Abstract
Neutrophils infiltration/activation following wound induction marks the early inflammatory response in wound repair. However, the role of the infiltrated/activated neutrophils in tissue regeneration/proliferation during wound repair is not well understood. Here, we report that infiltrated/activated neutrophils at wound site release pyruvate kinase M2 (PKM2) by its secretive mechanisms during early stages of wound repair. The released extracellular PKM2 facilitates early wound healing by promoting angiogenesis at wound site. Our studies reveal a new and important molecular linker between the early inflammatory response and proliferation phase in tissue repair process.
Wound repair is a complex pathophysiological process. Many biological events occur in an ordered and cooperative manner.1–3 Following cutaneous wound, the blood clots during homeostasis release cytokines to recruit neutrophils to the wound bed to clean possible invading of microorganisms. This event marks early inflammatory responses.4,5 Massive macrophages influx follows the decrease in neutrophils, which triggers the proliferation phase that is marked by formation of granulation and active angiogenesis at the wound site. Neutrophils are the first infiltrating immune cells at the wound site post injury. Functional role of neutrophils in wound repair process other than control of infections and removal of tissue debris is not well understood. It is argued that presence of neutrophils delays reepithelialization.6–9 However, other studies suggest that neutrophil infiltration plays an important role in promoting dermal microvasculature and fibroblast growth.10,11
Pyruvate kinase M2 is an enzyme that acts in the last step of glycolysis pathway. There are four isoforms of pyruvate kinases, L/R and M1/M2, which express in different tissue types and different physiological status. PKM2 can form homodimer and homotetramer. The tetramer is active as pyruvate kinase, while dimer is inactive.12,13 Pyruvate kinase inactive PKM2 actually provides a metabolic advantage to supply precursors for biosynthesis of rapidly proliferating cells.14 Interestingly, a number of recent studies showed that PKM2 is functionally involved in multiple cellular processes in different locations, including metabolism control, transcription regulation, and chromatin package.15–17
High serum levels of PKM2 have long been observed in cancer patients of many types. Studies show that there is a strong correlation between the serum levels of PKM2 and tumor progression.18–20 Our previous study demonstrated that extracellular PKM2 facilitates tumor growth by promoting angiogenesis. The extracellular PKM2 promotes angiogenesis by facilitating endothelial cell migration and extracellular matrix (ECM) attachment.21 High serum levels of PKM2 are also detected in patients of various inflammatory diseases,22–24 indicating a potential association of release of extracellular PKM2 with inflammations. How PKM2 is released from immune cells and any potential role of extracellular PKM2 in inflammation are not known.
MATERIALS AND METHODS
Reagents, antibodies and protein expression/purifications
Damnacanthal (used in 1 μM), fMLP (used in 10 μM), Piceatannol (used in 40 μM), Src inhibitor PP1 (used in 1 μM), and MAPK inhibitor SB203580 (used in 1 μM) were purchased from Billerica, Roche R&D, Invitrogen, and Sigma Aldrich respectively. Vanicream was purchased from Stacy’s Pharmacy (Atlanta). Antibodies against mouse CD31, Ly6G, CD11b, β-actin, and GAPDH were purchased from Cell Signaling, Danvers, Abcam, and Santa Cruz Biotechnology respectively. Rabbit monoclonal antibody against PKM2 was raised against recombinant PKM2 expressed/purified from E. coli. IgG of antibody was purified from cell culture of hybridoma B23 over a protein G beads column. The cDNAs that encode human PKM2 and PKM1 were purchased from Adgene. The cDNAs were subcloned into bacterial expression vector pET-32a. The recombinant proteins were purified from bacterial lysates by a two-column procedure.15,25,26
Mice cutaneous wound healing and treatments
All animal experiments were approved by IACUC of Georgia State University. Wound was generated by a punch biopsy with 6 mm diameter on the back of each mouse (healthy CD-1, Beige-J, or C57BL/6J). Hair around the wound area was removed before wound induction. Appropriate agents (rPKM2, rPKM1, anti-PKM2, Rabbit IgG, and buffer saline) mixed with vanicream (at final 0.04% w/w) were applied at wound site on day 0, day 3, and day 6. The wound was covered with Tegaderm film and tape after application of the vanicream. The size of wound was monitored on day 0, day 3, day 6, and day 8 while changing the tape each time. Tissue sections were prepared from collected wound or other tissues at appropriate times as indicated. The tissue sections were analyzed by immunofluorescence (IF), immunohistochemistry (IHC), or H&E stains using commercially available antibodies as indicated.
Immunofluorescence staining and IHC
Immunofluorescence
Snap frozen tissue blocks were sliced into 10 mM thick sections using a cryostat. Frozen tissue slides were fixed with ethanol for 15 min and blocked by incubating with 3% BSA/PBS at room temperature for 1 h. CD31 antibody was incubated with the slides overnight at 4 °C. The slides were washed with PBS for 10 min at room temperature before incubating with a 1:1000 dilution of Alexa-488 conjugated secondary antibody. The slides were mounted with Prolong-Gold antifade reagent that contained DAPI (Invitrogen, Carlsbad, CA).
Immunohistochemistry
The tissue sections were baked for 2 h at 60 °C and incubated with xylene and graduate change of concentration alcohols for hydration. After antigen retrieval with 10 mM sodium citrate buffer, the slides were blocked with Dual Endogenous Enzyme Block (DAKO) for 30 min at room temperature, and incubated with appropriate antibody overnight at 4 °C. The slides were then incubated with labeled polymer-HRP for 30 min and subsequently with DAB+ substrate chromogen solution. The slides were stained with hematoxylin and dehydrate through graduate change of alcohols. Mounted slides were visualized under microscope.
Neutrophil isolation and activation in culture
Mouse bones were cut out from 8 week old CD-1 mouse. The femur and the tibia were removed and the bones were cleaned with HBSS. HBSS-EDTA solution was forced through the bones using a syringe. The cells were suspended with HBSS-EDTA and laid on a three-layer Percoll gradient of 78%, 69% and 52%. After centrifugation, the cells from the 69%/78% interface and the upper fraction of the 78% were harvested. The harvested cells were washed with HBSS-EDTA to remove the remaining red blood cells. Neutrophils were resuspended in HBSS-EDTA solution for further application.
For activation, neutrophils (5 × 106 cells) were incubated with fMLP (10 μM) or Damnacanthal (1 μM) at 37 °C for different time. The medium were collected by centrifugation to remove neutrophils. The collected medium were analyzed by immunoblot or ELISA. For the inhibition tests, neutrophils were pretreated with inhibitors for 5 min before stimulation with fMLP at 37 °C. The culture media were collected and cells were lysed with RIPA buffer. The samples were then tested by Western Blot using appropriate antibodies.
Statistical calculations
Data were statistically analyzed by comparing appropriate experimental groups. The P values were calculated using unpaired two-tailed Student t test in the cases of comparing two experimental groups. In all cases of comparing multiples experimental groups, statistical significance was also calculated by ANOVA. In all figures and tables, NS means P >0.05 and statistically insignificant, * means P <0.05, ** means P <0.01, and *** means P <0.001.
RESULTS
Extracellular PKM2 facilitates early wound healing
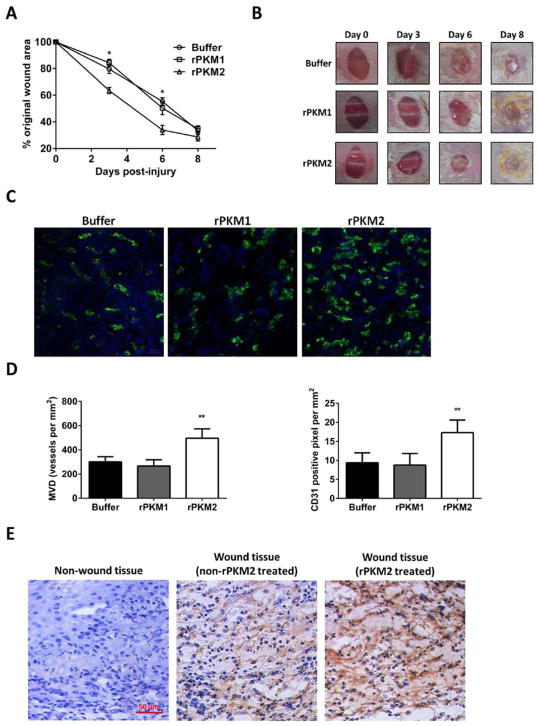
Extracellular PKM2 promotes angiogenesis.21 Angiogenesis is critically important for wound repair.27 Thus, we reasoned whether topical application of recombinant PKM2 (rPKM2) would facilitate wound healing by promoting angiogenesis. To test this speculation, we prepared a pharmacy cream (Vanicream) containing rPKM2, rPKM1, or buffer (0.04% by weight). Cutaneous wound was introduced to CD-1 mice. The prepared creams were topically applied to the wound. The wound closure was monitored. Clearly, topical application of rPKM2 facilitated wound closure early time, while, as controls, rPKM1 and buffer did not (Figure 1A, B). We subsequently prepared tissue sections from the wound and stained with an antibody against mouse CD31. Topical application of rPKM2 promoted higher levels of vessel growth compared to those treated with rPKM1 and buffer (Figure 1C, D). To confirm presence of rPKM2 at the wound site due to topical application, tissue sections were examined by IHC staining using antibody against PKM2. Topical application of rPKM2 led to accumulation of high levels of rPKM2 at the wound site (Figure 1E). Surprisingly, relative high levels of PKM2 were also detected in extracellular space of wound tissue in the buffer treated group, especially during early time of wound healing process, while no PKM2 stains were observed in nonwound tissues (Figure 1E).
Figure 1.
Extracellular PKM2 promotes wound healing. (A) The wound closure of CD-1 mice that were treated by the indicated agents. The wound closure is presented as % of original wound area, [(Original wound area - wound area)/original wound area]. (B) Representative pictures of wound areas at different days of treatments by indicated agents. (C) Representative images of immunostaining of wound tissue sections with antibodies against mouse CD31 (Green). The blues are DAPI stains. (D) Quantitative analyses of CD31 staining of the wound sections by manually counting using the software imaging-J. MVD (Left) is vessel density per mm2. The CD31 positive pixel (Right) is measured per mm2. The quantification was average of randomly selected 4 fields in randomly selected 3 sections. (E) Representative images of IHC staining of tissue sections from wound or un-wound area with/without application of rPKM2 using anti-PKM2 antibody. Blue is hematoxylin staining. Error bars in (A) and (D) are standard deviations from measurements of six mice.
Activated neutrophils release PKM2 at wound site
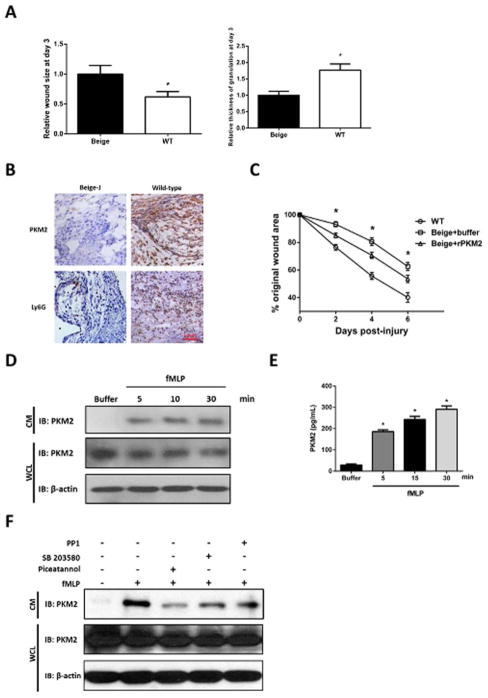
Presence of PKM2 in extracellular space of the wound tissues suggests a possible intrinsic mechanism of secretion of PKM2 during wound repair process. Thus, we questioned how PKM2 was released. Close examination of locations of PKM2 released at the wound site revealed that PKM2 localized in proximity of neutrophils (Figure 2A). Examination of extracellular PKM2 at different time points during wound repair indicated that PKM2 reached peak at the wound site around the 3rd day after wound induction and decreased thereafter (Figure 2B, C). The timing and location of extracellular PKM2 appearance matched well with that of neutrophil infiltration at wound site (Figure 2A–C), suggesting that PKM2 might be released from neutrophils during wound repair. Consistently, it is known that activated neutrophils express PKM2.28,29 Macrophages arrive the wound site following neutrophil infiltration. We examined the macrophage levels at wound site at day three. Immunostaining indicated that only very few residue macrophages presented at wound site at day three of wound induction (Figure 2D), suggesting that it is unlikely that PKM2 was released by macrophages. Fibroblast cells do not express PKM2. It is not possible that PKM2 was released from fibroblast cells. We next sought to test whether neutrophils or activated neutrophils released PKM2. Neutrophils were isolated from mouse bone marrow. The isolated neutrophils were activated by a treatment using fMLP. Presence of PKM2 in culture medium of inactivated and activated neutrophils were analyzed by immunoblot and ELISA. Evidently, PKM2 was released into the culture medium of activated neutrophils. Presence of PKM2 in the medium of inactivated neutrophils was substantially less (Figure 3A, B). It is well known that activated neutrophils release many different molecules by its secretive mechanism. If PKM2 is released by a secretive mechanism such as degranulation, we would expect presence of PKM2 in neutrophil granules. We employed an established sucrose gradient ultracentrifugation method to isolate different cellular fractions of both activated and inactivated neutrophils.30 Presence of PKM2 in each isolated fraction was examined by immunoblot. Apparently, PKM2 presented in the fractions of neutrophil granules of activated neutrophils (Figure 3C). To further test whether extracellular PKM2 at wound site is released from neutrophils, we used beige mouse that has deficiency of neutrophil migration due to mutations in LYST gene that cause deficiency in neutrophil specific protease, Elastase and Cathepsin G.31–35 Cutaneous wound were introduced to the beige mice and control wild-type mice of the same strain. Wound repair rate was reduced with the beige mice (Figure 4A). Neutrophils did not accumulate at the wound site of the beige mice, while neutrophils presented normally at the wound site of the control mice (Figure 4B). Extracellular PKM2 was detected at the wound site of the control mice but not at the wound site of the beige mice (Figure 4B). The results supported that PKM2 is released by neutrophils at the wound site. Interestingly, topical application of rPKM2 partially recovered wound repair deficiency of the beige mice. However, the wound repair was not effective as in wild type mice (Figure 4C). The incomplete rescue of the wound healing response in beige mice could perhaps be addressed by improving delivery methods of rPKM2 to the wound. To further verify release of PKM2 by neutrophil secretive mechanisms, we tested whether activation and inhibition of neutrophil degranulation would affect the release of PKM2. To this end, the isolated neutrophils were treated with Damnacanthal, a compound that activates primary and 2nd neutrophil degranulation.30 Presence of PKM2 in the culture medium was then analyzed. Release of PKM2 to the culture medium was strongly activated by Damnacanthal (Figure 3A, B). Analyses of a time course of PKM2 release upon fMLP treatment further supported that PKM2 was released by neutrophil vesicle secretion, such as degranulation (Figure 4C, D). We also employed several inhibitors that are known to inhibit various mechanisms of neutrophil vesicle secretion. Releases of PKM2 by neutrophils was reduced or completely abolished by the inhibitors (Figure 4E). Thus, we concluded that PKM2 was released by the mechanism of neutrophil vesicle secretion.
Figure 2.
Activated neutrophils release PKM2 at the wound site. (A, B) Representative images of IHC staining of tissue sections from wound or un-wound areas using antibodies against PKM2 or Ly6G, a neutrophil marker. (A) Sections were prepared from tissues of 3rd day post wound induction and stained by anti-PKM2 antibody. On the right is call-out enlargement of the indicated area. (B) Sections were prepared from wound tissues of different days (indicated) after wound induction. (C) Quantitation of extracellular PKM2 vs Ly6G stains in (B). Quantitations were done by using Frida software. Randomly selected three fields were analyzed. For quantitation of extracellular PKM2, extracellular PKM2 stains were manually selected in each view field and quantitated by the software. Error bars are standard deviations from measurements of five sections from five mice. (D) Representative images of IHC staining of tissue sections from wound areas on day 0 and day 3 using antibody against F4/80, a macrophage marker. Blue in (A), (B), and (D) is hematoxylin staining.
Figure 3.
PKM2 present in secretive vesicles of activated neutrophils. (A, B) Levels of PKM2 in culture medium (CM) of neutrophils treated with indicated agents were analyzed by immunoblot (A) of PKM2 (IB:PKM2) or ELISA analyses (B) using goat anti-human PKM2 as capture antibody and rabbit monoclonal anti-PKM2 as detection antibody. Immunoblot of PKM2 (IB:PKM2) and β-actin (IB:β-actin) in cell lysate (WCL) in (A) were loading controls indicate amounts of neutrophils used in each experiment. Damn is Damnacathal. Error bars in (B) are standard deviations from five independent measurements. (C) Presence of PKM2 in each fraction of sucrose gradient of extracts of activated (bottom) and inactivated (upper) neutrophils (10 ml in total) was analyzed by immunoblot of PKM2 (IB:PKM2). Numbers on top indicate fraction number (1 ml each fraction from top down). Immunoblot of cd11b (IB:cd11b) indicate the fractions containing neutrophil granules (fractions 13–19). Immunoblot of β-actin (IB:β-actin) indicate general cytosolic fractions (fractions 1–10).
Figure 4.
Activated neutrophils release PKM2 by secretive mechanism. (A) The wound closure (left) and granulation tissue thickness (right) of beige-J (Beige) mice or control mice (WT) at day three after wound induction. The wound closure granulation tissue thickness is presented as relative wound areas and relative thickness of granulation tissue respectively by define the average of wound area and granulation tissue thickness of Beige-J mice as 1. Error bars are standard deviations from measurements of six mice. (B) Representative images of IHC staining of sections from wound areas of beige-J (Beige-J) mice or control mice of the same strain without gene mutations (wild-type) using antibodies against PKM2 or Ly6G. Sections were prepared from tissues of the 3rd days post wound induction. Blue staining is hematoxylin staining. (C) The wound closure of beige-J (Beige) mice or control mice (WT) that were treated by rPKM2 or buffer. The wound closure is presented as % of original wound area as in Figure 1A. (D) and (E) Levels of PKM2 in culture medium (CM) of neutrophils treated with fMLP for different time (indicated) were analyzed by immunoblot (D) of PKM2 (IB:PKM2) or ELISA analyses (E) using goat anti-human PKM2 as capture antibody and rabbit monoclonal anti-PKM2 as detection antibody. (F) Levels of PKM2 in culture medium (CM) of neutrophils treated with indicated agents were analyzed by immunoblot of PKM2 (IB:PKM2). Immunoblot of PKM2 (IB:PKM2) and β-actin (IB:β-actin) in cell lysate (WCL) in (D, F) were loading controls indicate amounts of neutrophils used in each experiment. Error bars in (E) are standard deviations of five independent experiments, in (A, C) are standard deviations of experiment from five mice.
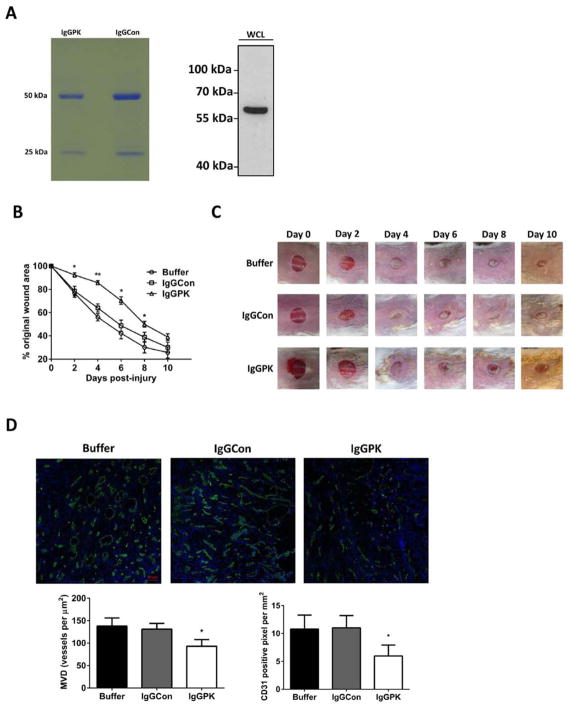
If release of PKM2 by neutrophils to the wound site plays a functional role in wound repair, we would expect that an antibody neutralizing PKM2 would affect wound healing. Thus, we tested effects of an in-house developed rabbit monoclonal antibody that specifically recognize PKM2. The antibody IgG was purified over a protein G/A beads column (Figure 5A). The cutaneous wound was introduced to CD-1 mice. The purified PKM2 antibody IgG was i.p injected (3 mg/kg) to the mice one day before wound induction. The antibody IgG mixed with the Vani-cream cream (0.04% weight) was also topically applied to the wound site. Wound closure was closely monitored. Clearly, the PKM2 antibody slowed down wound repair, especially at early time. As controls, buffer and purified rabbit IgG from pre-immune serum did not exhibit the effects (Figure 5B and C). The results suggested that PKM2 released to the wound site played a functional role in wound healing process. IF staining of tissue sections of the wound site using anti-CD31 antibody revealed that angiogenesis of the wound tissue was inhibited by the treatment of PKM2 antibody (Figure 5D).
Figure 5.
Effects of PKM2 antibody on wound closure and angiogenesis at wound site. (A) (Left) IgG purified from monoclonal antibody IgGPK or rabbit pre-immuno serum (IgGCon) by protein G/A beads. (Right) Specific recognition of PKM2 by the purified IgG of IgGPK in SW480 cell extracts. (B) Wound closure of CD-1 mice that were treated by topical application of indicated agents was monitored. The wound closure is presented as the % of original wound area. Error bars are standard deviations from measurements of six mice. (C) Representative pictures of wound areas at different days of treatment by indicated agents. (D) (Upper) Representative images of immunostaining of wound tissue sections with antibodies against mouse CD31 (Green). The blues are DAPI stains. The wounds were treated by the indicated agents. (Bottom) Quantitative analyses of CD31 staining of the wound sections by manually counting using the software imaging-J. MVD (bottom, left) is vessel density per mm2. The CD31 positive pixel (bottom, right) is measured per mm2. The quantification was average of randomly selected 4 fields in randomly selected 3 sections. Error bars are standard deviations from measurements of six mice.
DISCUSSION
Our study revealed an unexpected role of extracellular PKM2 in facilitating tissue repairing by promoting angiogenesis. Consistent with our previous report that extracellular PKM2 promotes tumor angiogenesis, extracellular PKM2 also plays a role in promoting angiogenesis in tissue regeneration. What is the molecular target for the effects of extracellular PKM2 in promoting angiogenesis has not been revealed. Does extracellular PKM2 targets the same receptor in promoting tumor angiogenesis and angiogenesis in wound healing? In an effort to answer this question, we are currently attempting to probe the possible PKM2 interaction partner(s) with HUVEC cells. Release of PKM2 by secretive pathways, such as degranulation, of activated neutrophils suggests a new and important link connecting the neutrophil infiltration/activation in early inflammation response to late proliferation phase during wound repair. This link provides an excellent explanation for a number of early observations concerning physiological roles of neutrophils in angiogenesis and proliferation during wound repair.10,11,36,37 Each protein secreted by activated neutrophils is targeted to neutrophil secretive vesicles by a specific molecular mechanism.38,39 PKM2 is a cytosolic protein that acts in the glycolysis. How does the cytosolic PKM2 end up in secretive vesicles of activated neutrophils is an unanswered question. Furthermore, what is the trigger to induce the release of PKM2 from neutrophils is an open question. Activated neutrophils secret high amounts of proteases, such as cathepsin G, neutrophil elastase, proteinase 3, and MMPs to degrade microorganisms and tissue debris.40 Apparently, PKM2 released by activated neutrophils, like many neutrophil released cytokines, chemokines, and growth factors,41 escapes from these protease cleavage. How the released PKM2 escapes the cleavage is intriguing. It is well documented that cancer cells release PKM2.20,42,43 Our studies indicate that stress and hypoxia conditions promote PKM2 release from cancer cells (unpublished observations). It is not known whether similar conditions play a role in PKM2 release by neutrophils. Apparently, functional role of extracellular PKM2 in promoting wound healing reveals a potential new therapeutically strategy for promoting wound healing, tissue regeneration, and tissue transplantation. An advantage of using extracellular PKM2 as a therapeutic agent for wound healing is that PKM2 is an intrinsic factor released by neutrophils in wound healing process.
Acknowledgments
We thank Jenny Yang, Ravi Chakra Turaga, Ganesh Satyanarayana for excellent suggestions for our studies. We thank Ms. Birgit Neuhaus for her assistance in microscopic imaging.
Source of Funding: This work is supported in part by research grants from National Institute of Health (CA175112, CA118113) and Georgia Cancer Coalition to Z.R. Liu.
Glossary
- ECM
Extracellular matrix
Footnotes
Conflict of Interest: All authors declare no conflict of interests related to publish of the article.
AUTHOR CONTRIBUTIONS
ZR Liu conceptualized, planned, and coordinated the study. ZR Liu wrote the paper. Yinwei Zhang conducted most of experiments, data analyses, and participated in paper writing; Liangwei Li participated the angiogenesis experiment and data analyses. Yuan Liu performed sucrose gradient isolation of neutrophil fractions. All authors discussed the results and commented on the paper.
References
- 1.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 2.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 3.Broughton G, 2nd, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7 Suppl):1e-S–32e-S. doi: 10.1097/01.prs.0000222562.60260.f9. [DOI] [PubMed] [Google Scholar]
- 4.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69(4):513–21. [PubMed] [Google Scholar]
- 5.Artlett CM. Inflammasomes in wound healing and fibrosis. J Pathol. 2013;229(2):157–67. doi: 10.1002/path.4116. [DOI] [PubMed] [Google Scholar]
- 6.Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol. 2003;73(4):448–55. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- 7.Nishio N, Okawa Y, Sakurai H, Isobe K. Neutrophil depletion delays wound repair in aged mice. Age (Dordr) 2008;30(1):11–9. doi: 10.1007/s11357-007-9043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brubaker AL, Schneider DF, Kovacs EJ. Neutrophils and natural killer T cells as negative regulators of wound healing. Exp Rev Dermatol. 2011;6(1):5–8. doi: 10.1586/edm.10.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson DM, Ross R. The neutrophilic leukocyte in wound repair a study with antineutrophil serum. J Clin Invest. 1972;51(8):2009–23. doi: 10.1172/JCI107007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong Y, Koh DR. Neutrophils promote inflammatory angiogenesis via release of preformed VEGF in an in vivo corneal model. Cell Tissue Res. 2010;339(2):437–48. doi: 10.1007/s00441-009-0908-5. [DOI] [PubMed] [Google Scholar]
- 11.Hubner G, Werner S. Serum growth factors and proinflammatory cytokines are potent inducers of activin expression in cultured fibroblasts and keratinocytes. Exp Cell Res. 1996;228(1):106–13. doi: 10.1006/excr.1996.0305. [DOI] [PubMed] [Google Scholar]
- 12.Elbers JR, van Unnik JA, Rijksen G, van Oirschot BA, Roholl PJ, Oosting J, et al. Pyruvate kinase activity and isozyme composition in normal fibrous tissue and fibroblastic proliferations. Cancer. 1991;67(10):2552–9. doi: 10.1002/1097-0142(19910515)67:10<2552::aid-cncr2820671027>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 13.Hacker HJ, Steinberg P, Bannasch Pyruvate kinase isoenzyme shift from L-type to M2-type is a late event in hepatocarcinogenesis induced in rats by a choline-deficient/DL-ethionine-supplemented diet. Carcinogenesis. 1998;19(1):99–107. doi: 10.1093/carcin/19.1.99. [DOI] [PubMed] [Google Scholar]
- 14.Dombrauckas JD, Santarsiero BD, Mesecar AD. Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis. Biochemistry. 2005;44(27):9417–29. doi: 10.1021/bi0474923. [DOI] [PubMed] [Google Scholar]
- 15.Gao X, Wang H, Yang JJ, Liu X, Liu ZR. Pyruvate kinase m2 regulates gene transcription by acting as a protein kinase. Mol Cell. 2012;45(5):598–609. doi: 10.1016/j.molcel.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo W, Hu H, Chang R, Zhong J, Knabel M, O’Meally R, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145(5):732–44. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang W, Xia Y, Ji H, Zheng Y, Liang J, Huang W, et al. Nuclear PKM2 regulates beta-catenin transactivation upon EGFR activation. Nature. 2011;480(7375):118–22. doi: 10.1038/nature10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider J. Tumor markers in detection of lung cancer. Adv Clin Chem. 2006;42:1–41. doi: 10.1016/s0065-2423(06)42001-1. [DOI] [PubMed] [Google Scholar]
- 19.Ugurel S, Bell N, Sucker A, Zimpfer A, Rittgen W, Schadendorf D. Tumor type M2 pyruvate kinase (TuM2-PK) as a novel plasma tumor marker in melanoma. Int J Cancer. 2005;117(5):825–30. doi: 10.1002/ijc.21073. [DOI] [PubMed] [Google Scholar]
- 20.Weinberger R, Appel B, Stein A, Metz Y, Neheman A, Barak M. The pyruvate kinase isoenzyme M2 (Tu M2-PK) as a tumour marker for renal cell carcinoma. Eur J Cancer Care (Engl) 2007;16(4):333–7. doi: 10.1111/j.1365-2354.2006.00753.x. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Zhang Y, Qiao J, Yang JJ, Liu ZR. Pyruvate kinase M2 in blood circulation facilitates tumor growth by promoting angiogenesis. J Biol Chem. 2014;289:25812–21. doi: 10.1074/jbc.M114.576934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeffery J, Lewis SJ, Ayling RM. Fecal dimeric M2-pyruvate kinase (tumor M2-PK) in the differential diagnosis of functional and organic bowel disorders. Inflamm Bowel Dis. 2009;15(11):1630–4. doi: 10.1002/ibd.20946. [DOI] [PubMed] [Google Scholar]
- 23.Staib P, Hoffmann M, Schinkothe T. Plasma levels of tumor M2-pyruvate kinase should not be used as a tumor marker for hematological malignancies and solid tumors. Clin Chem Lab Med. 2006;44(1):28–31. doi: 10.1515/CCLM.2006.006. [DOI] [PubMed] [Google Scholar]
- 24.Hathurusinghe HR, Goonetilleke KS, Siriwardena AK. Current status of tumor M2 pyruvate kinase (tumor M2-PK) as a biomarker of gastrointestinal malignancy. Ann Surg Oncol. 2007;14(10):2714–20. doi: 10.1245/s10434-007-9481-x. [DOI] [PubMed] [Google Scholar]
- 25.Gao X, Wang H, Yang JJ, Chen J, Jie J, Li L, et al. Reciprocal regulation of protein kinase and pyruvate kinase activities of pyruvate kinase m2 by growth signals. J Biol Chem. 2013;288(22):15971–9. doi: 10.1074/jbc.M112.448753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Zhang Y, Qiao J, Yang JJ, Liu ZR. Pyruvate kinase M2 in blood circulation facilitates tumor growth by promoting angiogenesis. J Biol Chem. 2014;289(37):25812–21. doi: 10.1074/jbc.M114.576934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5(1):40–6. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 28.Lominadze G, Powell DW, Luerman GC, Link AJ, Ward RA, McLeish KR. Proteomic analysis of human neutrophil granules. Mol Cell Proteomics. 2005;4(10):1503–21. doi: 10.1074/mcp.M500143-MCP200. [DOI] [PubMed] [Google Scholar]
- 29.Etiemble J, Boivin P. Pyruvate kinase isozymes among human organs and blood cells. Enzyme. 1976;21(4):296–303. doi: 10.1159/000458872. [DOI] [PubMed] [Google Scholar]
- 30.Chen CX, Soto I, Guo YL, Liu Y. Control of secondary granule release in neutrophils by Ral GTPase. J Biol Chem. 2011;286(13):11724–33. doi: 10.1074/jbc.M110.154203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zen K, Guo YL, Li LM, Bian Z, Zhang CY, Liu Y. Cleavage of the CD11b extracellular domain by the leukocyte serprocidins is critical for neutrophil detachment during chemotaxis. Blood. 2011;117(18):4885–94. doi: 10.1182/blood-2010-05-287722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeuchi K, Wood H, Swank RT. Lysosomal elastase and cathepsin G in beige mice. Neutrophils of beige (Chediak-Higashi) mice selectively lack lysosomal elastase and cathepsin G. J Exp Med. 1986;163(3):665–77. doi: 10.1084/jem.163.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeuchi KH, Swank RT. Inhibitors of elastase and cathepsin G in Chediak-Higashi (beige) neutrophils. J Biol Chem. 1989;264(13):7431–6. [PubMed] [Google Scholar]
- 34.Gardi C, Cavarra E, Calzoni P, Marcolongo P, de Santi M, Martorana PA, et al. Neutrophil lysosomal dysfunctions in mutant C57 Bl/6J mice: interstrain variations in content of lysosomal elastase, cathepsin G and their inhibitors. Biochem J. 1994;299(Pt 1):237–45. doi: 10.1042/bj2990237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pettipher R, Edwards J, Cruwys S, Jessup E, Beesley J, Henderson B. Pathogenesis of antigen-induced arthritis in mice deficient in neutrophil elastase and cathepsin G. Am J Pathol. 1990;137(5):1077–82. [PMC free article] [PubMed] [Google Scholar]
- 36.Ma Y, Yabluchanskiy A, Lindsey ML. Neutrophil roles in left ventricular remodeling following myocardial infarction. Fibrogenesis Tissue Repair. 2013;6(1):11. doi: 10.1186/1755-1536-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mori R, Shaw TJ, Martin P. Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J Exp Med. 2008;205(1):43–51. doi: 10.1084/jem.20071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dahlgren C, Karlsson A, Sendo F. Neutrophil secretory vesicles are the intracellular reservoir for GPI-80, a protein with adhesion-regulating potential. J Leukoc Biol. 2001;69(1):57–62. [PubMed] [Google Scholar]
- 39.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5(14):1317–27. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol. 2006;6(7):541–50. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 41.Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Front Immunol. 2014;5:508. doi: 10.3389/fimmu.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed AS, Dew T, Lawton FG, Papadopoulos AJ, Devaja O, Raju KS, et al. M2-PK as a novel marker in ovarian cancer. A prospective cohort study. Eur J Gynaecol Oncol. 2007;28(2):83–8. [PubMed] [Google Scholar]
- 43.Schneider J, Neu K, Grimm H, Velcovsky HG, Weisse G, Eigenbrodt E. Tumor M2-pyruvate kinase in lung cancer patients: immunohistochemical detection and disease monitoring. Anticancer Res. 2002;22(1A):311–8. [PubMed] [Google Scholar]