Abstract
Previous data has shown that the risk of nodal metastases is significantly greater for classical papillary thyroid carcinoma (PTC) as compared to the follicular variant (FVPTC). Given a recent change in diagnostic paradigm and definition of the noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) we intended to investigate if there remains a significant difference in nodal involvement between classical PTC and FVPTC. A 6-year retrospective review of all cases with FVPTC in the diagnostic line from the University of Utah/ARUP Laboratories was conducted. Two pathologists reviewed the remaining cases using the recently described histologic criteria of NIFTP to determine the total number the FVPTCs fitting the new classification paradigm. Histologic and clinical follow-up was tracked for all patients to determine the rate of nodal disease for all groups. 127 cases were identified using the above listed criteria. Forty-seven cases (37%) were classified as NIFTPs. None of the 47 patients had nodal disease either at the time of surgery or on follow-up. Twenty-eight cases met the current criteria for FVPTC (21%); of these 7/28 (25%) had evidence of nodal disease. By comparison, 17/45 (38%) of patients with mixed classical and FVPTC had nodal disease. Overall, there was no statistically significant difference in the risk of nodal metastasis between the pure FVPTC and mixed classical/FVPTC groups (p = 0.43). Our data indicates that implementing new definition for FVPTC will narrow the gap in the risk of nodal metastases between the classical PTC and FVPTC histologic subtypes.
Keywords: Thyroid, Cancer, Lymph node, Metastasis
Introduction
Thyroid cancer is the most common endocrine cancer and has an increasing incidence worldwide [1–3]. Increased surveillance is recognized as one of the major factors contributing to the rising incidence of thyroid carcinoma on a population scale. [4, 5] The vast majority of thyroid carcinomas fall into the well differentiated category, specifically follicular carcinoma and papillary carcinoma, and have a clinically indolent behavior with a 10-year disease-specific mortality of less than 5% [6]. Therefore, the concept of overtreatment is emerging in the literature with the hope that a more dynamic risk stratification can help minimize unnecessary management. The current clinical management of patients with well differentiated thyroid carcinomas is predicated on The American Joint Commission on Cancer (AJCC) staging system. To guide treatment, the AJCC system includes prognostic variables designed to predict the risk of recurrent disease as well as disease-specific mortality. These parameters include patient age, tumor size, extra-thyroidal tumor extension, as well as the presence and location (central neck versus lateral neck) of nodal metastases.
Lymph node status has been a controversial predictive factor in the aggressiveness of papillary thyroid cancer. However, recent literature has shown that positive lymph node status is a significant prognostic factor which influences recurrence rate and disease-free survival [7–11]. In light of the prognostic importance of nodal metastasis, the American Thyroid Association and Taskforce [12] recommends preoperative imaging using ultrasound for patients with thyroid carcinoma. In addition, fine needle aspiration (FNA) can be obtained at the time of ultrasound to aid in the evaluation of nodal status. In support of this strategy, Moreno et al. [13] showed that the presence of ultrasound-detected disease in the central and lateral compartments as well as the number of involved compartments influenced long-term disease-related survival.
Despite this information, the evaluation of lymph node status is not well standardized with opponents of elective dissections arguing that the potential for increased morbidity secondary to either recurrent laryngeal nerve injury or hypoparathyroidism offset the potential benefit [10].
Papillary thyroid carcinoma (PTC) accounts for approximately 85% of all thyroid cancers. The two most common subtypes of PTC are the classical variant and the follicular variant (FVPTC), which together account for the majority of the diagnosed PTCs [6]. Previous studies have demonstrated that the classical variant of PTC has a significantly higher rate of both central compartment lymph node involvement [14] and lateral neck nodal disease [7] as compared to the FVPTC. While studies vary on the exact relative risk of nodal involvement between these two most common forms of papillary carcinoma, the common thread is that the FVPTC has a significantly lesser risk of this progression. For instance, Hunt et al. showed that the risk for lateral neck lymph node metastasis was much higher in conventional PTC than the follicular variant [7]. Similarly, Lin and Bhattacharrya in a large studying encompassing over 45,000 patients showed that the rate of nodal metastasis for conventional PTC (27.8%) was about twice that of FVPTC (14.8%) [15].
Based on data from numerous studies showing that the encapsulated (or non-invasive) FVPTC behaves in a clinically indolent fashion akin to follicular adenoma, the tumor has been recently stripped of its malignant designation and is now termed ‘noninvasive follicular thyroid neoplasm with papillary-like nuclear features’ (NIFTP) [4, 6, 16, 17]. This reclassification changes the paradigm for the diagnosis of FVPTC as it restricts it to only include the invasive subtype. Given that the majority of previously diagnosed FVPTC would now be classified as NIFTPs [6] we intend to investigate if there remains a significant difference in lymph node involvement between classical PTC and the newly defined follicular variant of PTC.
Methods
A 6-year retrospective review of our laboratory information system at the University of Utah/ARUP Laboratories (from 1/1/2010 to 12/31/2015) was conducted to identify all surgical pathology cases with FVPTC in the diagnostic line. A total of 127 cases were identified during this time period. Forty-five cases had both a classical PTC and a FVPTC component upon further review of the pathology reporting and were removed from the histologic review aspect of the study. The remaining 82 cases diagnosed as FVPTC were histologically reviewed by two pathologists. Using the recently described histologic criteria of NIFTP [4] the 82 cases were assessed for to determine inclusion criteria for NIFTP (encapsulation/clear demarcation, follicular growth pattern, nuclear features of PTC, and size greater than 1.0 cm); as well as exclusion criteria for the entity (papillary architecture >1%, psammoma bodies, infiltrative border, tumor necrosis, high mitotic activity, and morphologic characteristics of other variants of PTC). Electronic medical records were then accessed for clinical and pathologic follow-up for all patients to determine the incidence of nodal disease in all groups. Statistical analysis using Fishers Exact test and ANOVA were performed to ascertain the risk of lymph node metastases between the different groups.
Results
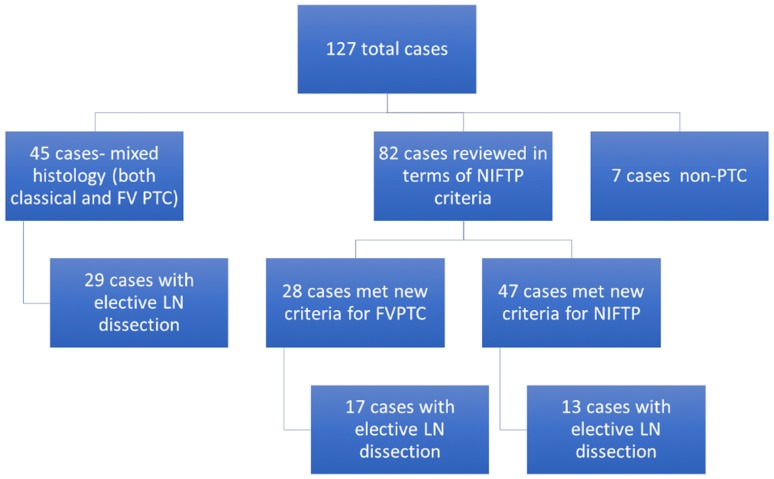
From 1/1/2010 to 12/31/2015 127 cases were identified as having FVPTC in the diagnostic line of a surgical pathology report at our institution. Forty-five (35%) cases had both a classical and a FVPTC component and were removed from the histologic review aspect of the study. Of the remaining 82 cases, 47 cases (37%) were classified as NIFTPs based on the newly-defined diagnostic parameters. From the remaining cases, 28 (22%) met the current standard for the diagnosis of FVPTC, and 7 cases were retrospectively determined as being neither (either a follicular adenoma or another type of thyroid carcinoma) (Fig. 1).
Fig. 1.
Algorithm for case evaluation
None of the 47 patients re-classified as NIFTP had nodal disease either at the time of surgery or on clinical follow-up. Lymph node excisions were performed in 13/47 (27.7%) cases at the time of surgery and all histologically evaluated lymph nodes were negative for metastatic disease. The remaining 34 patients did not have concerning evidence of lymph node metastases by ultrasound, at the time of surgery, or at follow-up. Seventeen of the 28 cases (60.7%) meeting the current diagnostic standard for FVPTC had lymph node excisions performed at the time of surgery. The remaining 11 patients did not have concerning evidence of lymph node metastases by ultrasound, at the time of surgery, or at follow-up. Seven out of 28 (25.0%) patients in the FVPTC category had histologic evidence of nodal disease. In the mixed classical PTC and FVPTC category, a lymph node excision was performed in 29/45 (64.4%) cases at the time of surgery. Seventeen out of 45 (37.7%) patients in this group had histologic evidence of nodal disease. The remaining 28 patients did not have concerning evidence of lymph node metastases by ultrasound, at the time of surgery, or at follow-up. Table 1 summarizes these findings.
Table 1.
Metastatic characteristics of evaluated thyroid tumors
| NIFTP | FVPTC | Mixed histology | p value | |
|---|---|---|---|---|
| Number of cases (total) | 47 (37%) | 28 (22%) | 45 (35%) | |
| Mean and range time of patient follow-up (in days) | 611.17 | 714.63 | 707.71 | 0.74 |
| (0–2310.0) | (0–2275.0) | (0–2104.0) | ||
| Average tumor size in cm (largest focus) | 1.72 | 1.43 | 2.10 | 0.20 |
| Average number of foci | 1.42 | 1.71 | 1.98 | 0.01 |
| Number of cases with nodal evaluation | 13/47 (27.7%) | 17/28 (60.7%) | 29/45 (64.4%) | <0.01 |
| Average number of LNs examined | 7.58 | 11.31 | 25.4 | <0.01 |
| Number of cases with positive lymph nodes | 0/47 (0%) | 7/28 (25.0%) | 17/45 (37.7%) | 0.43 |
| Location of positive lymph nodesa,b | pN1a = 0 cases | pN1a = 3 cases (10.7%) | pN1a = 5 cases (11.1%) | <0.01 |
| pN1b = 0 cases | pN1b = 4 cases (14.2%) | pN1b = 12 cases (26.6%) | ||
| Number of positive nodal cases with extra-thyroidal extension | 0/0 (0%) | 1/7 (14.2%) | 12/17 (70.6%) | 0.02 |
a pN1a metastasis to level VI (pretracheal, paratracheal and prelaryngeal/Delphian, perithyroidal) lymph nodes
b pN1b metastasis to unilateral, bilateral or contralateral cervical (levels I, II, III, IV, V) or retropharyngeal or superior mediastinal lymph nodes (level VII)
The average clinical follow-up periods were similar for all groups (the mean ranged from 611 days in the NIFTP group to 714 in the FVPTC groups). In addition, there was no significant difference in tumor size between the three groups (ranging from 1.4 cm in the FVPTC group to 2.1 cm in the mixed histology group). Lastly, the average number of tumor foci was similar between the FVPTC and mixed histology groups (1.71 and 1.98, respectively). Please refer to Table 1.
In the FVPTC group, only one case showed extra-thyroidal extension (ETE) out of the seven cases with nodal involvement (14.2%). However, in the mixed histology group 12 out of 17 (70.5%) cases with nodal involvement showed ETE. All of the cases newly re-classified as NIFTP were encapsulated and therefore by definition did not have ETE.
After application of strict criteria of invasion to the follicular variant of PTC category, we found no statistically significant difference in the nodal metastatic rates between the FVPTC and mixed classical/FVPTC groups (p = 0.43).
Discussion
The current literature holds that the FVPTC has a much lower chance of lymph node metastasis compared to the classical variant of PTC. [7, 15] With this backdrop, our data indicates that with the implementation of the more stringent definition for FVPTC the risk of lymph node metastatic rates between the FVPTC and tumors with a classical PTC component is more similar than previously published. In fact, our data shows that there is no statistically significant difference in nodal metastatic rates between tumors with a classical PTC component and the FVPTC (37.7 and 25.0% respectively, p = 0.43). Practically, this ‘narrowing of the nodal gap’ on a histologic basis suggests that other risk parameters, such as tumor size, location of tumor, patient age, and extra-thyroidal extension should play the most significant roles in follow-up clinical management. In our patient cohort, extra-thyroidal extension (ETE) was the most significant risk factor for nodal metastasis, as 54% of cases with ETE also had lymph node metastases across all three groups. The importance of evaluating for extra-thyroidal extension was highlighted by Youngwirth et al. who showed that ETE was an independent poor prognostic factor for differentiated cancer survival [18]. This group also found a strong positive correlation between ETE and neck nodal metastases.
There are several practical considerations that should be made in light of these findings. Ideally, in order to be most efficient in patient management, the diagnosis of NIFTP versus FVPTC would be made prior to surgery in order to allow the surgeon and patient to better weigh the risks of an elective lymph node dissection. However, such a differentiation is not currently possible using imaging modalities or fine needle aspiration as the diagnosis of NIFTP currently relies on solely histologic criteria. A recent retrospective review by Maletta et al. showed that most NIFTP lesions are classified in the indeterminate FNA categories (Bethesda 3, 4, or 5) and while FNA can reliably identify the papillary type nuclei in the NIFTP lesions, it cannot reliably distinguish between NIFTP and invasive FVPTC [19]. It remains to be seen how the subject of FNA and NIFTP may drive the direction of future research and the updated Bethesda Thyroid cytology categorizations (the next release is set for 2018). Additionally, molecular testing could continue to evolve as a helpful tool in patient risk stratification for pre-operative clinical management. Although a proposed algorithm for surgical decisions taking clinical history, FNA, ultrasound imaging and molecular testing has been recently proposed by Golding et al., there are currently no consensus guidelines on surgical management of suspected NIFTP lesions [20]. Nevertheless, surgeons may already be using a certain algorithmic formula in pre-operative management of patients with likely NIFTPs, as in our cohort a much small percentage of these patients received elective nodal neck dissections at the time of surgery.
There are some limitations of the current study. One limitation is that there were multiple treating surgeons with varied approaches. This could contribute to some cases having different patterns of lymph node evaluation. However, in general, the nodal assessments were similar in that only patients with imaging, FNA and/or clinical concern for nodal disease underwent neck dissection. Another limitation to our study is the relatively low number of patients it involved. Specifically, the number of patients who underwent lymph node dissections at the time of surgery represented less than half of the total cases. Although it’s worth noting that the majority of patients in the FVPTC (60.7%) and classical PTC component (64.4%) categories had histologic nodal assessments. In most patients imaging, clinical assessment as well as gross assessment of nodes at the time of surgery was used instead of FNA or biopsy to evaluate nodal status, as is the current standard of care, thereby limiting the number of histologically sampled nodes. Despite the limited study size, we were still able to garner statistically important results. Additionally, we did not make the distinction between micro-metastatic disease and macro-metastatic disease in this paper, one point of contention in terms of prognostic import in the current literature [18]. Lastly, other variants of PTC (tall cell variant, columnar cell variant, oncocytic variant, etc.) were not evaluated in this study so no conclusions regarding these histologic subtypes and their risk of lymph node metastases can be made.
In conclusion, it is likely that implementing the new definition for FVPTC will narrow the gap in the risk of nodal metastases between tumors with a classical PTC component and those with solely FVPTC histology. This suggests that other risk parameters, such as tumor size and perhaps most importantly, extra-thyroidal extension play a more significant role than histologic subtype in determining risk of nodal involvement in PTC.
Compliance with Ethical Standards
Conflict of interest
Aleksandra M. Sowder, Benjamin L. Witt and Jason P. Hunt declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Hundahl SA, Fleming ID, Fremgen AM, MenckHR A national cancer data base report on 53,856 cases of thyroid carcinoma treated in the US, 1985–1995. Cancer. 1998;83:2638–2648. doi: 10.1002/(SICI)1097-0142(19981215)83:12<2638::AID-CNCR31>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013;96:5212. doi: 10.1155/2013/965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kweon SS, Shin MH, Chung IJ, Kim YJ, Choi JS. Thyroid cancer is the most common cancer in women, based on the data from population-based cancer registries, South Korea. Jpn J Clin Oncol. 2013;43:1039–1046. doi: 10.1093/jjco/hyt102. [DOI] [PubMed] [Google Scholar]
- 4.Nikifirov YE, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2:1023–1029. doi: 10.1001/jamaoncol.2016.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearce EN, et al. Thyroid cancer overdiagnosis is a result of screening programs in South Korea. Clin Thyroidol. 2017;29:8–10. doi: 10.1089/ct.2017;29.8-10. [DOI] [Google Scholar]
- 6.Fagin JA, Wells SA., Jr Biologic and clinical perspectives on thyroid cancer. N Engl J Med. 2016;375:1054–1067. doi: 10.1056/NEJMra1501993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt JP, Buchmann LO, Wang L, Abraham D. An analysis of factors predicting lateral cervical nodal metastases in papillary carcinoma of the thyroid. Arch Otolaryngol Head Neck Surg. 2011;137:1141–1145. doi: 10.1001/archoto.2011.174. [DOI] [PubMed] [Google Scholar]
- 8.Lundgren CI, Hall P, Dickman PW, Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case- control study. Cancer. 2006;106:524–531. doi: 10.1002/cncr.21653. [DOI] [PubMed] [Google Scholar]
- 9.Wada N, Duh QY, Sugino K, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003;237:399–407. doi: 10.1097/01.SLA.0000055273.58908.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stulak JM, Grant CS, Farley DR, et al. Value of preoperative ultrasonography in the surgical management of initial and preoperative papillary thyroid cancer. Arch Surg. 2006;141:489–496. doi: 10.1001/archsurg.141.5.489. [DOI] [PubMed] [Google Scholar]
- 11.Harwood J, Clark OH, Dunphy JE. Significance of lymph node metastasis in differentiated thyroid cancer. Am J Surg. 1978;136:107–112. doi: 10.1016/0002-9610(78)90209-X. [DOI] [PubMed] [Google Scholar]
- 12.Cooper DS, Doherty GM, Haugen BR, et al. American Thyroid Association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer [published correction appears in Thyroid. 2010; 20(8):942] Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 13.Moreno MA, Agarwal G, de Luna R, et al. Preoperative lateral neck ultrasonography as a long-term outcome predictor in papillary thyroid cancer. Arch Otolaryngol Head Neck Surg. 2011;137:157–162. doi: 10.1001/archoto.2010.254. [DOI] [PubMed] [Google Scholar]
- 14.Salter KD, et al. Central nodal metastases in papillary thyroid carcinoma based on tumor histologic type and focality. Arch Otolaryngol Head Neck Surg. 2010;136:692–696. doi: 10.1001/archoto.2010.112. [DOI] [PubMed] [Google Scholar]
- 15.Lin HW, Bhattacharyya N. Clinical behavior of follicular variant of papillary thyroid carcinoma: presentation and survival. Cancer. 1994;73:424–431. doi: 10.1002/1097-0142(19940115)73:2<424::AID-CNCR2820730230>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 16.Daniels GH. What if many follicular variant papillary thyroid carcinomas are not malignant? A review of follicular variant papillary thyroid carcinoma and a proposal for a new classification. Endocr Prac. 2011;17(5):768–787. doi: 10.4158/EP10407.RA. [DOI] [PubMed] [Google Scholar]
- 17.Chan JKC. Strict criteria should be applied in the diagnosis of encapsulated follicular variant of papillary thyroid carcinoma. Am J Clin Pathol. 2002;117:6–18. doi: 10.1309/P7QL-16KQ-QLF4-XW0M. [DOI] [PubMed] [Google Scholar]
- 18.Youngwirth LM, Adam MA, Scheri PR, et al. Extrathyroidal extension is associated with compromised survival in patients with thyroid cancer. Thyroid. 2017;27:626–631. doi: 10.1089/thy.2016.0132. [DOI] [PubMed] [Google Scholar]
- 19.Maletta F, Massa F, Torregrossa L, et al. Cytological features of “noninvasive follicular thyroid neoplasm with papillary-like nuclear features” and their correlation with tumor histology. Human Pathol. 2016;54:134–142. doi: 10.1016/j.humpath.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Golding A, Shively D, Bimston DN, Harrell RM. Noninvasive encapsulated follicular variant of papillary thyroid cancer: clinical lessons from a community-based endocrine surgical practice. Int J Surg Oncol. 2017;2017:1–6. doi: 10.1155/2017/4689465. [DOI] [PMC free article] [PubMed] [Google Scholar]