Abstract
Background
Many cases of Fuchs’ uveitis have been associated with persistent rubella virus infection. A 73-year-old male patient with typical Fuchs’ Uveitis Syndrome (FUS) first experienced heterochromia of the left eye at the age fourteen, when rubella was endemic in the US.
Objectives
The purposes of this report are to describe the patient’s FUS clinical presentations and to characterize the virus detected in the vitreous fluid.
Study design
The patient underwent a therapeutic pars plana vitrectomy in May 2013. A real-time RT-PCR assay for rubella virus was performed on the vitreous fluid by Focus Diagnostics. Additional real-time RT-PCR assays for rubella virus detection and RT-PCR assays for generation of templates for sequencing were performed at the Centers for Disease Control and Prevention (CDC).
Results
The results from Focus Diagnostics were positive for rubella virus RNA. Real-time RT-PCR assays at CDC were also positive for rubella virus. A rubella virus sequence of 739 nucleotides was determined and phylogenetic analysis showed that the virus was the sole member of a new phylogenetic group when compared to reference virus sequences.
Conclusions
While FUS remains a clinical diagnosis, findings in this case support the association between rubella virus and the disease. Phylogenetic analysis provided evidence that this rubella virus was likely a previously undetected genotype which is no longer circulating. Since the patient had rubella prior to 1955, this sequence is from the earliest rubella virus yet characterized.
Keywords: Fuchs’ uveitis syndrome, Rubella virus, Genomic characterization of rubella virus
1. Background
Fuchs’ uveitis syndrome was originally described by Ernest Fuchs’ in 1906 [1]. It has variously been referred to as Fuchs’ heterochromic cyclitis, Fuchs’ heterochromic iridocyclitis, Fuchs’ heterochromic uveitis, and most recently Fuchs’ uveitis syndrome (FUS) [1,2]. The typical characteristics of FUS include iris heterochromia, cyclitis, and cataract. Other characteristics of FUS include a mild anterior chamber reaction, the occurrence of small to medium-sized diffusely distributed stellate keratic precipitates, iris atrophy with or without heterochromia, and late-onset ocular hypertension or glaucoma [1,3,4]. Hypothesized causes of FUS include hypersensitivity, autoimmunity reactions, and infectious agents such as Toxoplasma gondii, cytomegalovirus (CMV), and herpes simplex virus (HSV) [5–8]. Recent reports suggest that rubella virus is a leading cause of FUS as rubella antibodies and/or nucleic acid have been detected in the vitreous fluid in FUS patients [9–15]. The significant decline of FUS cases in rubella post-vaccinated communities is consistent with a causal relationship between rubella infection and FUS [16].
In the last 10 years, the genetic diversity of RuVs have been documented and in 2005 a systematic nomenclature for rubella viruses was adopted by the World Health Organization (WHO) [17] in which genetic characterization of rubella virus identified two clades which differ by 8–10% at the nucleotide level. The clades are divided into 13 genotypes with 3–6% genetic distance between the genotypes within a clade.
2. Objectives
This report describes the molecular detection and genotypic characterization of rubella virus nucleic acid recovered from the vitreous fluid of a 73-year-old Pennsylvania man with a clinical diagnosis of FUS. The patient first experienced heterochromia of the left eye at age fourteen; thus, the rubella virus nucleic acid detected is likely due to rubella infection prior to 1953.
3. Study design
3.1. Rubella virus RNA detection
The vitreous fluid from the patient’s left eye collected at the time of vitrectomy was submitted to Focus Diagnostics (Cypress, CA) for the detection of DNA of T. gondii and RNA of rubella virus. The specimen was shipped on cold packs and RNA extraction was performed within 48 h of the specimen collection. At Focus Diagnostics, DNA/RNA was extracted from the sample using the MagNa Pure System (Roche Diagnostics, Indianapolis, IN). The T. gondii DNA was tested using a qualitative real-time PCR assay developed by Quest Diagnostics (Nichols Institute, Chantilly, Virginia). The rubella RNA was tested with a real-time RT-PCR assay developed by Focus Diagnostics and run on an ABI 7900 platform (Life Technologies, Carlsbad, CA). The assay used Scorpion primers and targeted the E1 gene in the rubella virus genome.
The sample was shipped from Focus Diagnostics to CDC with ice packs and was cold on arrival 72 h later. For tests performed at the CDC, the RNA was extracted from vitreous fluid on arrival 26 days post vitrectomy using the QIAamp Viral RNA Extraction Mini Kit (Qiagen, Valencia, CA) following the manufacturer’s instructions. Two real-time RT-PCR assays were performed in order to detect and confirm the integrity of rubella virus RNA in the specimen. The first assay, a laboratory developed real-time RT-PCR standard curve assay which targets the region between nucleotides (nts) 8812–8996 in the E1 gene, was performed as described in Abernathy et al. [18] with the addition of an additional reverse primer to improve detection of clade 2 viruses [19]; RNAse P was used as a reference gene to verify the presence of intact RNA in the sample. A second real-time RT-PCR assay used primers targeting the 5′ terminus of the viral genome (nt 195–323) [20]. All real-time reactions were performed using a Superscript qRT-PCR kit (Life Technologies, Carlsbad, CA) and the specimen RNA was run in triplicate. The CDC laboratory developed test has not been validated on vitreous humor as a metrix. Vitreous humor is a rare specimen for rubella virus detection; thus we are unable to procure sufficient specimens to validate the assay. Additionally, multiple freeze-thaw cycles and non-optimal shipping conditions likely did result in a reduction in the level of virus present in the sample. However, a sufficient amount of RNA remained allowing us to perform the analyses described in this manuscript
3.2. Sequencing and phylogenetic analysis
Two nested set amplifications were used to obtain templates for sequencing. The first nested set amplified a fragment containing 601 nts from the 3′ end of the WHO recommended 739 nt genotyping window [17] using a two-step method. First-strand cDNA synthesis was performed with the SuperScript® III Reverse Transcriptase kit (Life Technologies) according to the manufacturer’s instructions. The cDNA was added to the first round PCR amplification using the Takara Ex-Taq DNA polymerase (Clontech Laboratories, Mountain View, CA) with primers RV11 (nts 8812–8831) and Rub3′ (nts 9745–9762 plus 18 Ts). Primers RV8823F (ACGGACAACTCGAGGTCC) and RV9549R (GCAGTGGTGTGTGTGCCATAC) were used for the second round. To obtain the sequence of the 138 nts at the 5′ end of the genotyping window, the SuperScript III High Fidelity kit (Life Technologies) was used with a second nested set. Primers RV8699F-2C (GTCCAGCACCCTCACAAGAC) and RV8986R-PA (CCACTCCCCTGACTGTTCG) and RV8711F (CACAAGACCGTCCGGGTCAA) [21] and RV8910R-2C (CACCGGGACTGTTGGTTG) were used for the first and second rounds, respectively. DNA products from the positive nested-set reactions were purified with a Charge Switch-Pro PCR cleanup Kit (Life Technologies). The sequences were determined bidirectionally (ABI Prism BigDye terminator, v 3.1, Life Technologies) and run on an AB 3130 DNA Sequencer (Life Technologies). Sequences were analyzed using Sequencher 5.2 (Gene Codes, Ann Arbor, MI) and phylogenetic and distance analyses was done using the MEGA 6 program [22].
4. Results
4.1. Patient history and presentation
The patient was a 73-year-old male, born and raised in the United States, with chronic iritis in the left eye for which he had been on and off topical steroids for many years. He had a history of a repaired left eye retinal detachment performed elsewhere 8 years prior with scleral buckle. On admission, he revealed that he had good vision for a year after the repair of the retinal detachment, but then developed persistent debris and floaters. The examination showed a vision of 20/25 and 20/40 in the right eye and the left eye, respectively. The heterochromia of his irises was noted as the right eye was darker than the left (Fig. 1). However, the patient indicated that his heterochromia was first noted at age 14. The left eye exhibited stellate keratic precipitates on the corneal endothelium but no cells in the anterior chamber. It also showed an old scleral buckle with chorioretinal scarring (Fig. 2) to the treated tears inferiorly and superiorly and punched out choroidal lesions suggestive of toxoplasmosis. The right eye was normal and the fundus examination was unremarkable. Additionally, the patient had notable vitreous debris and floaters in the left eye suggestive of FUS. He underwent a therapeutic pars plana vitrectomy in May 2013 to remove the debris and to improve his vision. Postoperatively, the patient’s vision improved, and the vitreous debris and floaters were resolved. His vision has remained intact as of this report.
Fig. 1.
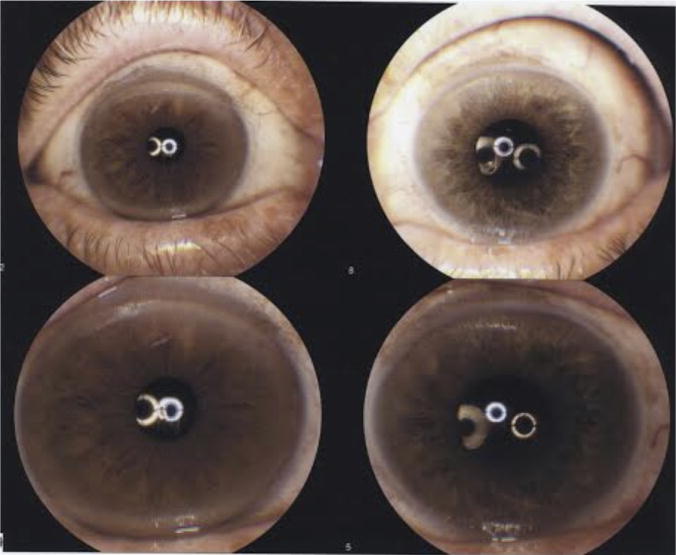
This photo depicts the heterochromia of iris, the lighter eye is the affected eye.
Fig. 2.
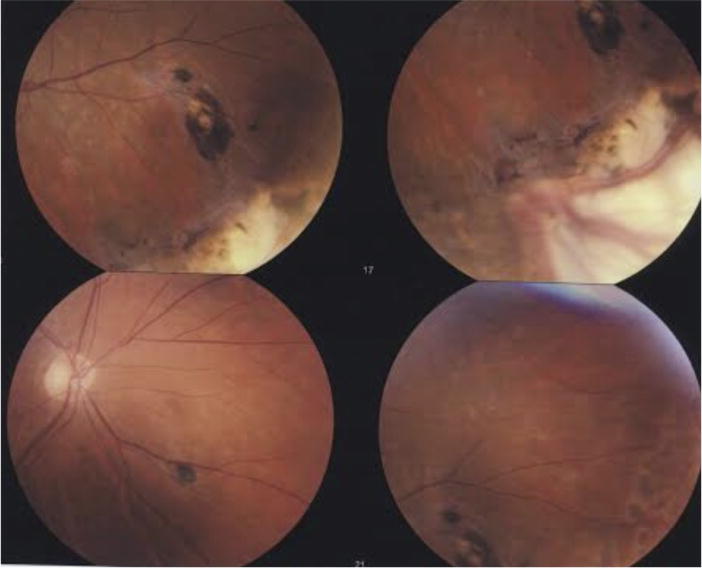
This photo shows the pigmented chorioretinal scars centrally from old inflammation. The white areas are previous cryotherapy scars placed at the time of the original retinal detachment. In the further periphery indentation from the scleral buckle is noted.
4.2. Laboratory findings
At Focus Diagnostics T. gondii DNA was absent in the vitreous fluid of the patient; however, rubella RNA was detected despite the fact that the assay had not been validated for use with vitreous fluid. The vitreous fluid sample was subsequently sent to the CDC to confirm the initial results and to attempt to sequence and genotype the virus.
The RNA extracted from the vitreous fluid was tested with the E1 coding region real-time RT-PCR assay, which produced a 185 base pair (bp) product. One of three triplicate reactions was positive with a cycle threshold (Ct) value of 39 out of 40 and the RNAse P signal for the sample was positive indicating that intact cellular RNA was present. To confirm the presence of viral RNA in the sample, a real-time assay which amplifies a 129 bp product in the nonstructural open reading frame, close to the 5′ end of the genome, was also used. This assay resulted in 2 out of 3 positive signals for the sample with an average Ct value of 36.5, thereby confirming the presence of rubella RNA in the specimen. Two nested primer sets were necessary to amplify enough template to sequence the WHO recommended 739 nt sequence window (nts 8731–9469). The first nested primer set, which amplified approximately the 3′ four-fifths of the sequence window, allowed 601 nts (8869–9469) to be sequenced. NCBI BLAST results for the sequence showed that genotype 2C viruses were the closest match. Utilizing known sequences of 2C viruses and the sequence of the 601 nts determined for this sample to design primers, a template for sequencing of the remaining 138 nts of the requisite sequence window was amplified by nested set and sequenced. The sequence was assigned a name using an adaptation of the WHO standard rubella virus naming convention: RVs/Scranton.PA.USA/19.13/FUS (KP982900). The addition of FUS to the name indicated that the rubella sequence identified was from a case of Fuchs’ uveitis syndrome. The date in the name reflects the date of sample collection, rather than the date of the virus infection. The mean distances between the 32 WHO reference viruses and the Scranton sequence were 6% (genotype 2C), 8% (genotype 1a), and 10–11% (remaining 11 genotypes) (Fig. 3). Of the 46 nt changes between the Scranton sequence and the 2C virus, Moscow RUS/67, 44 occurred at the 3rd codon position. The two changes that were seen at the 2nd codon position both resulted in an amino acid change in the E1 protein at positions 16/246 (V–A) and 150/246 (H–R). When compared to 190 other 739 nt sequences from all genotypes, the V–A change was observed in several other sequences, but the H–R change was unique (data not shown). The sequence of the Scranton virus was the single member of a deep branch from the same node as the genotype 2C viruses.
Fig. 3.

The genetic relationships of the 739 nt sequences were inferred using the Neighbor-Joining method. The percentage of replicate trees (70% or greater) in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The genetic distances were computed using the Maximum Composite Likelihood method and are in units of the number of base substitutions per site. All taxa are labeled with WHO names. The Scranton sequence is marked with a circle.
5. Discussion
Until recently, FUS remained a clinical diagnosis with an unknown etiology. The association between rubella infection and FUS was not recognized until 2004 [23].
On initial examination, the left eye showed an old scleral buckle with chorioretinal scarring and punched out choroidal lesions suggestive of toxoplasmosis. The patient also had notable vitreous debris and floaters suggestive of FUS. Therefore, the vitreous fluid was tested for the detection of T. gondii DNA and RuV RNA. An association between infectious agent(s) and FUS is not completely characterized, but in this case RuV was clearly found. The development of more sensitive nucleic acid detection tests for RuV, HSV, CMV, and toxoplasmosis will likely contribute to future diagnostic tests and may help with proper management of FUS [13,23].
Rubella was endemic in the United States during the case-patient’s entire childhood. Although the case-patient does not remember having rubella as a child, he reported having heterochromia at age 14 and, thus, presumably had rubella infection prior to that age. Therefore, his eye had likely been persistently infected with rubella virus for at least 60 years (since 1953). Due to the very low amount of viral genetic material present in the sample, it was difficult to detect and sequence the virus. Previous studies on sequence analysis of whole genome sequences of rubella viruses indicated that the nucleotides near the 5′ terminus are the most conserved among rubella viruses [24,25]. In this case, using highly conserved primer binding sites present in the 5′ real-time assay likely lowered the Ct values compared to the real-time assay targeting the more variable E1 coding region.
The first successful rubella virus isolation occurred in 1961 [26], and the sequence of the 1961 virus (known as M33) was the earliest known rubella virus sequence prior to this report. One previous report of FUS in a 28-year-old congenital rubella syndrome patient described 252 nt rubella sequences from both eyes which partially overlapped the standard genotyping window [13]. There were five nucleotide differences between the two FUS derived sequences which the authors attributed to independent rubella virus evolution in the right and left eyes. Despite the differences, both sequences were considered to belong to genotype 1G, a currently circulating genotype.
Phylogenetic analysis provided evidence that the Scranton virus described here was likely from a previously undetected genotype which is no longer circulating. Using the WHO recommended 739 nt sequence, the Scranton sequence was found to be the sole member of a new rubella virus group and shared the most genetic similarity to genotypes 2C and 1a, viruses in both genotypes were collected in the 1960s and viruses in neither genotype are known to be currently circulating [27]. The genetic distance from the 13 known rubella genotypes could have two possible explanations. The long period of persistent infection could have led to accumulated mutations from an ancestor of one of the current genotypes or the distance could be due to the fact that this virus belonged to unknown, now extinct genotype (possibly even a different clade due to the large (6%) genetic distance from closest virus). The node in common with 2C viruses was not supported by a significant bootstrap value, indicating that the relationship between the Scranton virus and genotype 2C was not reliable. This reinforced the conclusion that the Scranton virus could not be classified with any of the currently recognized groups. The very low amount of rubella virus genetic material present in the sample unfortunately limited the amount of sequence available for a more rigorous phylogenetic analysis. Specimen handling and transportation in this case were sub-optimal and likely contributed to the low level of viral RNA present in the sample. We hope in the future, if any additional procedures are performed on the patient or on other similar patients, that additional samples could be procured and handled in a more efficient manner, perhaps enabling us to extend our rubella virus genetic studies for Fuchs’ uveitis patients.
A previously published evolutionary rate for the rubella 1E genotype is 1.65 × 10−3 substitutions/site/year [28]. Although this rate is based on analysis of a different genotype, we used it to estimate the number of nucleotide changes that would be predicted in the Scranton virus. Based on the sequence length of 739 nts and a 60 year period, the number of nucleotides substitutions expected would be approximately 71. In this case, 46 changes are seen between the Scranton sequence and a genotype 2C sequence. We consider 46 to be the maximum number of changes from the actual parental virus (i.e., we assume the actual parental virus would be more closely related to the Scranton virus than the genotype 2C sequence); thus, we conclude that the actual substitution rate per year would likely be even lower. Little is known about the replication rate of virus in a long term persistent infection in human eye tissue; a very low rate of replication would likely reduce the number of predicted substitutions.
The fact that 44 of the 46 changes occurred in the 3rd codon position indicates that the changes were not random, and was in agreement with a previous report that 93% of RuV first and second codon positions were invariant, while only 48% of third codon positions were invariant in circulating viruses [24]. Although 2 amino acid changes were noted relative to other RuVs, the changes were to amino acids with similar properties: valine and alanine are both hydrophobic and histidine and arginine are both positively charged. The observed maintenance of the open reading frame and the observed amino acid substitutions were consistent with productive virus replication.
In summary, this study supports the role of rubella virus as a cause of FUS and suggests that persistent RuV presence in the eye can be the cause of chronic vision problems. In terms of relevance to public health, it would be interesting to investigate whether FUS patients are capable of shedding infectious virus (e.g., in tears), which would be a significant observation since rubella has been eliminated from the United States.
Acknowledgments
We thank Dr. Pierre Rivailler and Dr. Roman Tatusov for helpful discussions and reading of the manuscript. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Names of specific vendors, manufacturers, or products are included for public health and informational purposes; inclusion does not imply endorsement of the vendors, manufacturers, or products by the Centers for Disease Control and Prevention or the US Department of Health and Human Services.
Funding
None.
Ethical approval
Not required.
Footnotes
Conflict of interest
None.
Randomized controlled trial
N/A.
References
- 1.Muller A, McGhee CNJ. Professor Ernst Fuchs’ (1851–1930): a defining career in ophthalmology. Arch Ophthalmol. 2003;121:888–891. doi: 10.1001/archopht.121.6.888. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham ET, Jr, Baglio E. Fuchs’ heterochromic iridocyclitis: syndrome, disease, or both? Am J Ophthalmol. 2009;148:479–481. doi: 10.1016/j.ajo.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Bonfioli AA, Curi AL, Orefice F. Fuchs’ heterochromic cyclitis. Semin Ophthalmol. 2005;20(3):143–146. doi: 10.1080/08820530500231995. [DOI] [PubMed] [Google Scholar]
- 4.Kanavi MR, Soheilian M, Naghshgar N. Confocal scan features of keratic precipitates in Fuchs’ heterochromic iridocyclitis. Cornea. 2010;29(1):39–42. doi: 10.1097/ICO.0b013e3181acf674. [DOI] [PubMed] [Google Scholar]
- 5.Vasconcelos-Santos DV. Ocular manifestations of systemic disease: toxoplasmosis. Curr Opin Ophthalmol. 2012;23(6):543–550. doi: 10.1097/ICU.0b013e328358bae5. [DOI] [PubMed] [Google Scholar]
- 6.Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56(7):481–490. doi: 10.1136/jcp.56.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Multack RF, Genge MR, Skorin L. Immune Disease. In: Onofrey B, Skorin L, Holdeman NR, editors. Ocular Therapeutics Handbook: A Clinical Manual. 2nd. Lippincott Williams & Wilkins; Philadelphia, PA: 2005. pp. 236–288. [Google Scholar]
- 8.Bonfioli AA, Orefice F. Sarcoidosis. Semin Ophthalmol. 2005;20(3):177–182. doi: 10.1080/08820530500231938. [DOI] [PubMed] [Google Scholar]
- 9.de Groot-Mijnes JD, de Visser L, Rothova A, Schuller M, van Loon AM, Weersink AJ. Rubella virus is associated with Fuchs’ heterochromic iridocyclitis. Am J Ophthalmol. 2006;141:212–214. doi: 10.1016/j.ajo.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 10.de Visser L, Braakenburg A, Rothova A, deBoer JH. Rubella virus-associated uveitis: clinical manifestations and visual prognosis. Am J Ophthalmol. 2008;146(2):292–297. doi: 10.1016/j.ajo.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki J, Goto H, Komase K, Abo H, Fujii K, Otsuki N, Okamoto K. Rubella virus as a possible etiologic agent of Fuchs’ heterochromic iridocyclitis. Graefes Arch Clin Exp Ophthalmol. 2010;248:1487–1491. doi: 10.1007/s00417-010-1434-6. [DOI] [PubMed] [Google Scholar]
- 12.Van Gelder RN. Idiopathic no more: clues to the pathogenesis of Fuchs’ heterochromic iridocyclitis and glaucomatocyclitis crisis. Am J Ophthalmol. 2008;145:769–771. doi: 10.1016/j.ajo.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Winchester SA, Varga Z, Parmar D, Brown KE. Persistent Intraocular rubella infection in a patient with Fuchs’ uveitis and congenital rubella syndrome. J Clin Microbiol. 2013;51:1622–1624. doi: 10.1128/JCM.03239-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruokonen PC, Metzner S, Ucer A, Torun N, Hofmann J, Pleyer U. Intraocular antibody synthesis against rubella virus and other microorganisms in Fuchs’ heterochromic cyclitis. Graefes Arch Clin Exp Ophthalmol. 2010;248(4):565–571. doi: 10.1007/s00417-009-1239-7. [DOI] [PubMed] [Google Scholar]
- 15.Siemerink MJ, Sijssens KM, de Groot-Mijnes JD, de Boer JH. Rubella virus-associated uveitis in a nonvaccinated child. Am J Ophthalmol. 2007;143(5):899–900. doi: 10.1016/j.ajo.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 16.Birnbaum AD, Tessler HH, Schultz KL, Faber MD, Gao W, Lin P. Epidemiologic relationship between Fuchs’ heterochromic iridocyclitis and the United States rubella vaccination program. Am J Ophthalmol. 2007;144(3):424–428. doi: 10.1016/j.ajo.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Standardization of the nomenclature for genetic characterization of wild-type rubella viruses. Wkly Epidemiol Rec. 2005;80:126–132. [PubMed] [Google Scholar]
- 18.Abernathy E, Cabezas C, Sun H, Zheng Q, Chen MH, Castillo-Solorzano C, Ortiz AC, Osores F, Oliveira L, Whittenbury A, Andrus JK, Helfand RF, Icenogle J. Confirmation of rubella within 4 days of rash onset. J Clin Microbiol. 2009;47(1):182–188. doi: 10.1128/JCM.01231-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Namuwulya P, Abernathy E, Bukenya H, Bwogi J, Tushabe P, Birungi M, Sequya R, Kabaliisa T, Alibu V, Kayondo J, Rivailler P, Icenogle J, Bakamutumaho B. Phylogenetic analysis of rubella viruses identified in Uganda, 2003–2012. J Med Virol. 2014;86(12):2107–2113. doi: 10.1002/jmv.23935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hübschen JM, Kremer JR, De Landtsheer S, Muller CP. A multiplex TaqMan PCR assay for the detection of measles and rubella virus. J Virol Methods. 2008;149(2):246–250. doi: 10.1016/j.jviromet.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 21.Cooray S, Warrener L, Jin L. Improved RT-PCR for diagnosis and epidemiological surveillance of rubella. J Clin Virol. 2006;35:73–80. doi: 10.1016/j.jcv.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 22.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA 6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quentin CD, Reiber H. Fuchs’ heterochromic cyclitis: rubella virus antibodies and genome in aqueous humor. Am J Ophthalmol. 2004;138(1):46–54. doi: 10.1016/j.ajo.2004.02.055. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y, Ushijima H, Frey TK. Genomic analysis of diverse rubella virus genotypes. J Gen Virol. 2007;88:932–941. doi: 10.1099/vir.0.82495-0. [DOI] [PubMed] [Google Scholar]
- 25.Abernathy E, Chen MH, Bera J, Shrivastava S, Kirkness E, Zheng Q, Bellini W, Icenogle J. Analysis of whole genome sequences of 16 strains of rubella virus from the United States, 1961–2009. Virol J. 2013;10:32. doi: 10.1186/1743-422X-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parkman PD, Buescher EL, Artenstein MS. Recovery of rubella virus from army recruits. Proc Soc Exp Biol Med. 1962;111:225–230. doi: 10.3181/00379727-111-27750. [DOI] [PubMed] [Google Scholar]
- 27.Abernathy E, Hubschen J, Muller C, Jin L, Brown D, Komase K, Mori Y, Xu W, Zhu Z, Siqueira M, Shulfa S, Tikhonova N, Pattamadilok S, Incomserb P, Smit S, Akoua-Koffi C, Bwogi J, Lim W, Woo G, Triki H, Jee Y, Mulders M, de Filippis A, Ahmed H, Ramamurty N, Featherstone D, Icenogle J. Status of global virologic surveillance for rubella viruses. J Infect Dis. 2011;204:S524–S532. doi: 10.1093/infdis/jir099. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Z, Cui A, Wang H, Zhang Y, Liu C, Wang C, Zhou S, Chen X, Zhang Z, Feng D, Wang Y, Chen H, Pan Z, Zeng X, Zhou J, Wang S, Chang X, Lei Y, Tian H, Liu Y, Zhou S, Zhan J, Chen H, Gu S, Tian X, Liu J, Chen Y, Fu H, Yang X, Zheng H, Liu L, Zheng L, Gao H, He J, Sun L, Xu W. Emergence and continuous evolution of genotype 1E rubella viruses in China. J Clin Micro. 2012;50:353–363. doi: 10.1128/JCM.01264-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


