Abstract
Motor neurone disease (MND) is an adult-onset neurodegenerative disease which leads inexorably via weakness of limb, bulbar and respiratory muscles to death from respiratory failure three to five years later. Most MND is sporadic but approximately 10% is inherited. In exciting recent breakthroughs two new MND genes have been identified. Diagnosis is clinical and sometimes difficult – treatable mimics must be excluded before the diagnosis is ascribed. Riluzole prolongs life by only three to four months and is only available for the amyotrophic lateral sclerosis (ALS) form of MND. Management therefore properly focuses on symptom relief and the preservation of independence and quality of life. Malnutrition is a poor prognostic factor. In appropriate patients enteral feeding is recommended although its use has yet to be shown to improve survival. In ALS patients with respiratory failure and good or only moderately impaired bulbar function non-invasive positive pressure ventilation prolongs life and improves quality of life.
Key Words: amyotrophic lateral sclerosis, differential diagnosis, motor neurone disease, primary lateral sclerosis, progressive bulbar palsy, progressive muscular atrophy, riluzole
Motor neurone disease (MND) is an adult-onset neurodegenerative disorder that leads to progressive weakness of limb, bulbar and respiratory muscles. Death typically occurs three to five years later, most often due to respiratory failure. While a fatal outcome unfortunately remains inevitable, the unhelpful nihilism often encountered by patients in the past has now been replaced by a more positive approach to symptomatic management and the support of patients and their families.
Epidemiology and risk factors
MND is a common neurodegenerative disorder with an incidence of 1–2/100,000 and a prevalence of approximately 6/100,000. Onset is typically in late middle age. Increasing age, male sex (1.6:1) and the inheritance of a genetic susceptibility are the only proven risk factors although there is ongoing research interest in others including physical exercise. Approximately 90% of MND occurs sporadically. The remainder is inherited.
Familial motor neurone disease
Most familial MND is autosomal dominant. Mutations to a ubiquitous anti-oxidant enzyme, Cu/Zn superoxide dismutase (SOD1), are responsible for approximately 20% of inherited MND.1 How these mutations cause motor neuronal death is still unclear. The genetic basis of some uncommon MND variants has since been clarified and only in the last year mutations to two further genes, TARDBP and fused in sarcoma or FUS, were shown to cause classical ALS.2–5 Both gene products are ribonucleic acid (RNA)/DNA binding proteins with likely roles in RNA processing.6 Mutations to these two genes together account for a further ∼10% of familial MND. Their suspected common function sheds an entirely new light on the pathogenesis of the disease and a redirection of much MND research is likely to result.
Classification of motor neurone disease
In the UK the term MND collectively refers to three forms of the disease; amyotrophic lateral sclerosis (ALS), progressive muscular atrophy (PMA) and primary lateral sclerosis (PLS). ALS is by far the most common and involves loss of upper and lower motor neurones (UMN and LMN) producing a characteristic mixed picture. PMA is a disease predominantly of the LMN although late in disease some UMN features may develop. PLS is the rarest form of MND. It predominantly involves UMNs and is associated with the best prognosis. Some LMN features may eventually develop. Interestingly, motor neurons serving the extra-ocular muscles and pelvic floor are spared in all forms of MND. Uncommonly MND may be associated with extra-motor features. Ten per cent of classical ALS is associated with a fronto-temporal dementia while the rare Guamanian variant has additional extrapyramidal features and dementia. Progressive bulbar palsy is a form of ALS predominantly affecting the bulbar region while LMN disease predominantly affecting upper (flail arm) and lower limbs (flail leg) is also recognised.7 Monomelic forms of MND are prevalent in some geographical areas.
Diagnosis
In the absence of a definitive diagnostic test, the diagnosis of MND is made clinically. Electrophysiology lends support while other investigations are tailored to exclude MND mimics. Central to the diagnosis of ALS are progressive mixed UMN and LMN symptoms and signs. Sensory signs are not a feature of any form of MND, nor are persistent pain or sphincter disturbance. If any of these are prominent, MND is unlikely. Weakness out of proportion to wasting or an atypical pattern of weakness should trigger consideration of alternative diagnoses. Failure to progress as expected or the development of atypical features should prompt reconsideration of the diagnosis. Implicit in this is the assumption that MND patients will be regularly followed up.
Diagnostic criteria
Formal diagnostic criteria for ALS were first agreed in 1994. As their purpose was to standardise diagnosis of patients enrolled in clinical trials they were stringent by intent. A simplified version of the updated El Escorial revised criteria is given in Box 1.8
Box 1. Requirements for the diagnosis of amyotrophic lateral sclerosis (ALS) according to the revised EL Escorial criteria.
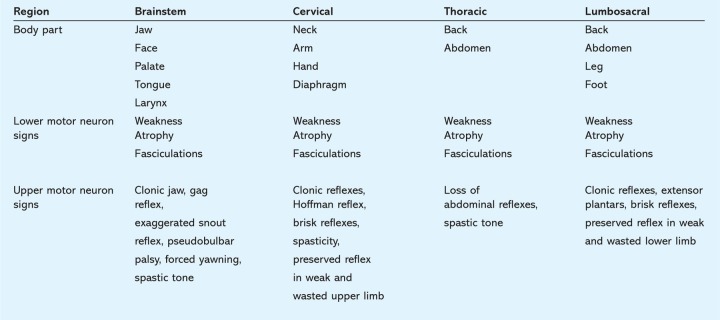
The criteria further specify degrees of diagnostic certainty dependent upon assessment of the extent of disease. To this end four central nervous system regions are defined – brainstem, cervical, thoracic and lumbosacral. The signs to be sought in each region are given in Table 1. Clinically definite ALS is defined on clinical evidence alone by the presence of UMN, as well as LMN signs, in three regions. Clinically probable ALS is defined on clinical evidence alone by UMN and LMN signs in at least two regions with some UMN signs above the LMN signs. The further refinements to this schema will not be discussed further here.
Table 1.
Upper and lower motor neuron signs in the four central nervous system regions simplified from Reference 8.
Pragmatism in diagnosis
By the time patients meet criteria for clinically definite ALS they are, by definition, at an advanced stage of disease. In everyday practice, however, a working diagnosis is often needed earlier. Diagnosis allows patients to plan for what lies ahead but more importantly allows them to access riluzole, the only available disease-modifying therapy. Under National Institute for Health and Clinical Excellence guidelines riluzole can only be prescribed for patients diagnosed by a specialist with the ALS form of MND.9 Logically the earlier neuroprotective treatment starts the better so a delicate balance must be struck between diagnostic certainty and early diagnosis. Achieving this can be difficult – diagnosis is best left to a neurologist with a specialist interest. If there is inadequate evidence to justify even a working diagnosis of MND the patient must be followed and developments awaited.
Differential diagnosis and misdiagnosis
One consequence of a clinical diagnosis and the need to make this diagnosis early is a significant rate of misdiagnosis. In a study of the Scottish ALS registry, 8% of patients initially categorised as clinically probable or clinically definite ALS were eventually given an alternative diagnosis, half of which were treatable.10 A similar study of the Irish ALS registry reported a misdiagnosis rate of 7.3%.11 A recent study of surgery performed on 260 confirmed ALS patients found that in the previous five years 13% had undergone inappropriate surgery for what, with hindsight, were the earliest symptoms of ALS.12
MND has a broad differential which is at its broadest early on in the course of disease (Table 2). There are more PMA mimics than there are ALS or PLS mimics. When Visser re-examined a cohort of prevalent PMA cases, 19% had another disorder of which 58% were treatable.13 It is clear from this that patients presenting with a pure LMN syndrome need a particularly careful work-up.
Table 2.
The clinically significant mimics of motor neurone disease (MND). ∗ = potentially treatable; EMG = electromyography; LMN = lower motor neuron; NCS = nerve conduction studies; UMN = upper motor neuron.
Investigation
Investigations are important adjuncts to the clinical diagnosis of MND. Properly used they can provide supportive evidence of the clinical findings and help delineate the extent of disease. Investigation is also important to identify benign or treatable MND mimics.
Electrophysiology
All suspected MND patients should undergo electrophysiological testing by an experienced neurophysiologist. Electrophysiology allows identification of LMN features of MND in both clinically affected and as yet clinically silent regions. In this way the disease may be shown to be more widespread than is evident clinically and an earlier working diagnosis made. The Awaji group recently recommended that electrophysiological evidence of LMN involvement carry the same weight as clinical evidence and that fasciculation potentials be accepted as evidence of active denervation. These changes would allow earlier diagnosis of ALS but are not yet widely accepted.14 Typical electromyographic features of MND include evidence of active denervation (positive sharp waves, fibrillation potentials, fasciculation potentials) and chronic denervation evidenced by large motor unit potentials. In pure LMN syndromes a careful search for evidence of alternative diagnoses, such as conduction block, demyelination, decrement and jitter, must be made. Where the extent of disease is inadequate to make a confident diagnosis interval studies should be performed. Conduction block can be particularly difficult to demonstrate and repeat studies are often required.
Imaging
Not all suspected MND patients require imaging but it is important in some presentations. Magnetic resonance imaging (MRI) of the spinal cord should be done in all limb-onset ALS patients without bulbar involvement as mixed cord and root compression is responsible for some 30% of ALS misdiagnoses. When symptoms and signs remain isolated to the bulbar region MRI can be useful in identifying infiltrative lesions of tongue and pharynx (Fig 1).
Fig 1.

A 60-year-old patient presented with progressive dysarthria and difficulty manipulating food in his mouth but normal swallowing. On examination he had a small, fasciculating, slowly moving tongue and no limb involvement. The lesion (arrowed above) was identified as a squamous carcinoma of the tongue.
Other tests
Our routine blood testing includes full blood count, erythrocyte sedimentation rate, C-reactive protein, urea and electrolytes, calcium, liver function tests (LFTs), glucose, Venereal Disease Research Laboratory, serum protein electrophoresis, thyroid function tests, creatine kinase and anti-GM1 (raised in multi-focal motor neuropathy with conduction block) and anti-MAG antibodies (raised in anti-MAG polyneuropathy). Cerebral spinal fluid helps exclude inflammatory mimics. Further testing is guided by the patient's presentation and might include acetylcholine receptor antibody testing and SOD1 mutation screening if familial disease is suspected. Rarer mimics must be considered in young patients who are tested for heavy metals, serum hexoseaminidase A and B and borrelia. Genetic testing for Kennedy's disease and spinal muscular atrophy may also be considered. Baseline respiratory function tests are performed on all patients.
Management of motor neurone disease
Multidisciplinary teams
MND is best managed within specialist centres offering multidisciplinary care. Patients managed in this way not only enjoy better care but survive longer while team members develop expertise which benefits professionals and patients alike.15 Teams are typically led by an interested neurologist and may include a general practitioner, nurse specialist, occupational therapist, physiotherapist, speech and language therapist or a dietitian. Most teams have access to a gastroenterologist expert in placing gastrostomy tubes in MND patients and a respiratory physician and technician to support home non-invasive ventilation. Close links are forged with local social workers and hospices. Care organised this way means patients have more of their needs met at one visit, important in advanced disease when hospital visits can become onerous.
Disease-modifying therapy
Riluzole is the only disease-modifying therapy licensed for use in MND. Chemically a benzothiazole developed as an anti-epileptic drug, riluzole was trialled in MND because it reduces presynaptic glutamate release. Other neuroprotective effects are likely to contribute. Two well-designed clinical trials support its use in ALS but as only ALS patients participated, its effects in PMA or PLS are unknown.16,17 On average riluzole extends the life of ALS patients by three to four months although subsets of patients may benefit more. Importantly riluzole does not prolong the pre-terminal phase of the disease.
Practicalities of riluzole use
Riluzole is given at 50 mg bd and is generally well tolerated. It may cause weariness, nausea and dizziness but these effects commonly wear off. Riluzole cannot be given in pregnancy or breastfeeding. Baseline LFTs should be checked. Monthly LFTs are recommended for the first three months then three-monthly thereafter. Riluzole should be withdrawn if LFTs increase beyond 5× normal. Increases over 3× normal can usually be managed by dose reduction or withdrawal followed by cautious reintroduction. Riluzole may rarely cause bone marrow suppression so patients should know to report febrile illnesses early.
Symptomatic therapy
In the absence of a cure, MND management must focus on symptom relief and the preservation of independence and quality of life. Nutritional and respiratory management of MND patients impacts upon prognosis and is considered separately. Table 3 summarises the palliative strategies available for other problems commonly encountered by MND patients.
Table 3.
Palliative measures employed in symptomatic care. NIPPV = non-invasive positive pressure ventilation.
Nutritional management
Oral intake must be actively managed as poor nutritional status adversely affects prognosis.18 Patients should be weighed at each clinic visit. MND patients without bulbar involvement may fail to eat enough for several reasons. Food must be shopped for, cooked and transferred from plate to mouth before bulbar function even comes into play. Patients with limb involvement may find these essential preliminaries difficult, especially if they live alone. Involvement of social services can prevent avoidable early weight loss. Mobile arm supports can prolong independent eating in those with arm weakness, as can modified cutlery. MND patients may suffer poor appetite independent of bulbar problems. Anorexia may be the first sign of respiratory insufficiency and although low mood is less prevalent than might be expected, this too may cause anorexia.
Most MND patients eventually develop progressive bulbar problems. Initially advice about optimal positioning and airway protective swallowing techniques suffices. Occasional coughing and spluttering is acceptable provided aspiration pneumonia does not result. As swallowing difficulties progress the texture of food attempted should be modified, fluids thickened and calorie content increased. If despite these measures weight loss continues, high calorie supplements are added between meals. Discussion about gastrostomy tube placement should be initiated well in advance. Weight loss of 10–15% warrants its consideration in suitable patients. Some patients will not want the procedure while others with weak hands may not be able to manage the tube. Few MND specialists would recommend enteral feeding in patients suffering associated fronto-temporal dementia. Gastrostomy does not preclude the enjoyment of small amounts of food by mouth but the ability to take most of their calories, fluids and medication by tube often comes as a relief to patients when mealtimes have become a struggle. Gastrostomy tubes are placed via endoscopy (PEG) or using ultrasound guidance (radiologically inserted gastrostomy (RIG)) (Fig 2). Tube placement is higher risk in patients with respiratory insufficiency. Ideally tubes should be placed before the patient's forced vital capacity (FVC) falls below 50% expected otherwise RIG placement is preferable. Skilled gastroenterologists may be happy to insert PEG tubes in more compromised patients – peri-procedure non-invasive positive pressure ventilation (NIPPV) can help. Dehydration or recurrent aspiration pneumonia are other indications for gastrostomy. Although there is no randomised controlled trial of enteral feeding in ALS, a Cochrane review of what evidence there is came to the view that it may convey a survival advantage.19 Re-feeding syndrome may occur in patients malnourished prior to gastrostomy.
Fig 2.

A percutaneous endoscopic gastrostomy tube in situ.
Respiratory management
A high index of suspicion is required to identify early respiratory insufficiency in MND as onset is typically insidious. Those rare patients in whom respiratory problems present first very often only reach a neurologist after referral elsewhere, delaying diagnosis. The symptoms and signs of respiratory involvement in MND are given in Box 2.
Box 2. Symptoms and signs of respiratory insufficiency in amyotrophic lateral sclerosis. FVC = forced vital capacity.
Baseline lung function tests should be performed at diagnosis and an FVC recorded at each subsequent clinic visit. Aspiration pneumonia must be identified and treated early. A cough assist machine can help with difficulty coughing up sputum. Patients generally become symptomatic of respiratory muscle weakness once their FVC reaches 50% expected. There is now good evidence that NIPPV confers a survival benefit and improves quality of life in patients with normal or moderately impaired bulbar function.20 Patients with severe bulbar dysfunction do not benefit but in practice such patients rarely tolerate NIPPV.
Discussion about NIPPV should be initiated well ahead of time. Patients who live alone and have significant upper limb weakness will not be able to readjust their masks. Patients with advanced disease may not be well served by an intervention that may prolong life when quality is already poor. Those with little or no bulbar involvement but early respiratory compromise are ideal candidates. This group tolerate NIPPV well, typically experiencing a dramatic improvement in wellbeing. Initially NIPPV is often only needed at night with carry over benefits extending well into the next day. As respiratory compromise worsens NIPPV is needed for more and more of the day. Ventilators capable of modulating inspiratory pressures to deliver a fixed tidal volume deal best with changes in respiratory muscle strength day to day and over time. Ventilator entrapment is rarely a problem with NIPPV in MND patients.
References
- 1.Rosen DR, Siddique T, Patterson D, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 2.Valdmanis PN, Rouleau GA. Genetics of familial amyotrophic lateral sclerosis. Neurology. 2008;70:144–52. doi: 10.1212/01.wnl.0000296811.19811.db. doi: 10.1212/01.wnl.0000296811.19811.db. [DOI] [PubMed] [Google Scholar]
- 3.Sreedharan J, Blair IP, Tripathi VB, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–72. doi: 10.1126/science.1154584. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vance C, Rogelj B, Hortobagyi T, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–11. doi: 10.1126/science.1165942. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwiatkowski TJ, Jr, Bosco DA, Leclerc AL, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–8. doi: 10.1126/science.1166066. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 6.Sleegers K, Van Broeckhoven C. Motor-neuron disease: Rogue gene in the family. Nature. 2009;458:415–7. doi: 10.1038/458415a. doi: 10.1038/458415a. [DOI] [PubMed] [Google Scholar]
- 7.Wijesekera LC, Mathers S, Talman P, et al. Natural history and clinical features of the flail arm and flail leg ALS variants. Neurology. 2009;72:1087–94. doi: 10.1212/01.wnl.0000345041.83406.a2. doi: 10.1212/01.wnl.0000345041.83406.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9. doi: 10.1080/146608200300079536. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Clinical Excellence . Guidance on the use of riluzole (Rilutek) for the treatment of motor neurone disease. London: NICE; 2001. 20. [Google Scholar]
- 10.Davenport RJ, Swingler RJ, Chancellor AM, Warlow CP. Avoiding false positive diagnoses of motor neuron disease: lessons from the Scottish Motor Neuron Disease Register. J Neurol Neurosurg Psychiatry. 1996;60:147–51. doi: 10.1136/jnnp.60.2.147. doi: 10.1136/jnnp.60.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Traynor BJ, Codd MB, Corr B, et al. Amyotrophic lateral sclerosis mimic syndromes: a population-based study. Arch Neurol. 2000;57:109–13. doi: 10.1001/archneur.57.1.109. doi: 10.1001/archneur.57.1.109. [DOI] [PubMed] [Google Scholar]
- 12.Srinivasan J, Scala S, Jones HR, et al. Inappropriate surgeries resulting from misdiagnosis of early amyotrophic lateral sclerosis. Muscle Nerve. 2006;34:359–60. doi: 10.1002/mus.20555. doi: 10.1002/mus.20555. [DOI] [PubMed] [Google Scholar]
- 13.Visser J, van den Berg-Vos RM, Franssen H, et al. Mimic syndromes in sporadic cases of progressive spinal muscular atrophy. Neurology. 2002;58:1593–6. doi: 10.1212/WNL.58.11.1593. doi: 10.1212/WNL.58.11.1593. [DOI] [PubMed] [Google Scholar]
- 14.Carvalho MD, Swash M. Awaji diagnostic algorithm increases sensitivity of El Escorial criteria for ALS diagnosis. Amyotroph Lateral Scler. 2009;10:53–7. doi: 10.1080/17482960802521126. doi: 10.1080/17482960802521126. [DOI] [PubMed] [Google Scholar]
- 15.Traynor BJ, Alexander M, Corr B, et al. Effect of a multidisciplinary amyotrophic lateral sclerosis (ALS) clinic on ALS survival: a population based study, 1996–2000. J Neurol Neurosurg Psychiatry. 2003;74:1258–61. doi: 10.1136/jnnp.74.9.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330:585–91. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 17.Lacomblez L, Bensimon G, Leigh PN, et al. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347:1425–31. doi: 10.1016/s0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- 18.Desport JC, Preux PM, Truong TC, et al. Nutritional status is a prognostic factor for survival in ALS patients. Neurology. 1999;53:1059–63. doi: 10.1212/WNL.53.5.1059. doi: 10.1212/WNL.53.5.1059. [DOI] [PubMed] [Google Scholar]
- 19.Langmore SE, Kasarskis EJ, Manca ML, Olney RK. Enteral tube feeding for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2006;4:CD004030. doi: 10.1002/14651858.CD004030.pub2. doi: 10.1002/14651858.CD004030.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Bourke SC, Tomlinson M, Williams TL, et al. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol. 2006;5:140–7. doi: 10.1016/S1474-4422(05)70326-4. doi: 10.1016/S1474-4422(05)70326-4. [DOI] [PubMed] [Google Scholar]