Abstract
Sinonasal tumors with neuroendocrine differentiation form a group of rare heterogeneous neoplasms of neuroectodermal and epithelial origin, consisting of olfactory neuroblastomas and neuroendocrine carcinomas. Because the natural history and biological behavior of this group of tumors vary, the morphological diagnosis coupled with grading/staging is important for prognostication, and the approach to treatment and rehabilitation is multidisciplinary. The identification of molecular abnormalities underlying these tumors is critical to the development of specific targeted therapies and the design of clinical trials.
Keywords: Sinonasal neuroendocrine tumors, Sinonasal small cell carcinoma, Sinonasal large cell carcinoma, Olfactory neuroblastoma
General Considerations on Sinonasal Neuroendocrine Neoplasms
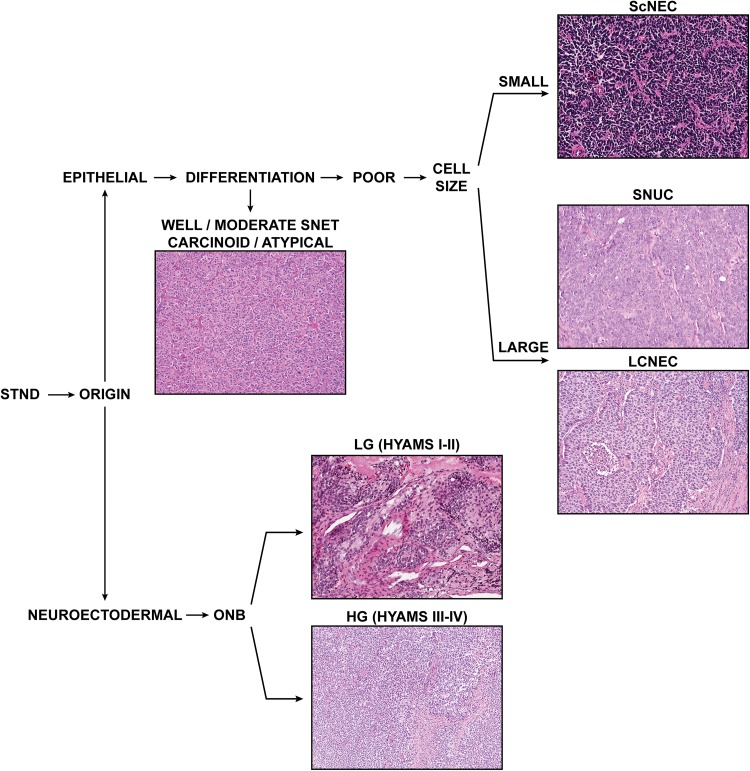
Sinonasal tumors with neuroendocrine differentiation comprise a group of rare heterogeneous neoplasms of neuroectodermal and epithelial origin, consisting of olfactory neuroblastomas (ONBs) and neuroendocrine carcinomas (NECs) [1–3]. The ONBs constitute the neuroectodermal group, while the NECs are divided into SNEC (including carcinoids and atypical carcinoids), small cell carcinoma, neuroendocrine type, and large cell carcinoma, neuroendocrine type, following the lung neuroendocrine neoplasm classification [4] (Fig. 1). Although cases of mixed neoplasms composed of neuroendocrine and non-neuroendocrine components have been described [2, 5], this nomenclature is controversial and these are not included in the current World Health Organization (WHO) classification. The natural history and biological behavior of this group of tumors vary considerably, and morphological diagnosis coupled with grading/staging is important for prognostication.
Fig. 1.
The current classification of sinonasal tumors with neuroendocrine differentiation (STND). SNET sinonasal neuroendocrine tumors, ScNEC small cell neuroendocrine carcinoma, SNUC sinonasal undiffererentiated carcinoma, LCNEC large cell neuroendocrine carcinoma, ONB olfactory neuroblastoma (low-grade and high-grade)
Carcinoid Tumor and Atypical Carcinoid
Typical and atypical carcinoids of the nasal cavity and paranasal sinuses are anecdotal, but they are similar to carcinoids in other sites [1, 6].
Small Cell Carcinoma, Neuroendocrine Type
Small cell carcinoma, neuroendocrine type, is a high-grade carcinoma composed of small to intermediate-sized cells resembling those of small cell carcinoma of pulmonary or extrapulmonary origin. The microscopic hallmarks of this tumor include necrosis, large numbers of apoptotic cells, high mitotic rate, and lack of neurofibrillary stroma. These tumors are also known as small cell NEC, oat cell carcinoma, and poorly differentiated NEC [7].
Small cell NEC of the sinonasal tract is a rare tumor. It has no predilection for a specific sex, race, or geographic area and no known association with smoking or radiation. These tumors most frequently arise in the superior or posterior nasal cavity, often extending into the maxillary or ethmoid sinuses. In a study of patients presenting to our institution with small cell NEC, the median age was 56 years, with equal sex distribution; the majority of patients presented with T3 or T4, node-negative disease (82%). The most common sites of tumor origin were the ethmoid sinus (64%), the nasal cavity (32%), and the maxillary sinus (14%) [8]. In general, a minority of cases have secondary involvement of the nasopharynx. Advanced tumors may invade the skull base, orbit, or brain. In rare cases, serum levels of ACTH and calcitonin are elevated [1, 9].
Small cell NECs are aggressive tumors with a poor prognosis, characterized by frequent local recurrence and distant metastasis despite multimodal therapy. Follow-up data have shown a local recurrence rate of 45% and a distant metastasis rate of 35% [6, 8, 10]. Common sites of metastasis include cervical lymph nodes, lung, liver, bone marrow, and vertebrae.
Large Cell Carcinoma, Neuroendocrine Type
Large cell NEC comprises two distinct types: (1) large, undifferentiated cells with only immunohistochemical or ultrastructural evidence of neuroendocrine differentiation; and (2) large cells as defined in the respiratory tract (organoid nests, trabeculae, and rosettes, with peripheral palisading of nuclei). The former is equivalent to sinonasal undifferentiated carcinoma [1, 7, 11], showing sheets, large nests, organoid trabeculae, and occasional rosettes. The cells are medium to large, and the nucleus often features a prominent single nucleolus. Comedonecrosis, lymphovascular invasion, and > 10 mitoses per 2 mm2 or 10 high-power fields are common. Squamous and glandular differentiation are absent, although an in situ component or pagetoid spread may be seen.
Treatment of Neuroendocrine Carcinomas
NEC, regardless of differentiation or subtype, accounts for 5% of sinonasal malignancies [12]. An ideal treatment strategy is elusive because of the rarity of cases and the heterogeneity of treatment approaches. In a study spanning 1990 to 2004 at MD Anderson Cancer Center, approximately half of the 28-patient NEC cohort underwent surgery as the primary treatment modality and another one-third received chemoradiation therapy [8]. The 5-year overall survival (OS), disease-specific survival, and disease-free survival (DFS) rates were 66.9, 78.5, and 43.8%, respectively. A total of 21, 25, and 18%, respectively, experienced local, regional, or distant treatment failure [8]. These results are better than generally reported [13, 14]. Predictors of poor outcomes were foveal or orbital involvement and tumor originating outside of the nasal cavity. A complete response to neoadjuvant chemotherapy correlated with a higher survival rate at 3 years. Given the high incidence of distant failure and the chemosensitivity of NEC, a promising treatment strategy is neoadjuvant chemotherapy followed by either chemoradiation or surgery with post-operative radiation therapy. In 8 of 18 patients treated with neoadjuvant chemotherapy and described in an earlier report from our institution, the OS and local recurrence rates were 64.2 and 27.4%, respectively [10].
A recent meta-analysis of 701 cases of sinonasal NECs (comprising 127 NECs, 459 sinonasal undifferentiated carcinomas, and 115 small cell NECs) concluded that the most important predictors of survival in sinonasal NECs are differentiation (grade) and the associated choice of treatment modality [15]. In contrast to other head and neck cancers, tumor staging appears of limited value in predicting survival or deciding on a treatment strategy. Surgery should be the cornerstone of treatment, supplemented by radiotherapy in poorly differentiated subtypes (undifferentiated and small cell types). Chemotherapy does not appear to prolong survival [15].
Olfactory Neuroblastoma
ONB was first described by Berger, Luc, and Richard in 1924 [16]; it has been characterized as a rare malignant neoplasm of the sinonasal cavity that arises in the superior portion of the nasal vault. Some cases are noted as having “ectopic” origin in the lower nasal cavity or within one of the paranasal sinuses (e.g., maxillary sinus) [17, 18]. Since its first description, ONB has been referenced under several names (esthesioneuroblastoma, esthesioneurocytoma, esthesioneuroepithelioma, olfactory placode tumor), but ONB is the term currently accepted by WHO [17]. The exact origin of this tumor, both the location and cell type, is under debate [19]. Proposed anatomic sites of origin include Jacobson’s organ, the sphenopalatine ganglion, the ectodermal olfactory placode, Loci’s ganglion, sympathetic ganglia of the nasal mucosa, and the nasal mucosa itself, but the most likely site of origin is the basal neural cells of the olfactory mucosa, and this is the one most generally accepted [19–21]. The olfactory neural epithelium is composed of different cell types, including Bowman’s gland cells, horizontal basal cells, globose basal cells, olfactory neurons (mature and immature), and sustentacular cells; each of these cell types expresses specific markers of differentiation [21, 22]. Basal cells are regarded as multipotent and/or neural precursor cells that proliferate and differentiate into either neural or non-neural cells in both humans and rodents [21, 22]. Mature and immature (transitional) olfactory neurons are located in the intermediate layer of the olfactory neural epithelium, while the apical layer contains sustentacular cells and sensory cilia that are projected from the dendrites of olfactory neurons [22]. Olfactory neurons are continuously replaced by neurogenesis in the olfactory neural epithelium throughout adult life. This process is regulated by growth factors that also control neurogenesis in the central nervous system [22]. The apparent neuronal or neural crest origin of ONB is supported by the fact that neural filaments are present in tumor cells, and molecular analysis suggests that ONB is derived from immature olfactory neurons [9, 19, 20].
ONB comprises 2–3% of tumors of the nasal cavity, with an incidence of 0.4 cases per million [17, 19]. It affects both sexes equally. Patients range in age from 2 to 90 years; although these tumors were first believed to present with a bimodal age distribution, recent reports support an even distribution across all ages with peaks in the 5th and 6th decades [23–25].
ONB typically presents as a unilateral nasal mass with symptoms of obstruction and bleeding. The classic imaging findings include a “dumbbell-shaped” mass extending across the cribriform plate, with the waist at the cribriform plate. MRI is most effective at delineating sinonasal and intraorbital extension or intracerebral extension [17]. The tumor appears hypointense relative to gray matter on T1-weighted images and isointense or hyperintense relative to gray matter on T2-weighted images, with avid homogeneous enhancement with contrast. Bony erosions are better demonstrated by CT images, necessitating careful evaluation of the lamina papyracea, cribriform plate, and fovea ethmoidalis. Peripheral tumor cysts and speckled calcifications are quite characteristic of ONB [17].
Several staging systems have been proposed for ONB, but no single system is universally accepted. The most widely applied is the Kadish system, which classifies local disease only [26]; it differentiates tumors that involve the nasal cavity alone (Kadish A), extend into the paranasal sinuses (Kadish B), or extend outside of the paranasal sinuses (Kadish C) [26]. Morita et al. modified the Kadish system [27], designating a new class for ONB with metastases; this added D class includes cases with regional nodal disease and distant metastasis. The Dulguerov classification system distinguishes patients with sphenoid sinus disease from those without sphenoid sinus disease; it also differentiates cases with intracranial and/or orbital extension from those with brain parenchymal invasion, while considering lymph node and distant metastasis separately [28]. The TNM staging system for paranasal sinus tumors can potentially be applied [29], but the biologically unique behavior of ONB compared to other sinonasal tumors makes the alternative classification systems more useful.
On gross examination, these unilateral tumors are usually polypoid, glistening, soft, red-grey masses with an intact mucosa; the cut surface appears grey-tan to pink-red and hypervascularized [17, 23]. Tumors range from < 1 cm to large masses involving the nasal cavity and intracranial region [17]. Tumors frequently expand into adjacent paranasal sinuses, orbits, and the cranial vault. Histologic examination reveals uniform lobules of small round blue cells with a neurofibrillary background. Pseudorosettes (Homer–Wright) are occasionally present, while true rosettes (Flexner–Wintersteiner) are uncommon. High-grade tumors are characterized by large pleomorphic cells and necrosis. The Hyams grading system [30] captures the spectrum of ONB maturation, from indolent disease to more aggressive behavior. The Hyams system assigns a score representing the degree of expression (1, least expression; 4, most expression) of key adverse features: mitotic activity, nuclear pleomorphism, rosette formation, necrosis, disorganized architecture, and sparse fibrillary matrix [30, 31]. Lately, histopathologic Hyams grade has been shown to accurately characterize the tumor’s biology and to be an independent predictor of locoregionally aggressive disease and poor DFS [23, 32–34]; thus it is considered to add value to clinical stage in clinical decision making. Hyams grade should therefore be determined and used in clinical management of ONB.
Typically, the diagnosis of ONB is established by positive staining for synaptophysin and other neuroendocrine markers combined with negative staining for keratin, muscle, melanoma, and lymphoma markers. S100 staining highlights sustentacular cells. Up to one-third of ONBs also stain focally for cytokeratin (Cam 5.2, CK 18) [1, 9, 20, 21, 23, 35]; Ki-67 staining reveals a variable proliferative index (2–50%) [9, 36, 37].
The differential diagnosis encompasses small blue round cell neoplasms, while identification of the cell lineage is crucial to the diagnosis. Within the clinical context and anatomic boundaries, the (ectopic) pituitary adenoma is on the differential list [38].
Molecular Pathogenesis
Holland et al. reported numerous chromosomal aberrations associated with ONB, predominantly involving chromosomes 2q, 5, 6q, 17, 19, 21q, and 22, as well as trisomy 8 [39]. In 22 ONBs analyzed by the same technique, deletions of 1p, 3p/q, 9p, and 10p/q and amplifications of 17q, 17p13, 20p, and 22q were frequent; this analysis also produced the interesting observation that specific deletion on chromosome 11 and gain on chromosome 1p were associated with metastasis and a worse prognosis [40]. Another study found amplification of the whole chromosome 19, partial gains of 1p, 8q, 15q, and 22q, and deletions of 4q and 6p in ONBs [41]. Gulled and colleagues, in an array comparative genomic hybridization study, identified gains at 7q11.22–q21.11, 9p13.3, 13q, 20p/q, and Xp/q and losses at 2q31.1, 2q33.3, 2q37.1, 6q16.3, 6q21.33, 6q22.1, 22q11.23, 22q12.1, and Xp/ in 13 ONB samples [42]. Gains were more frequent than losses, and high-stage tumors showed more alterations than low-stage ONB. Frequent changes in high-stage tumors were gains at 13q14.2–q14.3, 13q31.1, and 20q11.21–q11.23 and loss of Xp21.1. Gains at 5q35, 13q, and 20q, and losses at 2q31.1, 2q33.3, and 6q16–q22, were present in at least 50% of the cases. Gains in 20q and 13q may be important in the progression of this cancer; these regions may harbor genes with functional relevance in ONB. Valli et al., in their analysis of 11 samples from 10 patients by the same method, reported gains in chromosomes 19 and 20q13 and loss of segments of chromosomes 15 and 22 [43].
The detection of Patched1, Gli1, and Gli2 in 70, 70, and 65% of human ONB specimens suggests that the sonic hedgehog signaling pathway is involved in human ONB pathogenesis [44]. ONBs have been shown to express the olfactory marker protein and the RIC-8B genes, which are specifically expressed in mature olfactory neurons [45]. Transcription factor mammalian achaete-scute homologue (mASH-1), a member of the basic helix-loop-helix family, is essential for early development of the sympathetic nervous system, being transiently expressed in sympathetic neuroblasts during embryogenesis and contributing to differentiation and growth regulation [46]. Transcription of hASH1/ASCL1 is regulated by NOTCH and its downstream signal transducer HES1 [46, 47], and ASH1 was found to be highly expressed in various neuroendocrine tumors (gastrointestinal NEC, pheochromocytoma, esthesioneuroblastoma, medullary thyroid cancer, small cell lung cancer, and small cell prostate carcinoma) [47]. MASH1/HASH1/ASCL1 is expressed in immature olfactory neurons and is critical for their development [21, 48]. Mhawech et al. [49] showed for the first time distinct levels of expression of ASH1/ASCL1 in ONB samples, with an inverse correlation between ONB grade and ASH1/ASCL1 mRNA levels. An integrated molecular and phenotypic analysis of 52 skull base tumors confirmed expression of ASH1/ASCL1 by RT-PCR in small cell NEC, sinonasal undifferentiated carcinoma, and ONB [50]. A follow-up validation of ASH1 at the protein level in primary sinonasal tumors found ASH-1 immunoreactivity to correlate with the degree of neuroendocrine tumor differentiation [51]. High-grade neuroendocrine tumors show higher ASH1 expression, in support of previous reports indicating that expression of ASH1 appears to be restricted to immature cells [51].
Comprehensive genomic profiling of 41 relapsed or refractory ONBs revealed recurrent alterations or classes of mutations, including amplifications of tyrosine kinases encoded on chromosome 5q and mutations affecting genes in the mTOR/PI3K pathway [52]. In this cohort, the most frequently altered gene was TP53 (17%), and alterations in PIK3CA, NF1, CDKN2A, or CDKN2C were noted in 7% of samples. These results confirm prior anecdotal reports, supporting investigation into the use of tyrosine kinase inhibitors to treat these cancers. Another next-generation sequencing–based molecular profiling study on 23 ONBs found potential drivers such as CCND1 amplification, as well as potentially targetable FGFR3 amplifications [53, 54].
Treatment
One of the greatest challenges in the characterization and management of ONB is the broad biological variability of individual tumor course, from indolent disease to more aggressive and metastatic behavior. Generally, two systems are used to stratify these tumors, staging and grading, and the two are integrated to guide treatment planning. The variability in tumor course and classification has led to corresponding variability in treatment recommendations by different institutional series. Except for very early and limited disease, the best chance for cure is offered by multimodality management [19]; surgery followed by radiotherapy is considered the standard by most of the expert centers [19, 55]. In more advanced disease, however, the efficacy of neoadjuvant and adjuvant chemotherapy remains unknown and the impact of regional lymph node dissection or inclusion of lymph nodes in irradiation fields is controversial. Establishing definitively the prognostic value of staging vs. grading in ONB would allow better prediction of outcomes and more rational selection of adjuvant or neoadjuvant therapies [19, 31].
Low-grade (Hyams grades 1 and 2) and high-grade (Hyams grades 3 and 4) ONBs are reported as having clinically distinct patterns of presentation and recurrence [6]. Low-grade ONB is associated with late locoregional recurrence, whereas high-grade ONB is associated with T4 stage, frequent lymph node involvement, and leptomeningeal metastasis [33]. High-grade ONB, with its aggressive clinical course, low resectability rate, and poor survival, is a logical target for treatment escalation and evaluation for induction chemotherapy [23, 33, 35]. Pediatric ONB is a distinct clinical entity, as highlighted by Venkatramani et al. in a retrospective multicenter series [56]. In this series of 24 childhood ONBs, nodal metastasis (33%) at presentation and subsequent second malignancies (17%) were more frequent than in adults, the 5-year DFS rate was 74%, and the response to induction chemotherapy 84%. The investigators concluded that pediatric ONB is a chemosensitive tumor.
The recent MD Anderson Cancer Center experience with a small cohort of 15 patients with ONB suggests that induction chemotherapy is an acceptable alternative to up-front surgery for patients with either nonresectable, locally extensive tumor or nodal metastasis, and can be used in an organ-preservation approach [57]. Complete response to chemotherapy is associated with better patient outcomes. High Hyams grade may be a predictor of chemosensitivity [57]. Similar data were generated recently by a larger multicenter retrospective analysis of patients affected by sinonasal neuroendocrine neoplasms and ONBs [37]. In this cohort of 98 patients, ONB was the most frequent tumor type (68%); these tumors were evenly distributed by sex and most were locally advanced pT3-T4 stage at presentation. The poorly differentiated NECs represented another 22% (22/98), with slight predominance of small cell NEC (12/22) over large cell (10/22). Notably, no differences in OS or DFS were observed between these two high-grade NECs. The remainder of the tumors constituted mixed neuroendocrine/non-neuroendocrine (5.1%) and well-differentiated NEC/carcinoid (4.1%) types. The results of these studies reinforce the importance of incorporating induction chemotherapy into the treatment protocol for NECs, further suggesting that the response to induction chemotherapy might be a significant predictor both of tumor response to multimodal treatments and of overall outcomes. The rate of response to induction chemotherapy could be used to stratify “responder” patients who could be candidates for exclusive radiochemotherapy and “nonresponders” who may benefit from surgical resection followed by adjuvant radiotherapy or radiochemotherapy.
Special Issues: ONB with Divergent Differentiation and Mixed Types
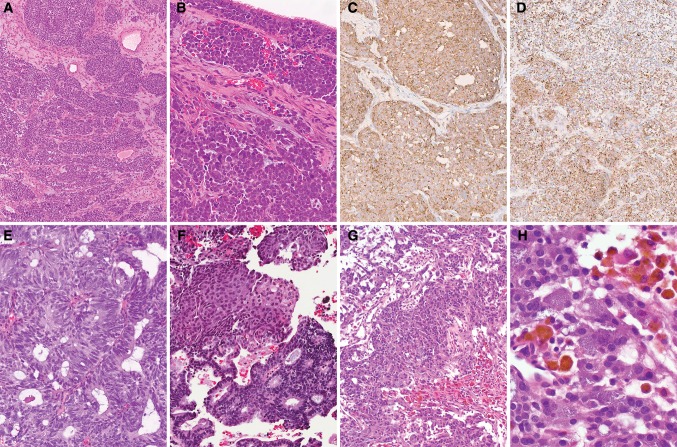
Rare ONBs exhibit divergent differentiation, in the form of melanocytic, myogenic, neural, or epithelial differentiation [20]. Some cases are described as having typical morphologic appearance but show purely immunophenotypic divergence (Figs. 2a–d, 3). Others show divergence in the form of glands, squamous morules, or rhabdomyoblastic or ganglioneuroblastic differentiation (Fig. 2e–h). This divergence may be encountered in pretreatment or post-treatment samples and can change after treatment. The significance of this divergence to prognosis and treatment is uncertain at this time, and such divergence should be accepted only when a pathognomonic feature of ONB is identified (neurofibrillary stroma or sustentacular cells) or in a recurrence/post-treatment resection of an otherwise typical ONB [20].
Fig. 2.
Olfactory neuroblastoma (ONB)-special issues. Phenotype divergence: a, b hematoxylin and eosin (H&E ×4 and ×10), ONB higher grade (Hyams 3) with submucosal lobular growth pattern, cytological atypia, pleomorphism, apoptotic bodies and necrosis, and intraepithelial pagetoid involvement (b). Diffuse immunoreactivity with anti-synaptophysin (c ×10), and significant keratin CAM5.2 positivity (perinuclear dot and focal membranous) (d ×4). Morphological divergence: e, f (H&E ×10), ONB higher-grade, with glandular (e) and squamoid (f) divergence. g, h (H&E ×4 and ×40), ONB lower grade (more conventional), with melanocytic component (dusky pigmentation, adjacent to hemosiderin laden histiocytes). This large (6.0 cm) olfactory neuroblastoma had classical imaging findings, with involvement across the cribriform plate
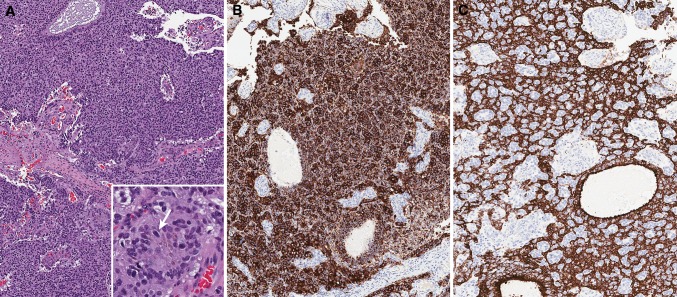
Fig. 3.
Cytokeratin 8/18 is found in the human olfactory tissue and up to one-third of ONB may also stain focally for cytokeratin. ONB involving the upper nasal septum, with a solid growth and vascularized stroma (H&E, a ×10). Inset: higher magnification (×40), arrow pointing focal melanin deposition. b Diffuse immunoreactivity with anti-synaptophysin (b, ×10) and anti-CK8/18 (c ×10)
Twelve cases of combined or collided squamous cell carcinoma (in situ or invasive) or adenocarcinoma with NEC have been reported to date, under the category of mixed neuroendocrine-non-neuroendocrine neoplasm [2, 3, 5, 13, 58]. The coexistence of neuroendocrine and non-neuroendocrine components in the same epithelial neoplasm is a recognized though rare phenomenon. The definition of these mixed types has been a matter of debate for years, as well as their inclusion in the classification of tumors of different sites [2]. The WHO classification of tumors of endocrine organs and of the digestive system proposed that mixed neoplasm are those in which each component represents at least 30% of the lesion [2]. Mixed neuroendocrine/non-neuroendocrine tumors have been interpreted either as the result of the combined growth of two different neoplastic clones, giving rise to the “collision theory,” or as the proliferation of a single precursor cell with divergent differentiation giving rise to the “common precursor theory” [2]. The molecular analysis of neoplasms in which the neuroendocrine component was represented by a high-grade NEC demonstrated a multistep progression from a common precursor lesion, showing a higher frequency of chromosomal and gene abnormalities in the neuroendocrine component than in the non-neuroendocrine component. This suggests that the molecular and morphological progression of mixed neuroendocrine/non-neuroendocrine tumors implies a pathway going from a non-neuroendocrine pathway toward a neuroendocrine cell pathway and not vice versa [2].
In a European multicenter study of sinonasal NECs, the most frequent diagnostic change was from ONB to NEC and was attributed to the expression of CK8/18 in tumor cells despite complete negativity for CKAE1/AE3, with the recommendation that CK8/18 immunohistochemistry be included in the work-up of sinonasal neoplasms with neuroendocrine differentiation [37]. The same study showed an independent prognostic role of the Ki-67 index (cutoff > 20%) [37], similar to prior work highlighting the capacity of the Ki-67 index to differentiate low-grade from high-grade ONB [36]. The utility of CK8/18 for diagnostic refinement in NEC has to be taken with caution, since CK18 labels both sustentacular cells and duct/gland cells of the olfactory mucosa [21].
Conclusion
Sinonasal neuroendocrine neoplasms are rare and heterogeneous in histophenotype and in clinical course and prognosis. Effective differentiation of these tumor types may have clinical impact, because advances in therapeutic intervention could prolong patient survival, improve quality of life, and even result in cures. Improved understanding of the biology of these tumors and their relevant markers will promote more individualized treatment approaches. Problems encountered in trials include small cohort size, heterogeneous histologic characteristics, and lack of surrogate biological endpoints for therapy effectiveness. To advance research and development in sinonasal cancer biology and clinical management, databases and banking cooperatives are needed to support comprehensive characterization of the genomic features of these tumors.
Compliance with Ethical Standards
Conflict of interest
The author declares that she has no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Bell D, Hanna EY, Weber RS, et al. Neuroendocrine neoplasms of the sinonasal region. Head Neck. 2016;38(Suppl 1):E2259–E2266. doi: 10.1002/hed.24152. [DOI] [PubMed] [Google Scholar]
- 2.La Rosa S, Sessa F, Uccella S. Mixed neuroendocrine-nonneuroendocrine neoplasms (MiNENs): unifying the concept of a heterogeneous group of neoplasms. Endocr Pathol. 2016;27(4):284–311. doi: 10.1007/s12022-016-9432-9. [DOI] [PubMed] [Google Scholar]
- 3.Uccella S, Ottini G, Facco C, et al. Neuroendocrine neoplasms of the head and neck and olfactory neuroblastoma. Diagnosis classification. Pathologica. 2017;109(1):14–30. [PubMed] [Google Scholar]
- 4.Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine tumors of the lung: current challenges and advances in the diagnosis and management of well-differentiated disease. J Thorac Oncol. 2017;12(3):425–436. doi: 10.1016/j.jtho.2016.11.2222. [DOI] [PubMed] [Google Scholar]
- 5.La Rosa S, Furlan D, Franzi F, et al. Mixed exocrine-neuroendocrine carcinoma of the nasal cavity: clinico-pathologic and molecular study of a case and review of the literature. Head Neck Pathol. 2013;7(1):76–84. doi: 10.1007/s12105-012-0379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su SY, Bell D, Hanna EY. Esthesioneuroblastoma, neuroendocrine carcinoma, and sinonasal undifferentiated carcinoma: differentiation in diagnosis and treatment. Int Arch Otorhinolaryngol. 2014;18(Suppl 2):S149–S156. doi: 10.1055/s-0034-1390014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson LDR, Bell D, Bishop JA. Neuroendocrine carcinomas. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of tumors of the head and neck. 4th ed. Tumors of the nasal cavity, paranasal sinuses and skull base. Lyon: IAC Press; 2017. pp. 21–23. [Google Scholar]
- 8.Mitchell EH, Diaz A, Yilmaz T, et al. Multimodality treatment for sinonasal neuroendocrine carcinoma. Head Neck. 2012;34(10):1372–1376. doi: 10.1002/hed.21940. [DOI] [PubMed] [Google Scholar]
- 9.Mills SE. Neuroectodermal neoplasms of the head and neck with emphasis on neuroendocrine carcinomas. Mod Pathol. 2002;15(3):264–278. doi: 10.1038/modpathol.3880522. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal DI, Barker JL, Jr, El-Naggar AK, et al. Sinonasal malignancies with neuroendocrine differentiation: patterns of failure according to histologic phenotype. Cancer. 2004;101(11):2567–2573. doi: 10.1002/cncr.20693. [DOI] [PubMed] [Google Scholar]
- 11.Bell D, Hanna EY. Sinonasal undifferentiated carcinoma: morphological heterogeneity, diagnosis, management and biological markers. Expert Rev Anticancer Ther. 2013;13(3):285–296. doi: 10.1586/era.13.1. [DOI] [PubMed] [Google Scholar]
- 12.Renner G. Small cell carcinoma of the head and neck: a review. Semin Oncol. 2007;34(1):3–14. doi: 10.1053/j.seminoncol.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 13.Babin E, Rouleau V, Vedrine PO, et al. Small cell neuroendocrine carcinoma of the nasal cavity and paranasal sinuses. J Laryngol Otol. 2006;120(4):289–297. doi: 10.1017/S0022215106000594. [DOI] [PubMed] [Google Scholar]
- 14.Fitzek MM, Thornton AF, Varvares M, et al. Neuroendocrine tumors of the sinonasal tract. Results of a prospective study incorporating chemotherapy, surgery, and combined proton-photon radiotherapy. Cancer. 2002;94(10):2623–2634. doi: 10.1002/cncr.10537. [DOI] [PubMed] [Google Scholar]
- 15.van der Laan TP, Iepsma R, Witjes MJ, van der Laan BF, Plaat BE, Halmos GB. Meta-analysis of 701 published cases of sinonasal neuroendocrine carcinoma: the importance of differentiation grade in determining treatment strategy. Oral Oncol. 2016;63:1–9. doi: 10.1016/j.oraloncology.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Berger L, Luc R, Richard D. “L’esthesioneuroepitheliome Olfactif.” Bulletin de l’Association Fran?aise pour l’étude du Cancer.vol 13. 1924. pp. 410–21.
- 17.Bell D, Franchi A, Gillison M, Thompson LDR, Wenig BM. Olfactory neuroblastoma. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of tumors of the head and neck. 4th ed. Tumors of the nasal cavity, paranasal sinuses and skull base. Lyon: IAC Press; 2017. pp. 57–59. [Google Scholar]
- 18.Holmes M, Su SY, Bell D. Ectopic primary olfactory neuroblastoma of the maxillary sinus. Ann Diagn Pathol. 2016;22:45–48. doi: 10.1016/j.anndiagpath.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Ow TJ, Bell D, Kupferman ME, Demonte F, Hanna EY. Esthesioneuroblastoma. Neurosurg Clin N Am. 2013;24(1):51–65. doi: 10.1016/j.nec.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Faragalla H, Weinreb I. Olfactory neuroblastoma: a review and update. Adv Anat Pathol. 2009;16(5):322–331. doi: 10.1097/PAP.0b013e3181b544cf. [DOI] [PubMed] [Google Scholar]
- 21.Holbrook EH, Wu E, Curry WT, Lin DT, Schwob JE. Immunohistochemical characterization of human olfactory tissue. Laryngoscope. 2011;121(8):1687–1701. doi: 10.1002/lary.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavoie J, Gasso Astorga P, Segal-Gavish H, et al. The olfactory neural epithelium as a tool in neuroscience. Trends Mol Med. 2017;23(2):100–103. doi: 10.1016/j.molmed.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bell D, Saade R, Roberts D, et al. Prognostic utility of Hyams histological grading and Kadish-Morita staging systems for esthesioneuroblastoma outcomes. Head Neck Pathol. 2015;9(1):51–59. doi: 10.1007/s12105-014-0547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jethanamest D, Morris LG, Sikora AG, Kutler DI. Esthesioneuroblastoma - A population-based analysis of survival and prognostic factors. Arch Otolaryngol Head Neck Surg. 2007;133(3):276–280. doi: 10.1001/archotol.133.3.276. [DOI] [PubMed] [Google Scholar]
- 25.Platek ME, Merzianu M, Mashtare TL, et al. Improved survival following surgery and radiation therapy for olfactory neuroblastoma: analysis of the SEER database. Radiat Oncol. 2011;6:41. doi: 10.1186/1748-717X-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadish S, Goodman M, Wang CC. Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer. 1976;37(3):1571–1576. doi: 10.1002/1097-0142(197603)37:3<1571::AID-CNCR2820370347>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 27.Morita A, Ebersold MJ, Olsen KD, Foote RL, Lewis JE, Quast LM. Esthesioneuroblastoma: prognosis and management. Neurosurgery. 1993;32(5):706–714. doi: 10.1227/00006123-199305000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Dulguerov P, Calcaterra T. Esthesioneuroblastoma: the UCLA experience 1970–1990. Laryngoscope. 1992;102(8):843–849. doi: 10.1288/00005537-199208000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 30.Hyams VJ. Olfactory neuroblastoma (case 6) In: Batsakis JG, Hyams VJ, Morales AR, editors. Special tumors of the head and neck. Chicago: ASCP Press; 1982. pp. 24–29. [Google Scholar]
- 31.Saade RE, Hanna EY, Bell D. Prognosis and biology in esthesioneuroblastoma: the emerging role of Hyams grading system. Curr Oncol Rep. 2015;17(1):423. doi: 10.1007/s11912-014-0423-z. [DOI] [PubMed] [Google Scholar]
- 32.Kaur G, Kane AJ, Sughrue ME, et al. The prognostic implications of Hyam’s subtype for patients with Kadish stage C esthesioneuroblastoma. J Clin Neurosci. 2013;20(2):281–286. doi: 10.1016/j.jocn.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malouf GG, Casiraghi O, Deutsch E, Guigay J, Temam S, Bourhis J. Low- and high-grade esthesioneuroblastomas display a distinct natural history and outcome. Eur J Cancer. 2013;49(6):1324–1334. doi: 10.1016/j.ejca.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Van Gompel JJ, Giannini C, Olsen KD, et al. Long-term outcome of esthesioneuroblastoma: Hyams grade predicts patient survival. J Neurol Surg Part B. 2012;73(5):331–336. doi: 10.1055/s-0032-1321512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandarano M, Colella R, Giansanti M, Sidoni A. Aberrant pattern of cytokeratin expression in olfactory neuroblastoma: a potential diagnostic pitfall. Head Neck Pathol. 2017;11(2):262–263. doi: 10.1007/s12105-016-0743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh L, Ranjan R, Madan R, Arava SK, Deepak RK, Singh MK. Microvessel density and Ki-67 labeling index in esthesioneuroblastoma: is there a prognostic role? Ann Diagn Pathol. 2015;19(6):391–396. doi: 10.1016/j.anndiagpath.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Turri-Zanoni M, Maragliano R, Battaglia P, et al. The clinicopathological spectrum of olfactory neuroblastoma and sinonasal neuroendocrine neoplasms: refinements in diagnostic criteria and impact of multimodal treatments on survival. Oral Oncol. 2017;74:21–29. doi: 10.1016/j.oraloncology.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Thompson LD, Seethala RR, Muller S. Ectopic sphenoid sinus pituitary adenoma (ESSPA) with normal anterior pituitary gland: a clinicopathologic and immunophenotypic study of 32 cases with a comprehensive review of the english literature. Head Neck Pathol. 2012;6(1):75–100. doi: 10.1007/s12105-012-0336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holland H, Koschny R, Krupp W, et al. Comprehensive cytogenetic characterization of an esthesioneuroblastoma. Cancer Genet Cytogenet. 2007;173(2):89–96. doi: 10.1016/j.cancergencyto.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 40.Bockmuhl U, You X, Pacyna-Gengelbach M, Arps H, Draf W, Petersen I. CGH pattern of esthesioneuroblastoma and their metastases. Brain Pathol. 2004;14(2):158–163. doi: 10.1111/j.1750-3639.2004.tb00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riazimand SH, Brieger J, Jacob R, Welkoborsky HJ, Mann WJ. Analysis of cytogenetic aberrations in esthesioneuroblastomas by comparative genomic hybridization. Cancer Genet Cytogenet. 2002;136(1):53–57. doi: 10.1016/S0165-4608(01)00659-8. [DOI] [PubMed] [Google Scholar]
- 42.Guled M, Myllykangas S, Frierson HF, Jr, Mills SE, Knuutila S, Stelow EB. Array comparative genomic hybridization analysis of olfactory neuroblastoma. Mod Pathol. 2008;21(6):770–778. doi: 10.1038/modpathol.2008.57. [DOI] [PubMed] [Google Scholar]
- 43.Valli R, De Bernardi F, Frattini A, et al. Comparative genomic hybridization on microarray (a-CGH) in olfactory neuroblastoma: analysis of ten cases and review of the literature. Genes Chromosom Cancer. 2015;54(12):771–775. doi: 10.1002/gcc.22288. [DOI] [PubMed] [Google Scholar]
- 44.Mao L, Xia YP, Zhou YN, et al. Activation of sonic hedgehog signaling pathway in olfactory neuroblastoma. Oncology. 2009;77(3–4):231–243. doi: 10.1159/000236047. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez-Kristeller DC, Gutiyama LM, Campos AH, Soares FA, Brentani H, Malnic B. Odorant receptor genes are expressed in olfactory neuroblastoma. Genet Mol Res. 2013;12(3):3479–3487. doi: 10.4238/2013.September.10.4. [DOI] [PubMed] [Google Scholar]
- 46.Ball DW, Azzoli CG, Baylin SB, et al. Identification of a human achaete-scute homolog highly expressed in neuroendocrine tumors. Proc Natl Acad Sci USA. 1993;90(12):5648–5652. doi: 10.1073/pnas.90.12.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Augustyn A, Borromeo M, Wang T, et al. ASCL1 is a lineage oncogene providing therapeutic targets for high-grade neuroendocrine lung cancers. Proc Natl Acad Sci USA. 2014;111(41):14788–14793. doi: 10.1073/pnas.1410419111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Persson P, Jogi A, Grynfeld A, Pahlman S, Axelson H. HASH-1 and E2-2 are expressed in human neuroblastoma cells and form a functional complex. Biochem Biophys Res Commun. 2000;274(1):22–31. doi: 10.1006/bbrc.2000.3090. [DOI] [PubMed] [Google Scholar]
- 49.Mhawech P, Berczy M, Assaly M, et al. Human achaete-scute homologue (hASH1) mRNA level as a diagnostic marker to distinguish esthesioneuroblastoma from poorly differentiated tumors arising in the sinonasal tract. Am J Clin Pathol. 2004;122(1):100–105. doi: 10.1309/QD0K9Q1JBH6B5GQQ. [DOI] [PubMed] [Google Scholar]
- 50.Cordes B, Williams MD, Tirado Y, et al. Molecular and phenotypic analysis of poorly differentiated sinonasal neoplasms: an integrated approach for early diagnosis and classification. Hum Pathol. 2009;40(3):283–292. doi: 10.1016/j.humpath.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taggart MW, Hanna EY, Gidley P, Weber RS, Bell D. Achaete-scute homolog 1 expression closely correlates with endocrine phenotype and degree of differentiation in sinonasal neuroendocrine tumors. Ann Diagn Pathol. 2015;19(3):154–156. doi: 10.1016/j.anndiagpath.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 52.Gay LM, Kim S, Fedorchak K, et al. Comprehensive genomic profiling of esthesioneuroblastoma reveals additional treatment options. Oncologist. 2017;22(7):834–842. doi: 10.1634/theoncologist.2016-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Czapiewski P, Kunc M, Haybaeck J. Genetic and molecular alterations in olfactory neuroblastoma: implications for pathogenesis, prognosis and treatment. Oncotarget. 2016;7(32):52584–52596. doi: 10.18632/oncotarget.9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de la LazoVega L, McHugh JB, Cani AK, et al. Comprehensive molecular profiling of olfactory neuroblastoma identifies potentially targetable FGFR3 amplifications. Mol Cancer Res. 2017;15(11):1551–1557. doi: 10.1158/1541-7786.MCR-17-0135. [DOI] [PubMed] [Google Scholar]
- 55.Ow TJ, Hanna EY, Roberts DB, et al. Optimization of long-term outcomes for patients with esthesioneuroblastoma. Head Neck. 2014;36(4):524–530. doi: 10.1002/hed.23327. [DOI] [PubMed] [Google Scholar]
- 56.Venkatramani R, Pan H, Furman WL, et al. Multimodality treatment of pediatric esthesioneuroblastoma. Pediatr Blood Cancer. 2016;63(3):465–470. doi: 10.1002/pbc.25817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su SY, Bell D, Ferrarotto R, et al. Outcomes for olfactory neuroblastoma treated with induction chemotherapy. Head Neck. 2017;39(8):1671–1679. doi: 10.1002/hed.24822. [DOI] [PubMed] [Google Scholar]
- 58.Franchi A, Rocchetta D, Palomba A, Degli Innocenti DR, Castiglione F, Spinelli G. Primary combined neuroendocrine and squamous cell carcinoma of the maxillary sinus: report of a case with immunohistochemical and molecular characterization. Head Neck Pathol. 2015;9(1):107–113. doi: 10.1007/s12105-013-0513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]