Abstract
Among the variants of squamous cell carcinoma (SCC) of the head and neck arising in mucosal surfaces, examples with sebaceous differentiation are exceedingly rare. We present a new case of SCC with sebaceous differentiation, developing in the larynx of a 64 year-old male, cigarette smoker and alcohol drinker. The tumor extended transglottically, metastasized to cervical lymph nodes, and killed the patient after 12 months. Comparing this case with four previously reported cases of SCC with sebaceous differentiation, two arising in the larynx and the other two in the adjacent pharynx, all five patients mostly shared the following features: appearance of the tumor in the seventh decade of life, heavy tobacco smoking, alcohol intake in three, surgery as mainstay treatment, tumor size between 2 and 4.7 cm, and regional lymph node metastases in four of them. Out of the four patients with a follow up of 12 months, two died of disease, one was alive with disease, and only one was alive without disease. One patient was lost for follow up. In conclusion, mucosal SCC with sebaceous differentiation is a very rare variant of SCC that when arising in the larynx and anatomically adjacent parts of the pharynx behaves aggressively and bears a dismal prognosis. The recognition of new cases of this entity requires special awareness of its phenotypic features and may be important for further assessment of its behavior.
Keywords: Larynx and adjacent pharynx, Squamous cell carcinoma variants, Sebaceous differentiation, Prognosis
Introduction
Sebaceous carcinomas (SbCs) are unusual malignancies with aggressive behavior that mainly originate from sebaceous glands in cutaneous territories of the head and neck [1]. About 75% of SbCs arise from the cutaneous appendages of the ocular adnexa, mainly from the tarsal meibomian glands and from the Zeis glands of the eyelashes, and to a lesser extent from the glands of the eyelids, eyebrows, conjunctiva and caruncle [2]. Of the non-cutaneous SbCs of the head and neck around 90% develop in the parotid gland [3, 4], and the rest in the submandibular, sublingual, and minor glands of various mucosal surfaces [5], the latter being referred to as mucosal squamous cell carcinomas with sebaceous differentiation (MSCCSb).
MSCCSb of the head and neck have been reported to occur in the oral cavity [6–8], vallecula [9], hypopharynx [10], and larynx [11, 12]. The purpose of this paper is to report on a new case of MSCCSb involving the larynx with review and discussion of the related literature in regard to the etiology, pathogenesis, clinical features, pathological diagnosis and management of this exceedingly rare type of mucosal tumor.
Case Report
A 64-year-old man presented at the Hospital Clinic of Barcelona with dysphonia, dyspnea, stridor, and discrete dysphagia, of 2-month duration. He reported a history of heavy smoking and alcohol consumption. Direct laryngoscopy revealed a sessile growth at the right hemi-larynx with involvement of the false vocal cord, fixation of the true vocal cord and extension to the ipsilateral aryepiglottic fold, and adjacent pyrifom sinus. The left hemi-larynx and the anterior commissure were also widely involved. Physical examination detected right latero-cervical lymphadenopathy. No cutaneous lesions were evident in the facial region or elsewhere. A laryngeal biopsy was carried out resulting in an initial diagnosis of poorly differentiated squamous cell carcinoma. A tracheostomy was performed due to increasing dyspnea and subsequently the patient underwent total laryngectomy, with selective bilateral neck dissection of level III.
Gross Examination
The surgical specimen contained a widespread transglottic tumor almost occupying the entire larynx, that extended to the right aryepiglottic fold, the adjacent pyriform sinus and arytenoid cartilage. The epiglottis, the anterior commissure and the left vocal cord were also involved by the tumor that in depth contacted without destruction with the perichondrium of the left wing of the thyroid cartilage, Fig. 1. Two cervical lymph nodes were found at level III of the right neck and one at level III left; the largest node was located at the right side and measured 1.5 cm.
Fig. 1.
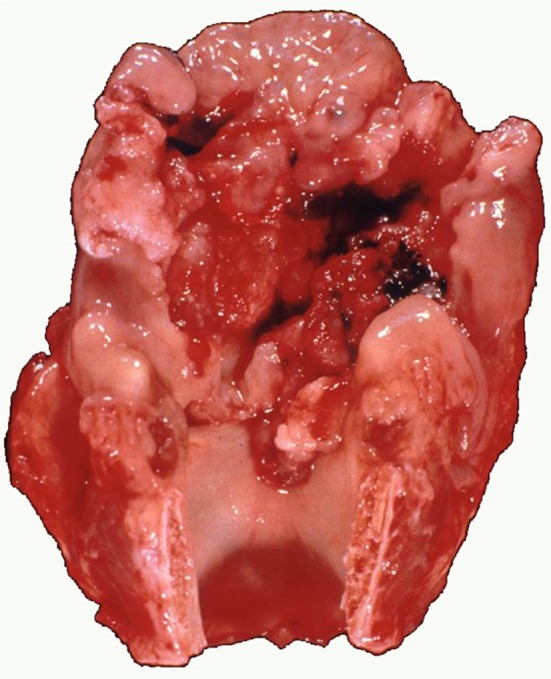
Extensive transglottic tumor, almost occupying the entire larynx
Microscopic Examination
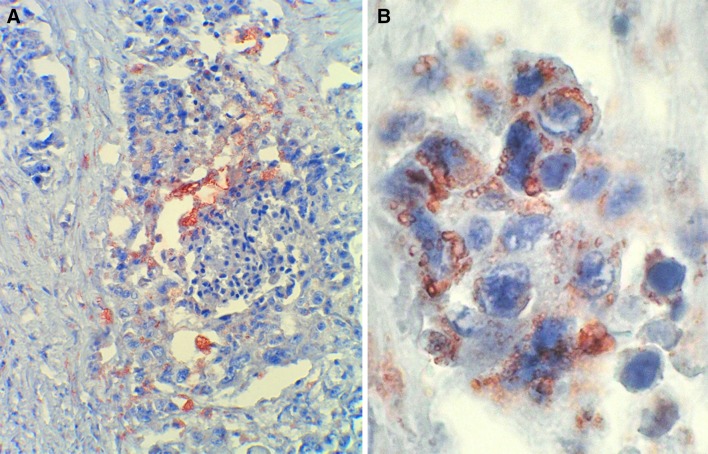
At low power, the tumor had the histological features of a conventional, high grade, squamous cell carcinoma (SCC). A distinct basal cell layer was often seen at the periphery of the interconnected nests and cords that composed the tumor. Inside these nests, the keratinocytes gave rise to foci of keratinizing pearl formation and to conspicuous areas of central necrosis, Fig. 2. Furthermore, at the surroundings of the necrotic foci, there were frequent areas with striking vacuolization of keratinocytes, Fig. 3. At high magnification, marked cell pleomorphism, prominent nuclear atypia, multinucleation and elevated mitotic count were commonly encountered, Fig. 4. Although these vacuoles were seemingly empty at first glance, closer inspection often disclosed a delicate intracytoplasmatic eosinophilic network, very suggestive of sebaceous differentiation, Fig. 5. The strong reactivity of these vacuoles and of the contiguous necrotic foci, with oil red-O stain, a specific marker of fat, confirmed this assumption. It also demonstrated holocrine type of secretion, which is a characteristic feature of sebaceous cellular secretion, Fig. 6 a and b. With all of these features, the diagnosis of SCC with prominent sebaceous differentiation was made. SCC metastases were found in two latero-cervical lymph nodes at level III, one right and one left, categorizing the tumor as pT4, N2c, M0. Stage: IVA.
Fig. 2.
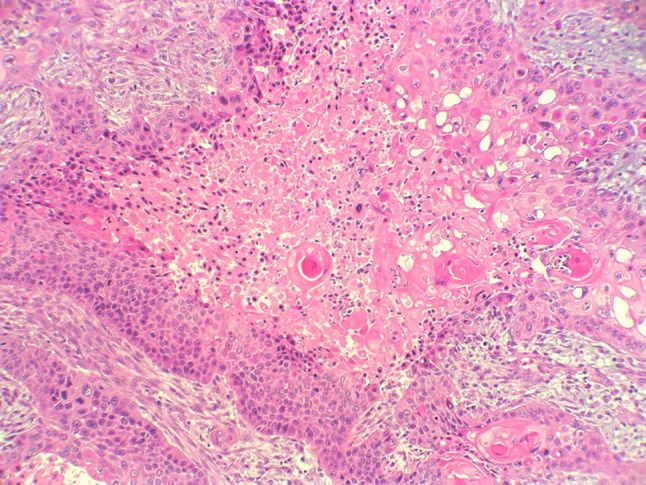
Conventional keratinizing SCC with distinct basal cell layer, foci of central necrosis and vacuolization of keratinocytes
Fig. 3.
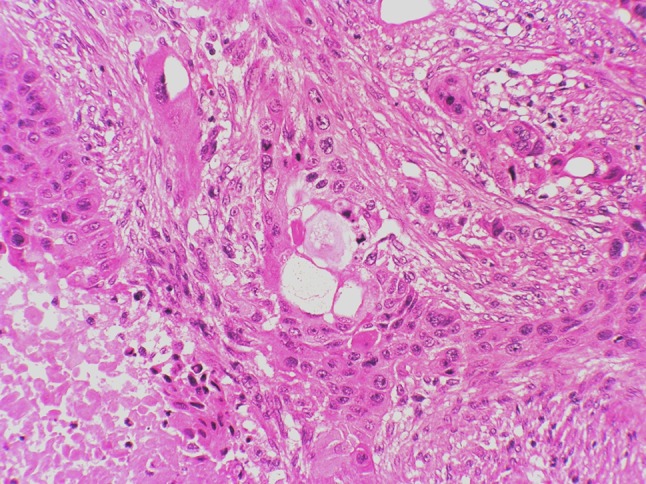
Striking vacuolization of keratinocytes, one of them of giant cell type, at the vicinity of a necrotic focus
Fig. 4.
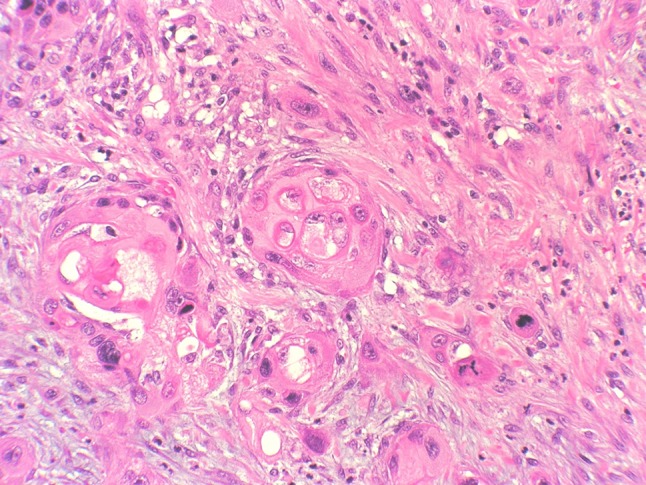
Vacuolization, pleomorphism, multinucleation, prominent nuclear atypia, and elevated mitotic count of keratinocytes
Fig. 5.
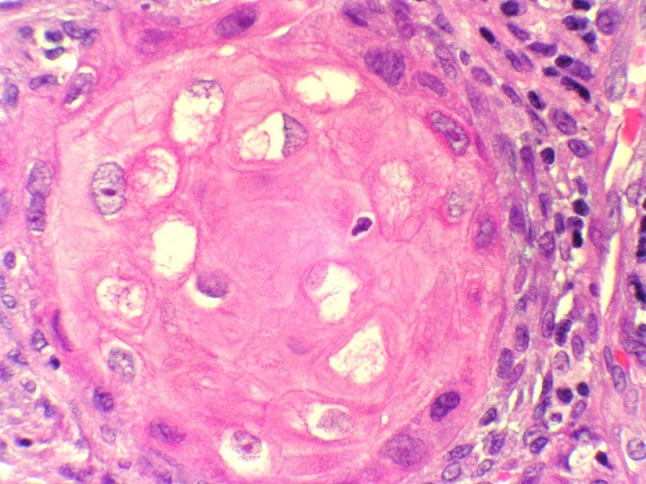
Keratinocytes displaying a delicate intracytoplasmatic eosinophilic network, very suggestive of sebaceous differentiation
Fig. 6.
a Strong reactivity of the intracytoplasmic vacuoles and of the contiguous necrotic foci, with oil red-O stain. b Detail of the staining of the intracytoplasmic vacuoles with oil red-O
Treatment and Follow Up
After surgery the patient received radio-chemotherapy and subsequently was referred to another Centre. He died of disease 1 year later.
Discussion
MSCCSb of the head and neck are extremely rare tumors. To the best of our knowledge only two such cases arising in the larynx and two others in the anatomically adjacent parts of the pharynx have been published (Table 1). Chronologically, the first case was reported by Assor in 1975, who documented one epidermoid carcinoma with sebaceous differentiation at the vallecula [9]. Martinez-Madrigal et al. reported in 1991 a case of hypopharyngeal sebaceous carcinoma [10]. However, since the first sentence of the abstract, the tumor was mostly addressed as squamous cell carcinoma with sebaceous differentiation. Baiocco et al. described in 1995 one case of squamous carcinoma of the epiglottis with sebaceous differentiation [11]. The case of Panayotides et al. in 1995 referred to a laryngeal squamous cell carcinoma with sebaceous differentiation [12]. All four cases were described as containing variable amounts of basaloid, squamous and sebaceous cells. In the oral cavity a total of ten cases of sebaceous carcinoma have been reported to occur up to 2015 according to Rowe et al. [8].
Table 1.
Summary of reported cases of sebaceous differentiation in squamous cell carcinoma of the larynx and adjacent pharynx
| Author | Patient age (years) | CS/AD | Location | Treatment | Tumor size (cm) | Metastases | Disease status and follow-up (months) |
|---|---|---|---|---|---|---|---|
| Assor et al [9] | 65 | CS + AD | Vallecula | Surgery | 2 | LN SbC | N/A |
| Martínez-Madrigal et al [10] | 74 | CS | Hypopharynx | Surgery | 5 | LN + skin SbC | AWD, 3 |
| Baiocco et al [11] | 64 | CS + AD | Larynx S | Surgery | 2.5 | No | AWOD, 8 |
| Panayiotides et al [12] | 68 | CS | Larynx S | Surgery | 2.3 | LN SCC | DOD, 7 |
| Present case | 64 | CS + AD | Larynx T | Surgery + chemoradiotherapy | 4.7 | LN SCC | DOD, 12 |
CS cigarette smoking, AD alcohol drinking, N/A not available, AWD alive with disease, AWOD alive without disease, DOD dead of disease, LN lymph node, SbC sebaceous carcinoma, SCC squamous cell carcinoma, S Supraglottic, T Transglottic
While the sebaceous glands of the ocular adnexa are considered the origin of the SbCs occurring in this territory, the collections of sebaceous glands known as Fordyce granules (spots) are plausibly related to the origin of the SbCs developing in the oral cavity, the parotid gland, and minor salivary glands, having all of these glandular structures in common with their ectodermal derivation [11]. However, this hypothesis seems less attractive for SbCs of endodermal derivation, as the vallecula, the larynx and the hypopharynx lack sebaceous glands. Nevertheless, very few examples of sebaceous heterotopia have been described, such as the presence of sebaceous glands in the lamina propria of the esophagus, which is of endodermal origin [12, 13]. In the four previously reported cases here discussed [9–12] the sebaceous transformation may be considered most likely due to metaplasia, since normal appearing sebaceous glands, similar to Fordyce granules were not found. Also in our present case, involving larynx and extending to hypopharynx, the images point to a process of sebaceous metaplasia in the absence of Fordyce-like granules. In this regard, as demonstrated some years ago, an epithelial cell line, harboring both sebaceous and squamous cells, was isolated from a cervical lymph node metastasis of a squamous cell carcinoma [14]. Although infrequently, sebaceous differentiation may also be seen in benign and malignant salivary gland tumors, mainly in Warthin tumor and pleomorphic adenoma, and less often in mucoepidermoid carcinoma, epithelial-myoepithelial carcinoma, carcinoma ex pleomorphic adenoma and oncocytoma [15].
The recognition of sebaceous differentiation in a SCC requires a high grade of suspicion, especially when the tumor cells lack a lobular arrangement. To achieve this, it is mandatory to discern between SCC with true clear cells and SCC with clear cytoplasm that additionally portray a delicate eosinophilic network insinuating sebaceous differentiation. In case of doubt, PAS, PAS-diastase, mucicarmine and alcian blue stains could be helpful to distinguish between sebocytes, glycogenated keratinocytes and mucus cells. When non-paraffin embedded tissue is available, classical stains for fat, as oil-red-O, or Sudan III, are diagnostic. In paraffin embedded tissue the staining of sebaceous glands by immunohistochemical markers such as EMA, as well as androgen receptor and GCDFP-15 has not proved to be specific enough. Adipophilin is a recently developed monoclonal antibody against a protein on the surface of intracellular lipid droplets expressed in sebocytes and sebaceous lesions, showing high sensibility and specificity when observed as membranous vesicular staining [16]. This new marker may provide a valuable help in the future recognition of new examples of mucosal SCC with sebaceous differentiation of the head and neck. This could be important in the management of patients with this type of SCC, especially those involving larynx and anatomically adjacent pharynx, as so far their outcome has been dismal, with reported survivals never over 12 months.
Concerning the epidemiology and etiology of laryngeal and pharyngeal SCC with sebaceous differentiation, all the five patients were males, in the seventh and eighth decades of life. There was history of heavy cigarette smoking in all of them. Moreover three of them were heavy drinkers of alcohol. Finally, it has to be taken into account the Muir–Torre syndrome, an autosomal dominant cancer syndrome, thought to be a subtype of Lynch’s syndrome that is associated with sebaceous tumors, and affects the genes hMLH1, hMSH2, hMSH6 and PMSH-2 [17]. Although so far no case with this syndrome has been reported in MSCCSb, the possibility of its participation needs further investigation.
Conclusion
Mucosal SCC with sebaceous differentiation is a very rare variant of SCC of the head and neck that when occurring in the larynx and in the anatomically adjacent parts of the pharynx behaves aggressively and bears dismal prognosis. Recognition of new cases of this entity, which requires special awareness of its phenotypic features, may be important to increase the knowledge on its behavior.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they do not have any conflict of interests.
Research Involving Human or Animal Participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article was written by members and invitees of the International Head and Neck Scientific Group (http://www.IHNSG.com).
Contributor Information
Antonio Cardesa, Phone: +34 932275450, Email: acardesa@clinic.ub.es.
Alfons Nadal, Email: lalos@clinic.ub.es.
Llucia Alos, Email: anadal@clinic.ub.es.
Josep Lloreta-Trull, Email: jlloreta@parcdesalutmar.cat.
Alfio Ferlito, Email: alfio.ferlito@uniud.it.
References
- 1.Rulon DB, Helwig EB. Cutaneous sebaceous neoplasms. Cancer. 1974;33:82–102. doi: 10.1002/1097-0142(197401)33:1<82::AID-CNCR2820330115>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Font RL, Croxatto JO, Rao NA. Atlas of tumor pathology. Tumors of the eye and ocular adnexa, 4th series, fascile 5. Washington, D.C.: Armed Forces Institute of Pathology; 2006. p. 15, 33, 183–191.
- 3.Ellis GL, Auclair PL. Atlas of tumor pathology. Tumors of the salivary glands, 4th series, fascicle 9. Washington, D.C.: Armed Forces Institute of Pathology; 2008. p. 377–380.
- 4.Gnepp DR. Sebaceous carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. WHO classification of head neck tumors. Lyon: IARC Press; 2005. p. 231. [Google Scholar]
- 5.Gnepp DR, Assaad A, Ro JY. Sebaceous adenocarcinoma. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of head neck tumors. Lyon: IARC Press; 2017. pp. 178–179. [Google Scholar]
- 6.Alawi F, Siddiqui A. Sebaceous carcinoma of the oral mucosa: case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:79–84. doi: 10.1016/j.tripleo.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Yao J, Solomon M, Axiotis CA. Sebaceous carcinoma of the oral cavity: case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:e37–e40. doi: 10.1016/j.tripleo.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 8.Rowe ME, Khorsandi AS, Urken GR, Wenig BM. Intraoral sebaceous carcinoma metastatic to the lung and subcutis: case report and discussion of the literature. Head Neck. 2016;38:E20–E24. doi: 10.1002/hed.24091. [DOI] [PubMed] [Google Scholar]
- 9.Assor D. Epidermoid carcinoma with sebacous differentiation in the vallecula. Am J Clin Pathol. 1975;63:891–894. doi: 10.1093/ajcp/63.6.891. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Madrigal F, Casiraghi O, Khapiech A, Nasr-Khapiech RB, Richard J-M, Micheau Ch. Hypopharyngeal sebaceous carcinoma. Human Pathol. 1991;22:929–931. doi: 10.1016/0046-8177(91)90186-S. [DOI] [PubMed] [Google Scholar]
- 11.Baiocco R, Palma O, Locatelli G. Squamous carcinoma of the epiglottis with sebaceous differentiation. Pathologica. 1995;87:531–533. [PubMed] [Google Scholar]
- 12.Panayiotides JG, Arapantoni-Dadioti P, Banis CG. Laryngeal squamous cell carcinoma with sebaceous differentiation. J Laryngol Otol. 1995;109:784–786. doi: 10.1017/S0022215100131329. [DOI] [PubMed] [Google Scholar]
- 13.Bambirra EA, de Souza Andrade J, Hooper de Souza LA, Savi A, Ferreira Lima G, de Oliveira CA. Sebaceous glands in the esophagous. Gastrointest Endosc. 1983;29:251–252. doi: 10.1016/S0016-5107(83)72605-2. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto K, Yamagawa T, Azuma M, et al. Establishment of a transformed human epithelial cell line with a sebaceous phenotype and effect of epidermal growth factor and dibutyryl cyclic adenosine 3′-5′ monophosphate on the cellular phenotype. Cancer J. 1989;3:414–422. [Google Scholar]
- 15.Hellquist H, Skalova A. Sebaceous carcinoma and sebaceous lymphadenocarcinoma. In: Hellquist H, Skalova A, editors. Histopathology of the salivary glands. Berlin: Springer; 2014. pp. 407–409. [Google Scholar]
- 16.Ostler DA, Prieto VG, Reed JA, Deavers MT, Lazar AJ, Ivan D. Adipophilin expression in sebaceous tumors and other cutaneous lesions with clear cell histology: an immunohistochemical study of 117 cases. Mod Pathol. 2010;23:567–573. doi: 10.1038/modpathol.2010.1. [DOI] [PubMed] [Google Scholar]
- 17.Ko CJ. Muir–Torre syndrome: facts and controversies. Clin Dermatol. 2010;28:324–329. doi: 10.1016/j.clindermatol.2009.07.001. [DOI] [PubMed] [Google Scholar]