Abstract
Multiple salivary gland tumors (MSGTs) are most common in the major than minor salivary glands. The most MSGTs are synchronous, either benign or malignant. A 61-year-old woman was referred presenting nine submucosal nodules, firm to fluctuant, being five nodules on the right side and four nodules on the left side of the upper lip. An incisional biopsy was performed. Hematoxylin and eosin staining was performed in 5-µm sections for histopathologic analysis. Immunohistochemical reactions were carried out in 3-µm sections in accordance with manufacturer’s instructions. The histopathological analysis showed focal area containing low-grade polymorphous adenocarcinoma (PAC) and multiple canalicular adenomas (CAs). Immunohistochemical analysis for each lesion was carefully investigated. Here, we present an unusual case of synchronous PAC and multiple CAs of the minor salivary glands, affecting the upper lip, which appears to be the first case showing PAC and CA.
Keywords: Minor salivary glands, Upper lip, Canalicular adenoma, Polymorphous adenocarcinoma, Multiple salivary glands tumors
Introduction
Multiple salivary gland tumors (MSGTs) can be classified by topographic distribution (unilateral or bilateral) and chronologic appearance (synchronous or metachronous) [1, 2]. MSGTs affect more commonly the major than minor salivary glands and can be observed the following histopathological patterns: (1) exclusively benign tumors, (2) benign and malignant tumors, and (3) exclusively malignant tumors [1]. To date, only 11 intraoral MSGTs (either benign or malignant, synchronous or metachronous) have been published in the English-language literature. The predominant pattern observed was synchronous tumors, with most cases showing benign histopathologic subtypes [1]. Here, we present an unusual case of synchronous polymorphous adenocarcinoma (PAC), the second most common malignant neoplasm of minor salivary glands, which affects more commonly the palate and rarely the upper lip, associated with multiple canalicular adenomas (CAs), a benign neoplasm exclusive of minor salivary glands that affect frequently the upper lip [2–5]. Additionally, we also present a histochemical and immunohistochemical analyses for each neoplasm.
Case Report
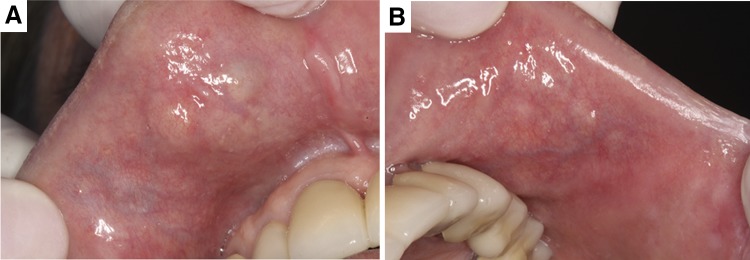
A 61-year-old woman was referred to the Oral Medicine Service of Araraquara Dental School—UNESP, Brazil, with a 12-month history of multiple nodules scattered on the upper lip, bilaterally (Fig. 1). Clinical examination revealed nine asymptomatic submucosal nodules, firm to fluctuant, being five nodules on the right side and four nodules on the left side. The nodules presented covered by normal-appearing mucosa, ranged from 0.2 to 0.8 cm in greatest dimension. During the surgical procedures, three nodular lesions were detached from adjacent normal-appearing tissue. The histopathologic evaluation on the hematoxylin and eosin (H&E) stain revealed a multifocal tumor growth surrounded by normal salivary gland tissue (Fig. 2a, c). In high-power view, columnar cells arranged in 1–2 cell layers thick and forming canal-like structures in a loose vascular stroma were visualized (Fig. 2b, d). Moreover, the characteristic beading with parallel rows joining, intraluminal squamous balls or morules (Fig. 2d, arrows), as well as, foci containing adenoid basal cell pattern [mimicking basal cell adenoma (BCA)] were also noticed. Noteworthy, a focal area showed a predominant trabecular growth pattern, which the ductal structures were single layered, without evident luminal and/or abluminal components. Indian filing and perineural invasion with concentric growth pattern supported by collagenous stromal component were also observed (Fig. 3). The diagnosis was synchronous multifocal CA and focal area containing low-grade PAC. The surgical specimen showed typical areas of low-grade PAC, but CA was not observed. After 2-year follow-up the patient is well, without recurrence or alterations in the lesional area.
Fig. 1.
Clinical aspects of the multiple submucosal nodules on the upper lip. Five nodules on the right side (a) and four nodules on the left side (b). All lesions covered by normal-appearing oral mucosa, with consistency firm to fluctuant and asymptomatic on palpation
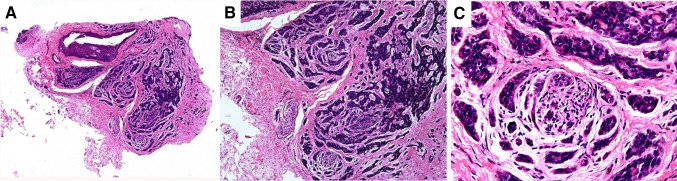
Fig. 2.
The histopathologic evaluation on the hematoxylin and eosin (H&E) stain revealed multifocal canalicular adenoma surrounded by normal-appearing salivary gland tissue (a, c, ×4). In high-power view, single columnar cells forming canal-like structures in a parvicellular and edematous stroma were visualized (b, d, ×40). Moreover, notice intraluminal squamous balls or morules (d, arrows) (the squares in a and c are shown at high magnifications in b and d, respectively)
Fig. 3.
The histopathologic evaluation on the hematoxylin and eosin (H&E) stain revealed focal area of low-grade polymorphous adenocarcinoma exhibiting a predominant trabecular growth pattern (a, ×4). Indian filing (b, ×10) and perineural invasion with concentric growth pattern (c, ×40) were also observed (Fig. 3)
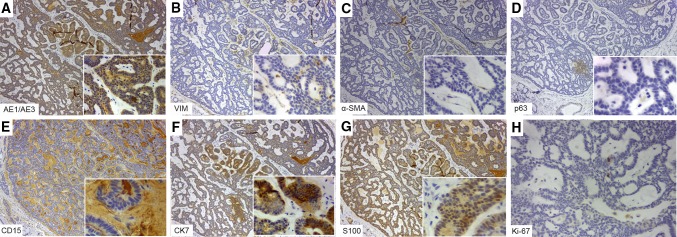
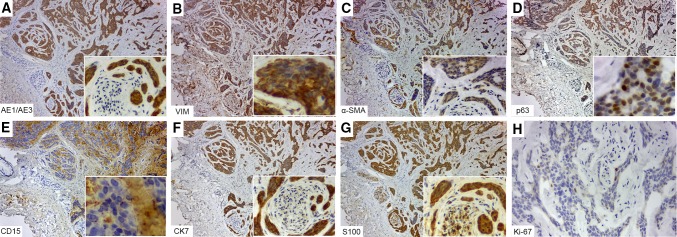
By immunohistochemistry both neoplasms were pan-cytokeratin (CK) AE1/AE3 positive, while that only CA was vimentin negative (Figs. 4, 5). Myoepithelial differentiation was only observed in low-grade PAC, which showed positivity for both alpha-smooth muscle antigen (α-SMA) and p63 (Fig. 4). Interestingly, CD15 highlighted the tumor stroma in both neoplasms (Figs. 4, 5). It was noticed a strong CK7 positivity in low-grade PAC than CA, while CK20 was negative in both tumors. S100 was positive in CA and low-grade PAC, in this latter highlighting the perineural invasion. The Ki-67 was 4% in low-grade PAC and 2% in CA. Glial fibrillary acidic protein (GFAP) and p53 were negative (Figs. 4, 5).
Fig. 4.
Immunohistochemical findings of canalicular adenoma. Pan-cytokeratin (CK) AE1/AE3 (a), CK7 (f) and S100 (g) were positive, whereas vimentin (b), α-SMA (c), p63 (d) were negative. Notice the CD15 positivity on the tumor stroma (e). The Ki-67 (h) proliferative index was low (>2%)
Fig. 5.
Immunohistochemical findings of low-grade polymorphous adenocarcinoma. Pan-cytokeratin (CK) AE1/AE3 (a), vimentin (b), α-SMA (c), p63 (d), CK7 (f) and S100 (g) were positive. Interestingly, CD15 positivity on the tumor stroma was observed (e). The Ki-67 (h) proliferative index was low (>4%)
Discussion
The PAC is regarded as the second most common intraoral malignant minor salivary gland tumor [3]. Most lesions occur in the palate, while that the upper lip is rarely affected. To date, approximately 20 cases of low-grade PAC affecting the upper lip have been reported [4]. These tumors are slightly more common in females, frequently between the sixth and seventh decades of life [6, 7]. Clinically, it reveals a firm, painless, submucosal mass covered by normal mucosa [6]. Histopathologically, PAC may be confused with other salivary gland neoplasms [7]. This tumor presents multiple growth patterns such as solid, cribriform, tubular, trabecular, fascicular and papillary [6, 7]. The differential diagnosis of PAC includes pleomorphic adenoma (PA), CA, BCA and adenoid cystic carcinoma (ACC) [6, 7].
The CA is a benign neoplasm preferentially involving the minor salivary glands. On the upper lip, CA accounts approximately for 80% of all cases, followed by buccal mucosa and palate [2, 5, 8]. There is slight female predilection, with a peak incidence in the fifth and seventh decades of life [2]. In about 13% of the cases, CA can present multifocal growth pattern; in these cases, recurrence or persistence is difficult to determine with certainty [2]. Clinically, it reveals an asymptomatic submucosal nodule that presents slowly growing. CA may be firm or slightly fluctuant on palpation and the color of the covering mucosa is normal or occasionally bluish [8]. Histopathologically, this tumor presents columnar or cuboidal cells, arranged in 1–2 cell layers thick, which form strands or ducts in a loose highly vascular stroma [5, 9].
By immunohistochemistry, the α-SMA and p63 positivity in PAC but not in CA is typical, such as demonstrated in the current case. Interestingly, while some studies have reported negativity for vimentin, recently, it was found positivity in 83% (43/52) of CA cases [9–11]. In the current case, different from PAC, CA was vimentin negative. Furthermore, CD15 positivity was observed in all but one CA cases (51/52), highlighting the tumor stroma [11]. In the present case, both PAC and CA showed also similar CD15 positivity. It is known that CD15, a carbohydrate adhesion molecule, in addition to inflammatory and tumor cells, is also expressed on glycoproteins, glycolipids and proteoglycans [12]. This latter can help understand the immunostaining pattern visualized in the current neoplasms. Moreover, the distinctive linear reaction of GFAP often reported at the tumor periphery in CA cases (81%, 42/52), as well as the variable GFAP immunopositivity in PAC [11], were not observed in the present study.
Frequently, salivary gland neoplasms are reported as single tumors, being the manifestation of MSGTs, chronologically synchronous or metachronous, uncommon. MSGTs affect more commonly major salivary glands, which can present as: (1) exclusively benign tumors, (2) benign and malignant tumors, and (3) exclusively malignant tumors [1]. After review of the English-language literature, to date, only 11 intraoral MSGTs (either benign or malignant, synchronous or metachronous) were identified. Five patients were men and six were women, with a mean age of 79 years. Six of these cases represented benign synchronous or metachronous tumors (5 with CAs and 1 with PAs); four cases represented synchronous or metachronous malignancies, being three PAC/PAC and one mucoepidermoid carcinoma/ACC cases; while that one case showed metachronous PAC/myoepithelioma. Overall, synchronous pattern was prevalent (7/11 MSGT cases) [1].
In summary, we report the first case of synchronous low-grade PAC and CA on the upper lip. Although they are very rare, the synchronous or metachronous presentation of MSGTs must be known by dentistry community, in order to establish proper treatment protocol with impact on the prognosis in this group of patients.
Acknowledgements
The authors thank the Laboratory of Applied Immunology of the Federal University of São Carlos—UFSCAR, Brazil, for technical support with digital whole slides (FAPESP 14/50256-4 and 11/52090-8). Financial support came from State of São Paulo Research Foundation Grants 2016/11419-0 (Jorge Esquiche León).
Compliance with Ethical Standards
Conflict of interest
There are no conflict of interest or disclosures related to this work.
Footnotes
This abstract was presented as a poster at the Brazilian Congress of Oral Medicine and Oral Pathology, Annual Metting, Manaus, AM, Brazil, on July 4–8, 2016.
References
- 1.Argyris PP, Gopalakrishnan R, Pambuccian SE, Tosios KI, Koutlas IG. Polymorphous low-grade adenocarcinoma of the upper lip with metachronous myoepithelioma of the buccal mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:e441–e448. doi: 10.1016/j.oooo.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 2.Samar ME, Avila RE, Fonseca IB, Anderson W, Fonseca GM, Cantín M. Multifocal canalicular adenoma of the minor labial salivary glands. Int J Clin Exp Pathol. 2014;7:8205–8210. [PMC free article] [PubMed] [Google Scholar]
- 3.Kimple AJ, Austin GK, Shah RN, Welch CM, Funkhouser WK, Zanation AM, Shockley WW. Polymorphous low-grade adenocarcinoma: a case series and determination of recurrence. Laryngoscope. 2014;124:2714–2719. doi: 10.1002/lary.24788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonseca FP, Brierley D, Wright JM, Santos-Silva AR, Almeida OP, Rocha AC, Van Heerden WF, Hunter KD. Polymorphous low-grade adenocarcinoma of the upper lip: 11 cases of an uncommon diagnosis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:566–571. doi: 10.1016/j.oooo.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Tyralik D, Dzierwa-Gawron A, Ryś J. Canalicular adenoma of the upper lip. Metachronous (multifocal) canalicular adenoma of the upper lip: a case report of an unusual finding. Pol J Pathol. 2013;64:71–74. doi: 10.5114/pjp.2013.34609. [DOI] [PubMed] [Google Scholar]
- 6.Abu El-Naaj I, Leiser Y, Wolff A, Peled M. Polymorphous low grade adenocarcinoma: case series and review of surgical management. J Oral Maxillofac Surg. 2011;69:1967–1972. doi: 10.1016/j.joms.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Fife TA, Smith B, Sullivan CA, Browne JD, Waltonen JD. Polymorphous low-grade adenocarcinoma: a 17 patient case series. Am J Otolaryngol. 2013;34:445–448. doi: 10.1016/j.amjoto.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Rousseau A, Mock D, Dover DG, Jordan RC. Multiple canalicular adenomas: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:346–350. doi: 10.1016/S1079-2104(99)70221-0. [DOI] [PubMed] [Google Scholar]
- 9.Siqueira CS, Fernandes KS, Vivas AP, Pinto Ddos S, Jr, de Sousa SC. Clinical and histological features of multifocal canalicular adenomas of the upper lip. Braz Dent J. 2013;24:542–546. doi: 10.1590/0103-6440201302264. [DOI] [PubMed] [Google Scholar]
- 10.Furuse C, Tucci R, Machado de Sousa SO, Rodarte Carvalho Y, Cavalcanti de Araújo V. Comparative immunoprofile of polymorphous low-grade adenocarcinoma and canalicular adenoma. Ann Diagn Pathol. 2003;7:278–280. doi: 10.1016/S1092-9134(03)00084-4. [DOI] [PubMed] [Google Scholar]
- 11.Thompson LD, Bauer JL, Chiosea S, McHugh JB, Seethala RR, Miettinen M, et al. Canalicular adenoma: a clinicopathologic and immunohistochemical analysis of 67 cases with a review of the literature. Head Neck Pathol. 2015;9:181–195. doi: 10.1007/s12105-014-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerr MA, Stocks SC. The role of CD15-(Le(X))-related carbohydrates in neutrophil adhesion. Histochem J. 1992;24:811–826. doi: 10.1007/BF01046353. [DOI] [PubMed] [Google Scholar]