132 malignant SGTs (one PA, one monomorphic adenoma, and two AdCC were missing from the AR TMA slides). The majority of benign (n = 105, 89%) and malignant (80, 61%) SGTs were negative for AR. Weak expression was seen in 11 (9%) benign and 9 (7%) malignant SGTs: 10 (11%) PA, 1 (4%) Warthin tumor, 2 (8%) SDC, 2 (8%) AdCC, 1 (6%) AcCC, 1 (6%) NOS, 2 (20%) CAexPA, and 1 (17%) PAC. Moderate expression was seen in 2 (2%) benign and 13 (10%) malignant SGTs: 2 (2%) PA, 3 (12%) SDC, 1 (4%) AdCC, 1 (6%) AcCC, 2 (13%) NOS, 3 (30%) CAexPA, 1 (17%) PAC, and 1 (20%) MASC. Strong expression was seen in no benign and 30 (23%) malignant SGTS: 20 (80%) SDC, 1 (6%) AcCC, 3 (19%) NOS, 2 (20%) CAexPA, 1 (13%) SqCC, 2 (50%) OnCA, and 1 (100%) intraductal carcinoma (Fig. 1). Of the 85 high grade/dedifferentiated carcinomas, 42 (49%) were positive for AR, five weak, nine moderate, and 28 strong.
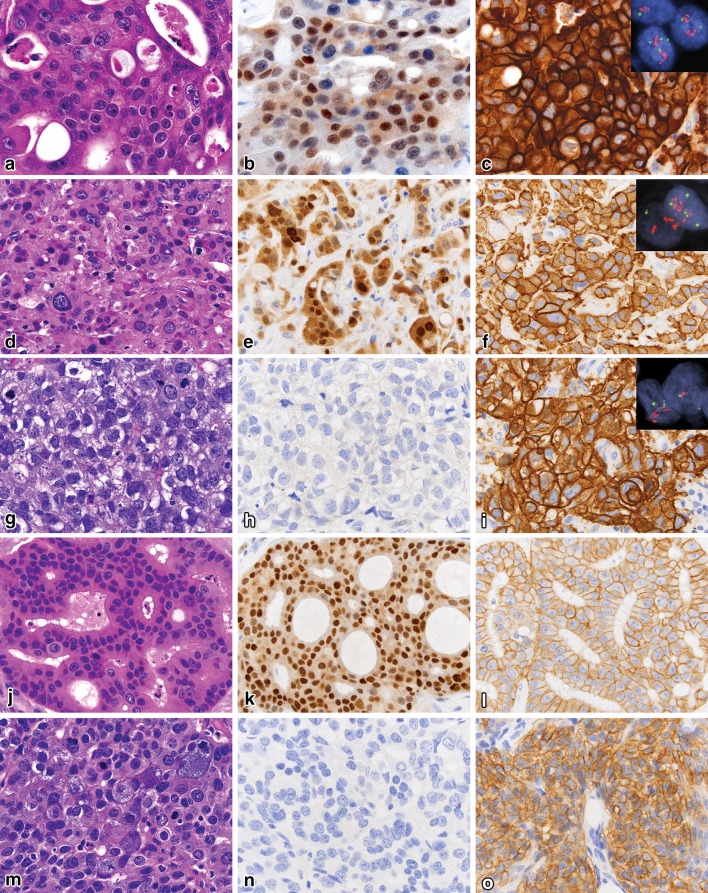
Fig. 1.
Representative images of salivary gland carcinomas with variable patterns of androgen receptor and HER-2 expression (all 600×). Salivary duct carcinoma (Case 6) (a) with strong AR expression (b), HER-2 IHC 3+ (c), and positive amplification with ratio 13.5 (inset). Carcinoma NOS (Case 10) (d) with strong AR expression (e), HER-2 IHC 3+ (f), and positive amplification with ratio 6.9 (inset). Dedifferentiated AdCC (Case 13) (g) with negative AR (h), HER-2 IHC 3+ (i), and positive amplification with ratio 11 (inset). Salivary duct carcinoma (Case 1) (j) with strong AR expression (k), HER-2 IHC 2+ (l), and unsuccessful FISH. High grade AcCC (Case 10) (m) with negative AR (n), HER-2 IHC 2+ (o), and negative amplification
HER-2 Immunohistochemistry and FISH
Tissue for HER-2 interrogation was present in 119 benign and 133 malignant SGTs (one PA and one AdCC were missing from the HER-2 TMA slides) (Table 2). Overexpression (2–3+ staining) was not seen in any benign SGT, but was seen in 40 (30%) malignant SGTs, 18 with 2+ expression and 22 with 3+ expression: 19 (76%) SDC, 1 (4%) AdCC, 4 (25%) AcCC, 2 (13%) MEC, 5 (31%) NOS, 2 (20%) CAexPA, 3 (38%) SCC, 3 (75%) OnCA, and 1 (100%) intraductal carcinoma. Of the 85 high grade/dedifferentiated carcinomas, 38 (45%) showed HER-2 overexpression.
HER-2 FISH was attempted on 16 cases with 2+ or 3+ HER-2 IHC expression (Table 3). All cases were high grade carcinomas. FISH was unsuccessful on one case of SDC (case 1) that showed 2+ HER-2 IHC expression as it did not hybridize well on the TMA section, and additional tissue blocks were unable to be located. In 15 successful cases, six were amplified. None of the cases with 2+ expression were amplified; all of the cases with 3+ expression were amplified. The amplified cases included one each of SDC, NOS, dedifferentiated AdCC, CAexPA, OnCA, and SCC (Fig. 1).
Table 3.
Summary of HER-2 FISH results in select IHC-positive cases
| Case | Diagnosis | IHC | HER-2/CEP17 ratio by FISH | Interpretation |
|---|---|---|---|---|
| 1 | Salivary duct carcinoma | 2+ | Unsuccessful; see results | |
| 2 | Salivary duct carcinoma | 2+ | 1.0 | Not amplified |
| 3 | Salivary duct carcinoma | 2+ | 1.0 | Not amplified |
| 4 | Salivary duct carcinoma | 2+ | 1.1 | Not amplified |
| 5 | Salivary duct carcinoma | 2+ | 1.1 | Not amplified |
| 6 | Salivary duct carcinoma | 3+ | 13.5 | Amplified |
| 7 | Poorly differentiated carcinoma, NOS | 2+ | 0.6 | Not amplified |
| 8 | Poorly differentiated carcinoma, NOS | 2+ | 0.3 | Not amplified |
| 9 | Poorly differentiated carcinoma, NOS | 2+ | 1.07 | Not amplified |
| 10 | Poorly differentiated carcinoma, NOS | 3+ | 6.9 | Amplified |
| 11 | Acinic cell carcinoma, high grade | 2+ | 0.6 | Not amplified |
| 12 | Acinic cell carcinoma, high grade | 2+ | 0.7 | Not amplified |
| 13 | Adenoid cystic carcinoma, dedifferentiated | 3+ | 11.0 | Amplified |
| 14 | Carcinoma ex PA, high grade | 3+ | 5.0 | Amplified |
| 15 | Oncocytic carcinoma, high grade | 3+ | 2.6 | Amplified |
| 16 | Squamous cell carcinoma | 3+ | 7.2 | Amplified |
Discussion
Expression of hormone receptors (ER, PR, and AR) or amplification of HER-2 in salivary gland tumors may have important ramifications for targeted therapy, and pathologists may receive requests to perform these markers in clinical practice. To date, a comprehensive study of all four markers in benign and malignant salivary gland tumors has not been undertaken, and the value of performing all four markers remains unknown. Additionally, co-expression of AR and HER-2 has been classically attributed to SDC. However, the expression pattern in other high grade salivary carcinomas has not been studied. Until now, the largest study evaluating ER, PR, and AR demonstrated no expression of ER and PR in 91% of SGTs and expression of AR in 54% of malignant SGTs but no benign SGTs [11]. Our dataset included 254 SGTs scored for ER, PR, AR, and HER-2: 120 benign and 134 malignant, of which 85 were high grade or “dedifferentiated.” ER, PR, and AR were scored using the Allred scoring system for hormone receptors, which takes into account the proportion of cells staining and the intensity of staining in order to determine the likelihood of response to hormone therapy (Table 1). In breast cancer, a total score of 0–1 (negative) predicted no effect, a score of 2–3 (weak) predicted small (20%) chance of benefit, a score of 4–6 (moderate) predicted a moderate (50%) chance of benefit, and a score of 7–8 (strong) predicted a good (75%) chance of benefit. We also scored HER-2 IHC and FISH with the same standards utilized in breast carcinoma.
ER & PR
Of 120 benign and 134 malignant SGTs in this study, the majority was negative for ER and PR (67% benign and 81% malignant were negative for ER; 96 and 93% were negative for PR). Very few malignant SGTs showed potentially clinically relevant expression. Eleven carcinomas showed moderate expression of ER, of which six were high grade: two AdCC (none high grade), two high grade NOS, one high grade CAexPA, three PAC (none high grade), one high grade SqCC, one high grade MASC, one high grade OnCA); two of these also showed moderate expression of PR. The response to treatment in SGTs with moderate expression has not been studied akin to breast carcinomas, however, the number of cases with any expression is very low.
Other authors have evaluated ER and PR expression in SGTs, predominantly in adenoid cystic carcinoma [9–13, 16]. Between 0 and 17% of AdCC showed ER expression, none strong (Table 4). All cases of MEC in one study were negative for ER [12], however, one MEC and one acinic cell carcinoma showed weak positivity in another study [11]. Between 7 and 50% of AdCC showed PR expression, only occasionally strong (Table 4). In these reported cases, the presence of PR in the absence of ER expression is unusual. In a large study of almost 6000 invasive breast cancers, none were ER-negative, PR-positive [24]. Our results are congruent with this profile, as all but one of the PR-positive cases were ER-positive (the exception was a high grade myoepithelial carcinoma ex pleomorphic adenoma in which up to 5% of cells showed strong nuclear PR expression).
Table 4.
Literature review of hormone receptor expression in salivary gland carcinomas
| Authors | Tumors evaluated | Scoring System | ER | PR | AR | |||
|---|---|---|---|---|---|---|---|---|
| Positive cases | Score | Positive Cases | Score | Positive Cases | Score | |||
| Barrera et al. [9] | 47 AdCC | 0, +1, +2, +3 | 8 (17%) | +1–2 | 4 (9%) | +1 | n/a | n/a |
| Dori et al. [10] | 27 AdCC | 1, 2, 3, 4 | 0 | n/a | 2 (7%) | 2–4 | n/a | n/a |
| Shick et al. [13] | 12 AdCC | 0, W, S | 0 | n/a | 6 (50%) | 5 W, 1 S | n/a | n/a |
| Pires et al. [12] | 72 AdCC 136 MEC |
n/a | 0 | n/a | n/a | n/a | n/a | n/a |
| Nasser et al. [11] | 52 carcinomas | 0, W, M, S | 2 (4%) | Wa | 5 (10%) | W-Mb | 28 (54%)c | 4 W, 5 M, 19 S |
| DiPalma et al. [16] | 35 SDC | Allred | 0 | n/a | 0 | n/a | 36 (86%) | 1 W, 10 M, 25 S |
| Butler et al. [14] | 45 SDC 59 MEC |
0, 1+, 2+, 3+, 4+ | n/a | n/a | n/a | n/a | 33 (73%) SDC, 0 MEC | 8 1+, 3 2+, 6 3+, 16 4+ |
ER estrogen receptor, PR progesterone receptor, AR androgen receptor, AdCC adenoid cystic carcinoma, MEC mucoepidermoid carcinoma, SDC salivary duct carcinoma, W weak, M moderate, S strong, AcCC acinic cell carcinoma, CAexPA carcinoma ex pleomorphic adenoma
a1/10 AcCC, 1/10 MEC
b4/14 CAexPA, 1/10 MEC
cStrong in 9/14 CAexPA, 1/10 MEC, 1/10 AcCC, 2/10 AdCC, 5/6 SDC, 1/2 Basal cell adenocarcinoma
It is difficult to directly compare these studies to the current study, as each use a different scoring system and different antibody clones. Though reported rates of ER and PR expression in salivary gland tumors are somewhat variable, the data suggest that only a minority of tumors express ER or PR in a predominantly weak to moderate pattern. The clinical significance of weak to moderate ER/PR expression in salivary gland tumors has not been studied. However, in breast carcinoma, some authors have found significant improvement in disease-free survival with endocrine therapy in cases with more than 0% IHC staining [25]. Targeted ER/PR therapy is currently not being used in the treatment of salivary gland carcinomas, likely due to the rarity of overexpression and the expected paucity of benefit.
AR
Of 118 benign and 132 malignant SGTs in this study, 89 and 61%, respectively, were negative for AR. Forty-three carcinomas showed moderate to strong expression of AR (including 23 SDCs), of which 37 were high grade. Of the 30 strong AR expressers, all but two were high grade (the exceptions were one intermediate grade oncocytic carcinoma and one low grade intraductal carcinoma without invasion). Salivary duct carcinoma is frequently associated with AR expression, with some series reporting 70–90% positivity rates (Table 4). We showed 100% positivity for AR in SDC (the majority being strong), as well as moderate to strong positivity in other carcinomas that did not exhibit salivary duct morphology. AR expression in other salivary gland tumors has been occasionally studied, with carcinoma ex pleomorphic adenoma being the second most frequent expresser [6, 11, 14, 16, 22, 26]. Focal AR expression has also been shown in up to 2% of benign pleomorphic adenomas [26].
The first report of androgen deprivation therapy in an SGT was in 1994 by van der Hulst et al. in the treatment of an adenocarcinoma NOS of the parotid with resultant partial remission [27]. In 2003, Locati et al. described a case of a 73-year-old man with an AR positive adenocarcinoma NOS of the parotid with complete clinical remission status post androgen deprivation therapy and then proceeded to find an overall 64.5% response rate in a series of 17 patients with AR positive adenocarcinoma NOS or SDC [28, 29]. Jaspers et al. treated ten patients with AR positive SDC with bicalutamide, some of whom had local recurrence and/or distant metastases, finding a response rate of 20% and a median progression-free survival of 12 months [30]. These findings suggest that androgen deprivation therapy could be used not only in adenocarcinoma NOS and SDC, but other salivary gland carcinomas expressing AR. Currently available androgen deprivation therapy includes luteinizing hormone-releasing hormone (LHRH) agonists (leuprolide, goserelin, triptorelin, histrelin), LHRH antagonists (degarelix), CYP17 inhibitors (abiraterone), and anti-androgens (flutamide, bicalutamide, and nilutamide). In fact, European clinical trials of androgen deprivation in salivary carcinomas are underway (NCT01969578, NCT02867852).
HER-2
Of 119 benign and 133 malignant SGTs in this study, 100 and 70%, respectively, were negative for HER-2. Forty carcinomas overexpressed HER-2 (including 19 SDCs), of which 38 were high grade (the exceptions were the same two carcinomas that had strong AR expression: one intermediate grade oncocytic carcinoma and one low grade intraductal carcinoma without invasion). FISH for HER-2 was successful in 15 of 16 cases, six of which were amplified, all 3+ by IHC (one each of dedifferentiated AdCC, carcinoma NOS, CAexPA, SDC, SCC, and high grade OnCA). Some studies have shown overexpression of HER-2 in 4–15% of salivary gland carcinomas, specifically in 20–34% of SDC [14, 16, 31, 32]. Of cases with positive IHC, the majority (77%) did not show amplification by FISH [32]. Tumors originating from the excretory ducts (MEC, SCC, and SDC) may show higher rates of HER-2 overexpression than tumors originating from intercalated ducts (AdCC, acinic cell carcinoma, adenocarcinoma NOS, myoepithelial carcinoma) [18, 19].
In a large prospective clinical trial of breast carcinoma patients, Press et al. favored FISH to IHC for determination of HER-2 status. They found a 92% agreement rate when comparing individual FISH lab results to the Breast Cancer International Research Group (BCIRG) central lab, but only a 77.5% agreement rate using IHC [33]. Furthermore, they claim that IHC is more prone to false negatives or false positives due to interlaboratory variability in tissue fixation, processing, and antigen retrieval whereas FISH is less affected by tissue processing. The benefit of targeted HER-2 therapy based on HER-2 status by IHC versus FISH requires further study in SGTs.
Treatment of HER-2 positive cases may include monoclonal antibodies (trastuzumab, pertuzumab) or HER-2 tyrosine kinase inhibitors (lapatinib). These targeted HER-2 therapies have been used in salivary gland carcinomas, with several case series and case reports demonstrating the benefit of trastuzumab-based combination therapy in patients with recurrent and/or metastatic SDC. Some patients have even experienced complete clinical response [1, 2, 19]. Additionally, some reports demonstrate the benefit of trastuzumab in patients with recurrent and/or metastatic CAexPA, MEC, and adenocarcinoma NOS [17, 32, 34]. Though HER-2 targeted therapy has been more frequently employed in SDC, it may demonstrate utility for all SGTs with HER-2 overexpression. Some authors argue that HER-2 gene amplification is the best predictor of response to trastuzumab, although patients with HER-2 overexpression by IHC alone have been found to experience a significant response [15]. The prognostic value of HER-2 overexpression has also been studied, with some studies reporting more aggressive behavior and others finding no association with behavior [1, 5].
Combined HER-2 and AR overexpression was seen in 22 carcinomas: 14 (56%) SDC, three poorly differentiated carcinomas NOS, two oncocytic carcinomas (one high and one intermediate grade), one squamous cell carcinoma, one high grade carcinoma ex pleomorphic adenoma, and one intraductal carcinoma. There is a recognized oncocytic variant of SDC, which may account for some cases of high grade oncocytic carcinoma [6]. It is possible that our cases of oncocytic carcinoma or carcinoma NOS represent oncocytic or poorly differentiated variants of SDC. However, diagnostic slides for these cases were reviewed and there was no evidence of classic SDC morphology (such as apocrine cells, large nests, cribriforming, central necrosis). Additionally, the rare intraductal carcinoma (formerly known as low grade salivary duct carcinoma or low grade cribriform cystadenocarcinoma) has not been definitively shown to represent a non-invasive form of SDC. In our single case, both strong AR and HER-2 expression was seen. Finally, we found isolated strong AR expression or isolated HER-2 overexpression in 26 additional high grade carcinomas, 15 of which were not morphologically salivary duct. Specifically, eight high grade carcinomas had isolated strong AR expression with HER-2 staining of 0–1+ (6 SDC, 1 AcCC, 1 CAexPA) and 18 high grade carcinomas had HER-2 overexpression with negative to moderate AR expression (5 SDC, 4 AcCC, 2 NOS, 2 MEC, 2 SqCC, 1 OnCA, 1 CAexPA, 1 AdCC). Of 48 carcinomas showing strong AR expression and/or HER-2 overexpression, approximately 50% were morphologically salivary duct. We do not advocate rendering a diagnosis of salivary duct carcinoma in all cases of high grade carcinoma that show isolated or combined expression of androgen receptor and HER-2. However, we suggest staining high grade carcinomas with AR and HER-2 in patients that might benefit from targeted therapies, as tumors with morphologies other than salivary duct may show expression.
There are limitations to the current study. First, in the majority of cases, IHC was performed on TMA slides, which represents a small portion of the entire tumor—an issue with all TMA studies. Duplicate cores were used to cover a somewhat larger tumor area than a single core. Second, it is difficult to compare this study to other reports of hormone receptor expression in SGTs, and it may also be difficult to compare results from a single tumor to another. Variation in antibody clones and scoring systems may make inter-study comparison difficult, while tissue ischemic times, fixation processes (including times and agents), and ages of FFPE blocks may make both inter- and intra-study comparison difficult. However, these issues occur in all IHC studies, especially those requiring quantification of staining. The Allred system used in the current study has the advantage of taking into account both intensity and proportion of staining, whereas many studies take into account the proportion or intensity of staining. Lastly, there were two carcinomas represented only by a single case (myoepithelial carcinoma and intraductal carcinoma). Both are relatively rare, and therefore, were included in the study. Results from tumors with single or small sample numbers may not be generalizable to all tumors of that subtype.
In conclusion, evaluation of AR and HER-2 should be considered for high grade salivary gland carcinomas in patients that might benefit from targeted therapies. Both salivary duct and non-salivary duct carcinomas may show AR expression or HER-2 amplification and may benefit from androgen deprivation or HER-2 antagonists. Conversely, hormone therapy targeting ER/PR is not being used in the treatment of SGTs, and does not appear to be a promising therapeutic option in light of the very few cases with positive expression, most of which are weak.
Acknowledgements
We would like to thank the staff at the Human Tissue Resource Center at the University of Chicago, particularly Terri Li, for their assistance with this project.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Contributor Information
Nhu Thuy Can, Email: nhuthuy.can@gmail.com.
Mark W. Lingen, Email: Mark.Lingen@uchospitals.edu
Heather Mashek, Email: Heather.Mashek@uchospitals.edu.
James McElherne, Email: James.McElherne@uchospitals.edu.
Renee Briese, Email: Renee.Briese@uchospitals.edu.
Carrie Fitzpatrick, Email: cfitzpat@bsd.uchicago.edu.
Annemieke van Zante, Email: Annemieke.VanZante@ucsf.edu.
Nicole A. Cipriani, Phone: 773-702-4974, Email: Nicole.Cipriani@uchospitals.edu
References
- 1.Alotaibi AM, Alqarni MA, Alnobi A, Tarakji B. Human epidermal growth factor receptor 2 (HER2/neu) in salivary gland carcinomas: a review of literature. J Clin Diagn Res. 2015;9(2):ZE04–ZE8. doi: 10.7860/JCDR/2015/11289.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Limaye SA, Posner MR, Krane JF, Fonfria M, Lorch JH, Dillon DA, et al. Trastuzumab for the treatment of salivary duct carcinoma. Oncologist. 2013;18(3):294–300. doi: 10.1634/theoncologist.2012-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masubuchi T, Tada Y, Maruya S, Osamura Y, Kamata SE, Miura K, et al. Clinicopathological significance of androgen receptor, HER2, Ki-67 and EGFR expressions in salivary duct carcinoma. Int J Clin Oncol. 2015;20(1):35–44. doi: 10.1007/s10147-014-0674-6. [DOI] [PubMed] [Google Scholar]
- 4.Nabili V, Tan JW, Bhuta S, Sercarz JA, Head CS. Salivary duct carcinoma: a clinical and histologic review with implications for trastuzumab therapy. Head Neck. 2007;29(10):907–912. doi: 10.1002/hed.20614. [DOI] [PubMed] [Google Scholar]
- 5.Perissinotti AJ, Lee Pierce M, Pace MB, El-Naggar A, Kies MS, Kupferman M. The role of trastuzumab in the management of salivary ductal carcinomas. Anticancer Res. 2013;33(6):2587–2591. [PubMed] [Google Scholar]
- 6.Simpson RH. Salivary duct carcinoma: new developments—morphological variants including pure in situ high grade lesions; proposed molecular classification. Head Neck Pathol. 2013;7(Suppl 1):S48–S58. doi: 10.1007/s12105-013-0456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vidal L, Tsao MS, Pond GR, Cohen EE, Cohen RB, Chen EX, et al. Fluorescence in situ hybridization gene amplification analysis of EGFR and HER2 in patients with malignant salivary gland tumors treated with lapatinib. Head Neck. 2009;31(8):1006–1012. doi: 10.1002/hed.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto N, Minami S, Fujii M. Clinicopathologic study of salivary duct carcinoma and the efficacy of androgen deprivation therapy. Am J Otolaryngol. 2014;35(6):731–735. doi: 10.1016/j.amjoto.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Barrera JE, Shroyer KR, Said S, Hoernig G, Melrose R, Freedman PD, et al. Estrogen and progesterone receptor and p53 gene expression in adenoid cystic cancer. Head Neck Pathol. 2008;2(1):13–18. doi: 10.1007/s12105-007-0032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dori S, Trougouboff P, David R, Buchner A. Immunohistochemical evaluation of estrogen and progesterone receptors in adenoid cystic carcinoma of salivary gland origin. Oral Oncol. 2000;36(5):450–453. doi: 10.1016/S1368-8375(00)00029-4. [DOI] [PubMed] [Google Scholar]
- 11.Nasser SM, Faquin WC, Dayal Y. Expression of androgen, estrogen, and progesterone receptors in salivary gland tumors. Am J Clin Pathol. 2003;119(6):801–806. doi: 10.1309/RVTP1G0Q727WJUQD. [DOI] [PubMed] [Google Scholar]
- 12.Pires FR, Perez DEDC, de Almeida OP, Kowalski LP. Estrogen receptor expression in salivary gland mucoepidermoid carcinoma and adenoid cystic carcinoma. Pathol Oncol Res. 2004;10(3):166–168. doi: 10.1007/BF03033746. [DOI] [PubMed] [Google Scholar]
- 13.Shick PC, Riordan GP, Foss RD. Estrogen and progesterone receptors in salivary gland adenoid cystic carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80(4):440–444. doi: 10.1016/S1079-2104(05)80338-5. [DOI] [PubMed] [Google Scholar]
- 14.Butler RT, Spector ME, Thomas D, McDaniel AS, McHugh JB. An immunohistochemical panel for reliable differentiation of salivary duct carcinoma and mucoepidermoid carcinoma. Head Neck Pathol. 2014;8(2):133–140. doi: 10.1007/s12105-013-0493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornolti G, Ungari M, Morassi ML, Facchetti F, Rossi E, Lombardi D, et al. Amplification and overexpression of HER2/neu gene and HER2/neu protein in salivary duct carcinoma of the parotid gland. Arch Otolaryngol Head Neck Surg. 2007;133(10):1031–1036. doi: 10.1001/archotol.133.10.1031. [DOI] [PubMed] [Google Scholar]
- 16.Di Palma S, Simpson RH, Marchio C, Skalova A, Ungari M, Sandison A, et al. Salivary duct carcinomas can be classified into luminal androgen receptor-positive, HER2 and basal-like phenotypes. Histopathology. 2012;61(4):629–643. doi: 10.1111/j.1365-2559.2012.04252.x. [DOI] [PubMed] [Google Scholar]
- 17.Ghazali N, Parker L, Settle K, Lubek JE. Sustained response of HER2-positive metastatic salivary adenocarcinoma, not otherwise specified, treated with trastuzumab. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(3):292–299. doi: 10.1016/j.oooo.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Glisson B, Colevas AD, Haddad R, Krane J, El-Naggar A, Kies M, et al. HER2 expression in salivary gland carcinomas: dependence on histological subtype. Clin Cancer Res. 2004;10(3):944–946. doi: 10.1158/1078-0432.CCR-03-0253. [DOI] [PubMed] [Google Scholar]
- 19.Kaidar-Person O, Billan S, Kuten A. Targeted therapy with trastuzumab for advanced salivary ductal carcinoma: case report and literature review. Med Oncol. 2012;29(2):704–706. doi: 10.1007/s12032-011-9884-1. [DOI] [PubMed] [Google Scholar]
- 20.Prat A, Parera M, Reyes V, Peralta S, Cedres S, Andreu J, et al. Successful treatment of pulmonary metastatic salivary ductal carcinoma with trastuzumab-based therapy. Head Neck. 2008;30(5):680–683. doi: 10.1002/hed.20714. [DOI] [PubMed] [Google Scholar]
- 21.Skalova A, Starek I, Vanecek T, Kucerova V, Plank L, Szepe P, et al. Expression of HER-2/neu gene and protein in salivary duct carcinomas of parotid gland as revealed by fluorescence in-situ hybridization and immunohistochemistry. Histopathology. 2003;42(4):348–356. doi: 10.1046/j.1365-2559.2003.01600.x. [DOI] [PubMed] [Google Scholar]
- 22.Soper MS, Iganej S, Thompson LD. Definitive treatment of androgen receptor-positive salivary duct carcinoma with androgen deprivation therapy and external beam radiotherapy. Head Neck. 2014;36(1):E4–E7. doi: 10.1002/hed.23383. [DOI] [PubMed] [Google Scholar]
- 23.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11(2):155–168. [PubMed] [Google Scholar]
- 24.Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5993 breast cancers. Am J Clin Pathol. 2005;123(1):21–27. doi: 10.1309/4WV79N2GHJ3X1841. [DOI] [PubMed] [Google Scholar]
- 25.Honma N, Horii R, Iwase T, Saji S, Younes M, Ito Y, et al. Proportion of estrogen or progesterone receptor expressing cells in breast cancers and response to endocrine therapy. Breast. 2014;23(6):754–762. doi: 10.1016/j.breast.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 26.DeRoche TC, Hoschar AP, Hunt JL. Immunohistochemical evaluation of androgen receptor, HER-2/neu, and p53 in benign pleomorphic adenomas. Arch Pathol Lab Med. 2008;132(12):1907–1911. doi: 10.5858/132.12.1907. [DOI] [PubMed] [Google Scholar]
- 27.van der Hulst RW, van Krieken JH, van der Kwast TH, Gerritsen JJ, de Jong RB, Nijeholt AL, et al. Partial remission of parotid gland carcinoma after goserelin. Lancet. 1994;344(8925):817. doi: 10.1016/S0140-6736(94)92372-8. [DOI] [PubMed] [Google Scholar]
- 28.Locati LD, Quattrone P, Bossi P, Marchiano AV, Cantu G, Licitra L. A complete remission with androgen-deprivation therapy in a recurrent androgen receptor-expressing adenocarcinoma of the parotid gland. Ann Oncol. 2003;14(8):1327–1328. doi: 10.1093/annonc/mdg331. [DOI] [PubMed] [Google Scholar]
- 29.Locati LD, Perrone F, Cortelazzi B, Lo Vullo S, Bossi P, Dagrada G, et al. Clinical activity of androgen deprivation therapy in patients with metastatic/relapsed androgen receptor-positive salivary gland cancers. Head Neck. 2016;38(5):724–731. doi: 10.1002/hed.23940. [DOI] [PubMed] [Google Scholar]
- 30.Jaspers HC, Verbist BM, Schoffelen R, Mattijssen V, Slootweg PJ, van der Graaf WT, et al. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29(16):e473–e476. doi: 10.1200/JCO.2010.32.8351. [DOI] [PubMed] [Google Scholar]
- 31.Haddad R, Colevas AD, Krane JF, Cooper D, Glisson B, Amrein PC, et al. Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral Oncol. 2003;39(7):724–727. doi: 10.1016/S1368-8375(03)00097-6. [DOI] [PubMed] [Google Scholar]
- 32.Clauditz TS, Reiff M, Gravert L, Gnoss A, Tsourlakis MC, Munscher A, et al. Human epidermal growth factor receptor 2 (HER2) in salivary gland carcinomas. Pathology. 2011;43(5):459–464. doi: 10.1097/PAT.0b013e3283484a60. [DOI] [PubMed] [Google Scholar]
- 33.Press MF, Sauter G, Bernstein L, Villalobos IE, Mirlacher M, Zhou JY, et al. Diagnostic evaluation of HER-2 as a molecular target: an assessment of accuracy and reproducibility of laboratory testing in large, prospective, randomized clinical trials. Clin Cancer Res. 2005;11(18):6598–6607. doi: 10.1158/1078-0432.CCR-05-0636. [DOI] [PubMed] [Google Scholar]
- 34.Sharon E, Kelly RJ, Szabo E. Sustained response of carcinoma ex pleomorphic adenoma treated with trastuzumab and capecitabine. Head Neck Oncol. 2010;2:12. doi: 10.1186/1758-3284-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]