Abstract
Staphylococcus aureus bacteraemia remains a significant cause of morbidity and mortality. National guidelines recommend that a minimum of 14 days of antibiotics should be used to treat uncomplicated bacteraemia. Five hospitals in the East Midlands region conducted a retrospective audit to assess compliance to these guidelines before and after the introduction of extra text to laboratory reports of S. aureus bacteraemia advising clinicians on the minimum length of treatment. Introduction of this extra text resulted in an increase in compliance with the national recommendation from 44% to 60%. This increase in compliance was noted in both methicillin-sensitive S. aureus (45% versus 58%) and methicillin-resistant S. aureus (42% versus 62%) bacteraemia. This audit demonstrated a simple and effective intervention that has improved the treatment of this potentially life-threatening condition.
Key Words: antibiotic therapy, audit, duration, Staphylococcus aureus
Introduction
Staphylococcus aureus is an important pathogen in bacteraemia that carries significant morbidity and mortality.1 The Department of Health has not only conducted mandatory reporting for methicillin-resistant S. aureus (MRSA) bacteraemia since April 2001, but also voluntary surveillance of all S. aureus bacteraemias (SABs). The rate of SAB remains high with over 14,000 episodes in the UK in 2007 with approximately 30% of those being MRSA.2 In order to reduce the clinical burden of these infections, it is therefore important that the duration of treatment is adequate and any local focus of infection is eradicated.
Some debate remains about the optimal duration of antibiotic treatment for SAB. The length of treatment will depend upon the source of infection. One study recommended 10 to 21 days of intravenous (iv) antibiotics for uncomplicated bacteraemia, which included superficial infections (no evidence of deep infection or metastatic seeding) and where the source, such as an intravascular device, had been removed.3 Another study suggested a 10- to 14-day course of antibiotics for uncomplicated bacteraemia.4 The British Society for Antimicrobial Chemotherapy (BSAC) guidelines recommend a minimum treatment duration of 14 days for uncomplicated bacteraemia.5 This is also supported by the Infectious Diseases Society of America.6
There is some evidence that an increased rate of relapse of staphylococcal bacteraemia is due to inadequately treated infection and one of the factors related to death in SAB was duration of treatment being less than 14 days.7,8 However, there is still a need for multicentre prospective comparative trials in selected patient groups to evaluate the duration of antibiotics for the treatment of S. aureus, including MRSA, bacteraemia.
Aims and objectives
The aim of this audit was to assess compliance of hospitals in the East Midlands region with national guidelines for the treatment of SAB.
Method
This retrospective audit involved data from five hospitals in the East Midlands region: Leicestershire, Lincolnshire and Nottinghamshire. A medical microbiologist reviewed case notes and drug charts retrospectively for patients with SAB in the three-month period from 1 January 2002 to 31 March 2002, and then again from 1 January 2008 to 31 March 2008. Data were gathered on a specially designed proforma and included patient's demographics, whether the blood culture was methicillin-sensitive S. aureus (MSSA) or MRSA, the dosage and length of antibiotic treatment (iv and oral). The inclusion criterion was a positive blood culture for S. aureus in a patient who survived 14 days or more after the date of the positive culture. An adequate dose of antibiotics included 4 g or more of flucloxacillin daily, or vancomycin or teicoplanin with therapeutic levels. If other antibiotics were prescribed (eg cefuroxime, erythromycin) their appropriateness and dosage was assessed by the microbiologist reviewing the case notes. Exclusion criteria included patients who died within 14 days of the positive blood culture result, when case notes were unavailable or incomplete, or when patients were transferred to another hospital preventing follow up. An adequate length of treatment was set at 14 days or more of antibiotics. Data were entered locally into a Microsoft Access database and these were then merged to form a regional database for subsequent analysis.
Results (2002)
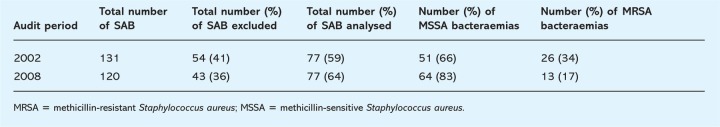
The number of SABs over the three-month period from 1 January to 31 March 2002 is represented in Table 1. There was a total of 131 SABs in this period of which 59% were included for analysis. Out of the SABs analysed 66% were MSSA bacteraemias and 34% were MRSA bacteraemias.
Table 1.
Number of Staphylococcus aureus bacteraemias (SABs) over a three-month period in 2002 (1 January 2002 to 31 March 2002) and 2008 (1 January 2008 to 31 March 2008).
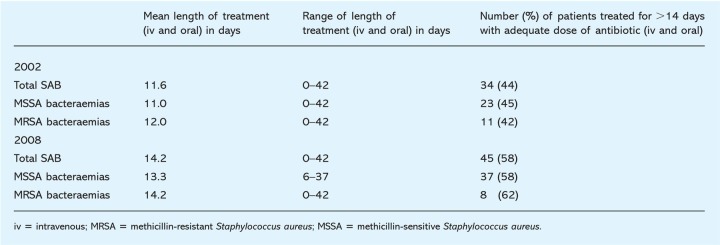
The duration of antibiotic therapy for SABs in this three-month period is represented in Table 2. The range of length of treatment was 0–42 days for all SABs. The mean length of treatment for total SABs, MSSA bacteraemias and MRSA bacteraemias was 11.6, 11.0, and 12.0 days respectively. The percentage of patients with SAB who were treated for 14 days or more at an adequate antibiotic dose was 44%; the individual breakdown was 45% for MSSA bacteraemias and 42% for MRSA bacteraemias.
Table 2.
Duration of antibiotic treatment for Staphylococcus aureus bacteraemias (SABs) over a three-month period in 2002 (1 January 2002 to 31 March 2002) and 2008 (1 January 2008 to 31 March 2008).
Discussion (2002)
Analysis of the results revealed 44% of the patients with SAB were treated for 14 days or more at an adequate antibiotic dose. The percentage of bacteraemias treated appropriately was similar for both the MSSA and MRSA arm (45% versus 42%). The range of length of treatment was wide (0–42 days) and the mean length of treatment for all bacteraemias was less than 14 days.
Two main reasons were considered for patients not receiving the full course of antibiotics. Firstly, some isolates were considered to be possible contaminants by the microbiologist and/or clinician. There are a few published estimates of the exact rate of S. aureus in blood culture representing contamination, and it is considered to indicate true bacteraemia in the overwhelming majority of cases. In a study that included 204 blood culture isolates of S. aureus, after an infectious disease physician reviewed the patient, 87% were thought to represent true bacteraemia, 6% contamination and 6% ‘unknown’.9 Since SAB may indicate occult infection, such as endocarditis, these ‘unknown’ cases may have, in fact, represented true bacteraemia. The study does not state whether echocardiography or other investigations were performed to identify the source of infection or whether there was any follow up. Therefore, it is likely that at least 90% of blood cultures positive with S. aureus represent true bacteraemia.
Secondly, many clinicians may not have realised that aggressive treatment was required. Given that the recording of telephoned blood culture advice in case notes has been found to occur in less than two-thirds of cases, a verbal recommendation by a microbiologist may have been forgotten.10
In conclusion, this audit highlighted non-compliance with national recommendations on duration of treatment for SAB.
Recommendations for improvement
-
1
Introduction of extra text to laboratory reports of SAB stating ‘Minimum two weeks antibiotic treatment required. Source of infection should be investigated’.
-
2
Education of clinicians at induction and through maintaining close liaison between microbiology and clinical teams.
-
3
Re-audit after these interventions to see if treatment compliance improves.
Results of re-audit (2008)
The number of SABs over the three-month period from 1 January to 31 March 2008 is represented in Table 1. There were a total of 120 SABs in this period of which 64% were included for analysis after application of exclusion criteria. Out of the SABs analysed, 83% were MSSA bacteraemias and 17% were MRSA bacteraemias.
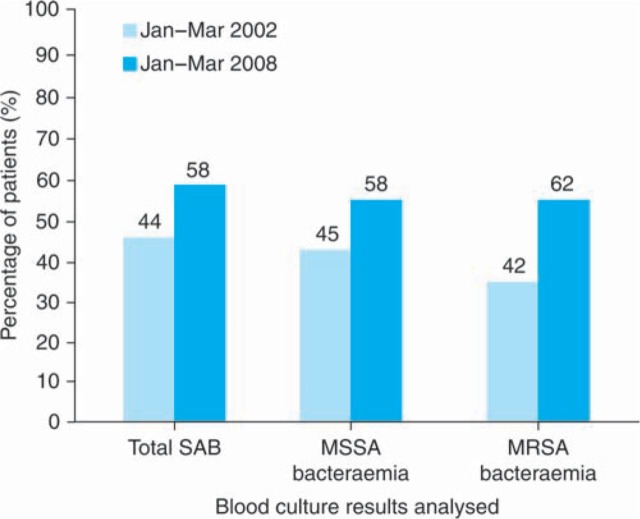
The duration of antibiotic therapy for SABs in this three-month period is represented in Table 2. The range of length of treatment for all SABs and MRSA bacteraemias was 0–42 days and for MSSA bacteraemias was 6–37 days. The mean length of treatment for all SABs, MSSA bacteraemias and MRSA bacteraemias was 14.2, 13.3 and 14.2 days, respectively. The percentage of patients with SAB who were treated for 14 days or more at an adequate antibiotic dose was 58%; the individual breakdown was 58% for MSSA bacteraemias and 62% for MRSA. These results are shown in Fig 1 with comparison of duration of antibiotics therapy for SABs before and after the interventions described.
Fig 1.
A comparison between 2002 and 2008 for percentage of patients treated for at least 14 days with adequate doses of antibiotic. SAB = Staphylococcus aureus bacteraemia; MSSA = methicillin-sensitive Staphylococcus aureus; MRSA = methicillin-resistant Staphylococcus aureus.
The intervention of adding extra text onto the laboratory report implemented after the first audit was also reviewed for each SAB analysed. The advice – ‘Minimum two weeks antibiotic treatment required. Source of infection should be investigated’ – was found in 72 out of the 76 laboratory reports (95%).
Discussion (2008)
Analysis of the re-audit results revealed that there was an increase in compliance with the BSAC recommendation of a minimum of 14-days antibiotic treatment for SAB from 44% to 58%. This compliance was noted in both MSSA (45% versus 58%) and MRSA (42% versus 62%) bacteraemia.
Overall, the majority of hospitals in the East Midlands region had added the extra text instructing clinicians that SAB should be treated for at least 14 days onto laboratory reports. Of laboratory reports, 95% (72/76) were sent out with this advice – this was because one trust did not have the advice on four laboratory reports. Out of these four cases only one patient did not receive antibiotics for at least 14 days.
In order to further increase compliance to national recommendations it should be possible to add the clinical advice to all SAB reports by programming the laboratory computer system to automatically add the extra text to a SAB result. The recommended minimum two weeks of antibiotics for SAB could be explained at doctors' induction seminars and also written in the antibiotics guidelines available to all clinicians on hospital websites. It is important to continue to educate clinicians by maintaining good communication between microbiology and clinical teams, a process which could be enhanced by appropriate bedside reviews. All SAB patients could be initially assessed by a multidisciplinary bacteraemia team, whose roles could include follow up during the duration of treatment as well as facilitating antimicrobial stewardship in line with local guidelines.
It was interesting to note that there were fewer SABs in the re-audit than in the first (120 versus 131). The percentage of MRSA bacteraemias in 2008 was also lower than in 2002 (17% versus 34%). The most reasonable explanation for this reduction in cases may be that the re-audit was performed after a number of interventions were put in place to reduce the number of methicillin-resistant SABs. This involved a change in antimicrobial prescribing policy and implementation of infection control measures. Thus, in addition to changes in the epidemiology of SAB, there appears to be improved management of the condition, which should lead to a cost saving benefit and less medical complications. However, it would be prudent to continue to audit the duration of SAB treatment yearly in order to assess continued compliance with national recommendations.
References
- 1.Lautenschlager S, Herzog C, Zimmerli W. Course and outcome of bacteraemia due to Staphylococcus aureus: evaluation of different clinical case definitions. Clin Infect Dis. 1993;16:567–73. doi: 10.1093/clind/16.4.567. [DOI] [PubMed] [Google Scholar]
- 2.Health Protection Agency . Voluntary reporting of Staphylococcus aureus bacteraemia in England, Wales, and Northern Ireland January – December 2007. London: HPA; 2008. pp. 1–6. www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1235032870553. [Google Scholar]
- 3.Iannini PB, Crossley K. Therapy of Staphylococcus aureus bacteraemia associated with a removable focus of infection. Ann Intern Med. 1976;84:558–60. doi: 10.7326/0003-4819-84-5-558. [DOI] [PubMed] [Google Scholar]
- 4.Raad II, Sabbagh MF. Optimal duration of therapy for catheter-related Staphylococcus aureus bacteraemia: A study of 55 cases and review. Clin Infect Dis. 1992;14:75–82. doi: 10.1093/clinids/14.1.75. [DOI] [PubMed] [Google Scholar]
- 5.Gould K, Brindle R, Chadwick PR, et al. Guidelines (updated in 2008) for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the United Kingdom. J Antimicrob Chemother. 2009;63:849–61. doi: 10.1093/jac/dkp065. [DOI] [PubMed] [Google Scholar]
- 6.Mermel LA, Farr BM, Sherertz RJ, et al. Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis. 2001;32:1249–72. doi: 10.1086/320001. [DOI] [PubMed] [Google Scholar]
- 7.Blyth CC, Darragh H, Whelaan A, et al. Evaluation of clinical guidelines for the management of Staphylococcus aureus bacteraemia. Int Med J. 2002;32:224–32. doi: 10.1046/j.1445-5994.2001.00205.x. [DOI] [PubMed] [Google Scholar]
- 8.Jensen AG, Wachmann CH, Espersen F, et al. Treatment and outcome of Staphylococcus aureus bacteraemia: a prospective study of 278 cases. Ann Intern Med. 2002;162:25–32. doi: 10.1001/archinte.162.1.25. [DOI] [PubMed] [Google Scholar]
- 9.Weinstein MP, Towns ML, Quartey SM, et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24:584–602. doi: 10.1093/clind/24.4.584. [DOI] [PubMed] [Google Scholar]
- 10.Greig JR. Accuracy and completeness of the documentation of blood culture results. J Clin Path. 2003;56:558. doi: 10.1136/jcp.56.7.558. doi: 10.1136/jcp.56.7.558. [DOI] [PMC free article] [PubMed] [Google Scholar]