Abstract
The purpose of this article is to describe acute complications associated with adhesive cyanoacrylate deposition in the peripheral circulation and their management. Despite best efforts, n-butyl cyanoacrylate glue embolization is inherently unpredictable and complications do occur. An understanding of preparation techniques that minimize adverse event rates and the technical skillset required to manage complications are necessary for the safe and efficient use of liquid embolic agents.
Cyanoacrylate glues are liquid alkyl-2-cyanoacrylate monomers that, on contact with ionic mediums (e.g., water, blood), form flexible polymers with strong adhesive bonds to soft tissues. These liquid monomers in isolation are nonviscous, radiolucent, and rapidly polymerize. Consequently, they are combined as a two-component embolic agent with either tantalum powder or ethiodized oil. The added agent prolongs polymerization time, opacifies the liquid agent, and allows for its visualization under fluoroscopy.
With super-selective catheter delivery in a flow-directed fashion, the n-butyl-2-cyanoacrylate (n-BCA) liquid embolic system (Trufill, Cordis Neurovascular) is approved by the Food and Drug Administration (FDA) for embolization of cerebral arteriovenous malformations (AVMs) (1). Through novel implementation, interventional radiologists have proven its safe and effective off-label use for the embolization of vascular and lymphatic pathology, arterial pseudoaneurysms, endoleaks, and vascular tumors. Because injected glue cannot be precisely controlled, the result of each deposition may be unpredictable and its use requires technical knowhow.
Procedure-related adverse events documented in prospective, randomized trials and use guidelines from the Japanese Society of Interventional Radiology fit into three categories: inadvertent vascular embolization, suboptimal agent polymerization time, and catheter retention (2–4). This article presents a pictorial essay highlighting these complications as they were encountered during adhesive cyanoacrylate deposition and discusses primary prevention strategies and management options.
Potential complications
Nontarget embolization
Nontarget vascular occlusion via inadvertent embolization is the most clinically significant n-BCA complication with the potential to precipitate end-organ ischemia and bacterial sepsis (5). When glue fragmentation and embolization is detected, one should identify the size, shape, and location of fragments. After characterization of the fragment burden, management options include relocating the embolus back to the target site (Fig. 1), parking the embolus in a suitable vascular bed beneath an endograft (Fig. 2), or retracting the glue embolus through a catheter (Fig. 3). If the embolic fragment is small enough to be retrieved through a sheath, this may be achieved with the use of a loop snare, thromboembolectomy balloons, or the Solitaire stent retriever device. Loop snares have an excellent safety profile, are relatively atraumatic, and are simple to use, providing good perceptual feedback. Using any one of the many purpose-designed snares, the loop is closed around the rogue fragment (Fig. 4) by advancing the catheter, and the whole system is retrieved back into the sheath (6). If the fragment is larger than a reasonable sheath diameter and is settled in close proximity to the target lesion, relocation may be attempted. By advancing the catheter system with charge of the fragment, the embolus may be redirected back toward the target lesion. If the fragment is not stable at the newly deposited location or if a large fragment is found far from the target, it may be easier, safer, and quicker to identify a suitable vascular bed within which to safely and permanently park the glue embolus. After redirecting the adhesive fragment to a designated location, an endograft may be deployed across the embolic cast, trapping it against the vascular wall (Fig. 2). This technique eliminates the risk of fragmentation of the n-BCA embolus and downstream vessel compromise.
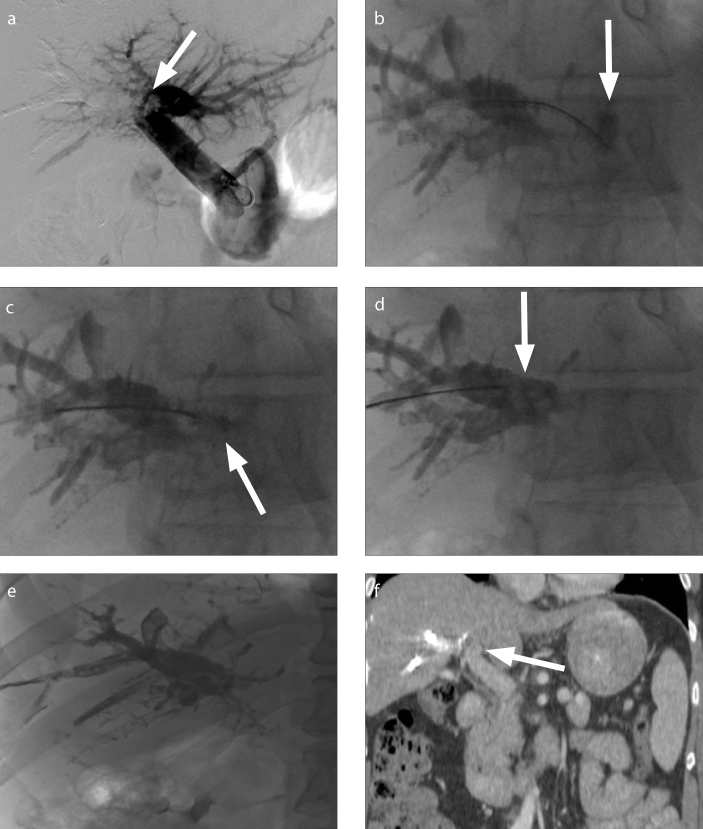
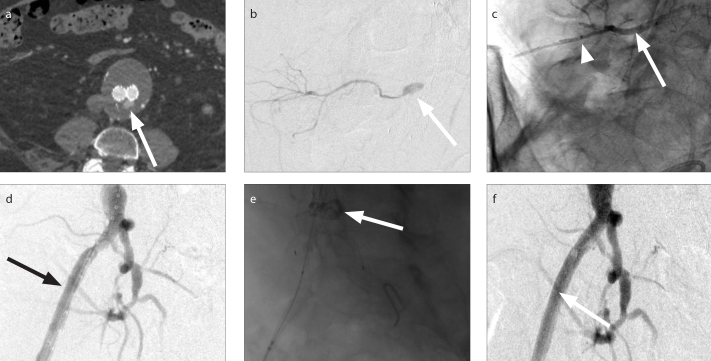
Figure 1. a–f.
A 50-year-old male with sigmoid carcinoma and right hepatic metastases. IR was consulted for right portal vein embolization prior to right hepatectomy. After successful right portal vein embolization with a 3:1 ethiodized oil: n-BCA mixture, digital subtraction portography demonstrated migration of a 13 mm glue embolus into the left portal vein (a, arrow). Initial attempts to maneuver the glue embolus using partially inflated balloons were unsuccessful. A 6 F 20 mm Amplatz GooseNeck Snare (Medtronic) was advanced into the left portal vein. The glue embolus was grasped via the proximal grab technique (b, c, arrows) and relocated to the right portal vein with no residual n-BCA in the contralateral vein (d, e). Postprocedural coronal contrast-enhanced CT showed no thrombus or n-BCA within the left portal vein (f, arrow).
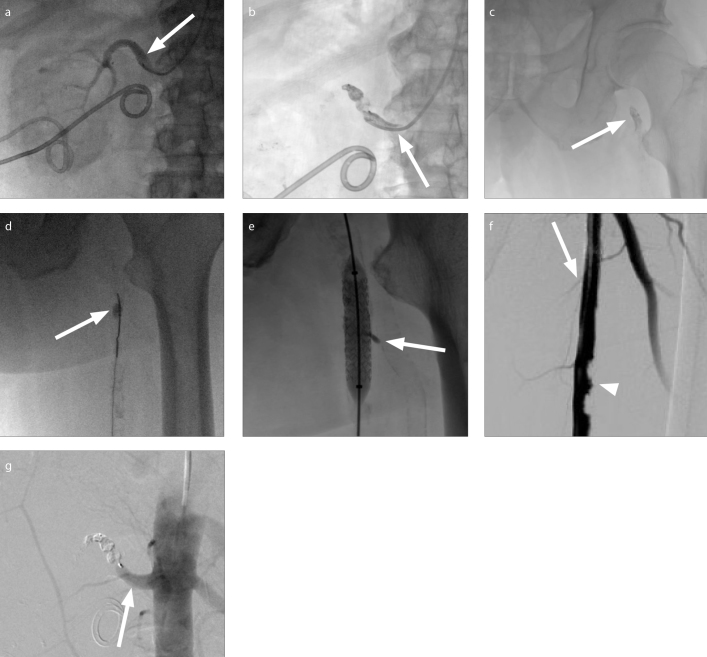
Figure 2. a–g.
A 72-year-old male with a history of left ureteroenteric fistula complicated by recurrent infectious nephritis was referred to IR for renal artery and ureteral embolization. The patient was placed prone on the table and a radial approach was used to facilitate both renal artery and ureteral embolization in the same procedure. Following successful intrarenal vasculature embolization with alcohol, a 7 mm Amplatzer Vascular Plug 4 (St. Jude Medical) was deployed in the left main renal artery. Spot fluoroscopy demonstrated continued perfusion past the plug (a, arrow). Reinforcement of the vessel occlusion via injection of additional 2:1 ethiodized oil:n-BCA around the plug was attempted. Reflux of glue around the microcatheter tip resulted in catheter occlusion with an attached pedicle of polymerized glue (b, arrow). An attempt to retract the cast resulted in mobilization and embolization of a glue embolus (c, arrow) to the origin of the right superficial femoral artery (SFA) as depicted on spot fluoroscopy (c). Via right popliteal access, an Amplatz gooseneck snare (Medtronic) was used to redirect the glue embolus (d) further into the proximal-SFA (d, arrow) to ensure the profunda femoris was not covered. An iCAST balloon expandable covered stent (Atrium Medical) was deployed across the embolus, trapping it against the vessel wall. A small amount of glue extruded into a small branch vessel (e, arrow). Completion arteriograms demonstrated patency of the right SFA (f, arrow) with calcified plaque (f, arrowhead) and stasis of the proximal left renal artery (g, arrow).
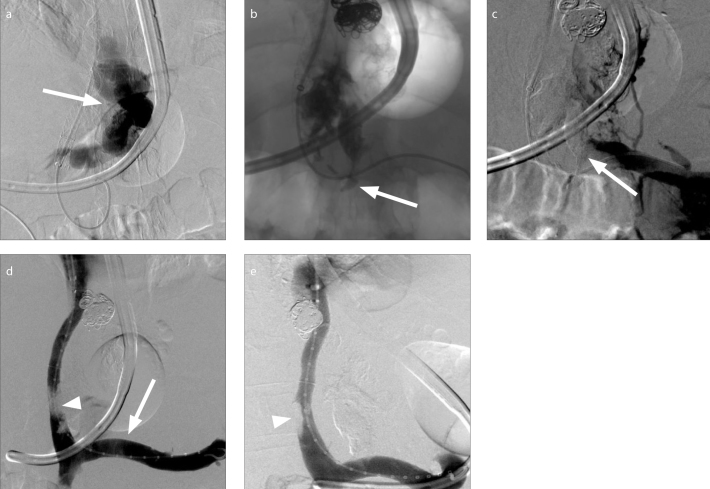
Figure 3. a–e.
A 56-year-old male with cirrhosis complicated by ascites and hemorrhaging gastroesophageal varices was referred to IR for transjugular intrahepatic portosystemic shunt (TIPS) creation and variceal embolization. Following successful TIPS creation, attempted embolization of a large, tortuous type-1 gastroesophageal varix arising from the coronary vein (a, arrow) was complicated by variceal rupture. A 2:1 n-BCA:ethiodized oil mixture was injected using the continuous column technique at the rupture site. Glue migrated into the origin of the coronary vein and splenic vein (b, arrow). Venogram (c) demonstrated glue within the coronary vein and the distal splenic vein, partially occluding the lumen of the latter vessel (c, arrow). A loop snare was used for transvenous retrieval of a section of the protruding polymer as depicted on spot fluoroscopy. Given persistent near-occlusion of the splenic vein at its junction with the superior mesenteric vein, a 13 mm Viabahn endoprosthesis (Gore Medical) was deployed (d, arrow) across the origin of the coronary vein from which glue was protruding. Completion venograms demonstrated a patent splenic vein (d, e) and TIPS without extravasation. Apparent filling defect was created by superimposed glue (d, arrowhead) and right portal thrombus (e, arrowhead), present on preoperative imaging.
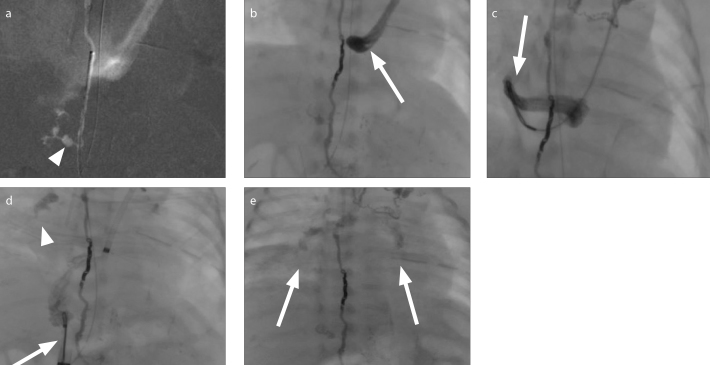
Figure 4. a–e.
A 6-week-old female with a high-output postsurgical chylothorax following congenital diaphragmatic hernia repair was referred to IR for thoracic duct leak characterization and interventional management. Following groin lymphangiography, the thoracic duct was cannulated via the cisterna chyli. Digital subtracted contrast injection through a microcatheter demonstrated the chyle leak from the caudal aspect of the duct (a, arrow). The thoracic duct was occluded with coils and a 2:1 ethiodized oil:n-BCA glue mixture, the glue forming a cast within the coils. On continued surveillance, glue migrated towards the coronary sinus (b, arrow). Via access in the left upper extremity and right groin, a 10 mm loop snare was used to successfully remove polymerized fragments as depicted on fluoroscopy (c, d, arrows). Following removal of larger polymer fragments from the right atrium, glue was noted to have migrated into the bilateral pulmonary arteries (d, arrowhead; e, arrows). Due to refractory hypoxia this was removed by surgical pulmonary embolectomy.
Delayed polymerization causing venous migration
Embolic agent migration past the target lesion due to delayed polymerization is a well-described and serious complication during AVM embolization where blockage of venous outflow increases hemorrhage risk (7). By similar hemodynamic tenets, occlusion of portosystemic collaterals in cirrhotic patients may be clinically significant. Partial or complete occlusion of the portal (Fig. 1) or splenic (Fig. 3) veins during gastroesophageal varix embolization may preclude liver transplantation and should be addressed prophylactically. Placement of venous stents may be used to ensure vessel patency (Fig. 3).
Several factors impact the depth of glue penetration including the direction and speed of blood flow past the catheter tip, injection technique, and cyanoacrylate dilution (ratio of n-BCA to ethiodized oil). Ethiodized oil is mixed with n-BCA in various dilution ratios depending on the application to control polymerization rate. A glue mixture with a higher proportion of n-BCA polymerizes more quickly on contact with ionic mediums and would be preferred if over-penetration were more concerning than complete lesion occlusion procedurally.
Premature polymerization
Injected n-BCA may polymerize prematurely blocking the microcatheter or refluxing around the catheter tip, potentially leading to retention to either the vascular wall or deployed devices, e.g., vascular plugs (Fig. 2), endografts (Fig. 5), or coils. These complications generally do not have untoward clinical consequences, but should nonetheless be managed with care.
Figure 5. a–f.
A 77-year-old male with a history of endovascular abdominal aortic aneurysm (AAA) repair was referred to IR for embolization of a type II endoleak in the setting of an enlarging aneurysm sac. Preprocedural CT (a) demonstrated a AAA with an infrarenal aorto-bi-iliac endograft in place with suspected sac filling (a, arrow) via a posterior lumbar branch vessel. Bilateral femoral access was gained and digital subtraction images confirmed CT findings with contrast injection into a right iliolumbar artery arising from the right internal iliac artery filling the L4 lumbar artery via a collateral network (b, arrow). Following successful coil and glue embolization (arrow), the tip of the anchoring catheter was found to be trapped in the deposited polymer. During catheter retraction, mechanical shearing of the microcatheter resulted in tip retention (c, arrowhead). A pigtail catheter was placed through the right femoral artery access (d, arrow) and digital subtraction arteriography demonstrated no thrombus within the major branch vessels. The glue cast (e, arrow) and trapped microcatheter were again identified and overlapping wall stents were deployed from the right common to external iliac artery across the retained foreign body effectively trapping it against the vessel wall. Completion arteriography (f) showed patent right internal and external iliac arteries without obstruction, dissection, or extravasation.
Premature polymerization causing microcatheter blockage
Premature polymerization of adhesives in the feeding pedicle is most often the result of suboptimal component mixture, delivery system use, or volume and speed of injection. Thus, avoidance is primarily a matter of preventative planning. Prior to injection of the n-BCA mixture, the microcatheter should be flushed with nonheparinized 5% dextrose (D5). This nonionic solution is used in place of a saline flush to prevent polymerization of the mixture on contact with residual blood or saline in the catheter tip (8). Injecting dextrose also creates a local nonionic environment at the catheter tip enabling more distal n-BCA progression (8). Regarding injection technique, polymerization may be delayed using the flood technique. In this approach, the inner diameter of the guide catheter is sufficiently larger than the outer diameter of the microcatheter to allow for a positive-pressure continuous flush with dextrose around the microcatheter. This will create a local nonionic environment at the catheter tip, slowing glue polymerization and allowing for deeper penetration of the mixture and more distal embolization. This technique is particularly useful when embolizing around a placed device or into a bleeding artery that is difficult to reach using the current generation of microcatheters. Once the injection is complete, the microcatheter is aspirated and withdrawn rapidly.
Should the catheter become occluded, the microcatheter should be replaced. Attempts to clear the lumen of the catheter could result in uncontrolled expulsion of polymerized glue fragment or the release of small amounts of liquid n-BCA from the catheter tip and possible nontarget embolization.
Premature polymerization causing catheter retention
Catheter entrapment within polymerized glue is a long-acknowledged risk associated with the use of adhesive cyanoacrylates. As primary prevention, care should be taken to remove redundant loops in the catheter to ensure the availability of quick and controlled pullback as needed. Permanent catheter fixation is less likely to occur with the use of hydrophilic-coated microcatheters given weaker n-BCA bonding and reduced catheter friction (9). Attention should be paid to cyanoacrylate ratio and the prevention of premature polymerization as described above when injecting liquid adhesives around placed devices.
If polymerized glue refluxes around the microcatheter tip, the tip should be pulled back sufficiently to free it prior to polymerization. Should the catheter adhere to surrounding structures, avoid forceful movement of the catheter to limit soft tissue trauma. Should the microcatheter tip fracture (Fig. 5), it may be left in place provided flow to critical structures is not compromised. Even complete microcatheters may safely remain in the vascular lumen attached to the adherent tip with the proximal end cut and left in the subcutaneous tissue (3). Fear of vessel thrombosis surrounding the retained foreign body, while perhaps an indication for catheter removal in the cerebral circulation (10), is less clinically significant in the periphery. Here, removal depends on characteristics of the catheter location and structures involved.
Conclusion
The behavior of liquid embolic agents during deposition in a biological system can be unpredictable. Understanding strategies to prevent and manage complications are required for their safe clinical use.
Main points.
The n-butyl cyanoacrylate liquid embolic system is approved by the Food and Drug Administration for embolization of cerebral arteriovenous malformations, but is also used in the peripheral circulation for embolization of vascular and lymphatic pathology, arterial pseudoaneurysms, endoleaks, and vascular tumors.
Despite best efforts, n-butyl cyanoacrylate glue deposition is inherently unpredictable and complications including nontarget embolization, venous migration, microcatheter blockage, and catheter retention do occur.
An understanding of techniques used to minimize adverse events and manage complications, including embolus relocation and stent deployment, are requisite for using liquid embolic agents safely and efficiently.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Mattamal GJ. US FDA perspective on the regulations of medical-grade polymers: cyanoacrylate polymer medical device tissue adhesives. Expert Rev Med Devices. 2008;5:41–49. doi: 10.1586/17434440.5.1.41. https://doi.org/10.1586/17434440.5.1.41. [DOI] [PubMed] [Google Scholar]
- 2.Loh Y, Duckwiler GR Onyx Trial I. A prospective, multicenter, randomized trial of the Onyx liquid embolic system and N-butyl cyanoacrylate embolization of cerebral arteriovenous malformations. J Neurosurg. 2010;113:733–741. doi: 10.3171/2010.3.JNS09370. https://doi.org/10.3171/2010.3.JNS09370. [DOI] [PubMed] [Google Scholar]
- 3.n-BCA Trail Investigators. N-butyl cyanoacrylate embolization of cerebral arteriovenous malformations: results of a prospective, randomized, multi-center trial. AJNR Am J Neuroradiol. 2002;23:748–755. [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi Y, Morishita H, Sato Y, et al. Guidelines for the use of NBCA in vascular embolization devised by the Committee of Practice Guidelines of the Japanese Society of Interventional Radiology (CGJSIR), 2012 edition. Jpn J Radiol. 2014;32:500–517. doi: 10.1007/s11604-014-0328-7. https://doi.org/10.1007/s11604-014-0328-7. [DOI] [PubMed] [Google Scholar]
- 5.Liao SC, Ko CW, Yeh HZ, Chang CS, Yang SS, Chen GH. Successful treatment of persistent bacteremia after endoscopic injection of N-butyl-2-cyanoacrylate for gastric varices bleeding. Endoscopy. 2007;39(Suppl 1):E176–177. doi: 10.1055/s-2007-966556. https://doi.org/10.1055/s-2007-966556. [DOI] [PubMed] [Google Scholar]
- 6.Woodhouse JB, Uberoi R. Techniques for intravascular foreign body retrieval. Cardiovasc Intervent Radiol. 2013;36:888–897. doi: 10.1007/s00270-012-0488-8. https://doi.org/10.1007/s00270-012-0488-8. [DOI] [PubMed] [Google Scholar]
- 7.Debrun GM, Aletich V, Ausman JI, Charbel F, Dujovny M. Embolization of the nidus of brain arteriovenous malformations with n-butyl cyanoacrylate. Neurosurgery. 1997;40:112–121. https://doi.org/10.1097/00006123-199701000-00026. [PubMed] [Google Scholar]
- 8.Moore C, Murphy K, Gailloud P. Improved distal distribution of n-butyl cyanoacrylate glue by simultaneous injection of dextrose 5% through the guiding catheter: technical note. Neuroradiology. 2006;48:327–332. doi: 10.1007/s00234-006-0059-2. https://doi.org/10.1007/s00234-006-0059-2. [DOI] [PubMed] [Google Scholar]
- 9.Mathis JM, Evans AJ, DeNardo AJ, et al. Hydrophilic coatings diminish adhesion of glue to catheter: an in vitro simulation of NBCA embolization. AJNR Am J Neuroradiol. 1997;18:1087–1091. [PMC free article] [PubMed] [Google Scholar]
- 10.Debrun GM, Aletich VA, Shownkeen H, Ausman J. Glued catheters during embolisation of brain AVMs with acrylic glue. Interv Neuroradiol. 1997;3:13–19. doi: 10.1177/159101999700300102. https://doi.org/10.1177/159101999700300102. [DOI] [PubMed] [Google Scholar]