Abstract
Individuals with chronic temporal lobe epilepsy (TLE) experience episodic memory deficits that may be progressive in nature. These memory decrements have been shown to increase with the extent of hippocampal damage, a hallmark feature of TLE. Pattern separation, a neural computational mechanism thought to play a role in episodic memory formation, has been shown to be negatively affected by aging and in individuals with known hippocampal dysfunction. Despite the link between poor pattern separation performance and episodic memory deficits, behavioral pattern separation has not been examined in patients with TLE. We examined pattern separation performance in a group of 22 patients with medically-refractory TLE and 20 healthy adults, using a task hypothesized to measure spatial pattern separation with graded levels of spatial interference. We found that individuals with TLE showed less efficient spatial pattern separation performance relative to healthy adults. Poorer spatial pattern separation performance in TLE was associated with poorer visuospatial memory, but only under high interference conditions. In addition, left hippocampal atrophy was associated with poor performance in the high interference condition in TLE. These data suggest that episodic memory impairments in patients with chronic, refractory TLE may be partially due to less efficient pattern separation, which likely reflects their underlying hippocampal dysfunction.
Keywords: episodic memory, hippocampal dysfunction, medial temporal lobe, progressive memory decline, spatial memory
1.1. Introduction
Temporal lobe epilepsy (TLE) is the most common treatment resistant partial epilepsy in adults (Engel, 1996). Hippocampal dysfunction is a hallmark feature of refractory TLE, and is often characterized by neuronal loss within the hippocampus and surrounding medial temporal lobe structures (de Lanerolle, et al., 1989). Due to the role of the hippocampus in memory, the most common cognitive deficits observed in patients with TLE are in episodic memory (Hermann, et al., 1997; Viskontas, et al., 2000). These deficits can be quite debilitating and have been shown to increase with the extent of hippocampal damage or following surgical resection of an intact hippocampus (for review see Helmstaedter, 2013; Lee, et al., 2002).
Converging evidence from animal and human studies indicates that pattern separation is a potential neural mechanism that might facilitate accurate memory encoding (for review see Kesner & Rolls, 2015; Yassa & Stark, 2011). Pattern separation is involved in the orthogonalization of partially overlapping neural representations and may allow for one pattern (or episode) to be retrieved separately from another similar pattern, increasing the potential for successful encoding and subsequent retrieval (O'Reilly & McClelland, 1994; O'Reilly & Norman, 2002). Based on animal models and human imaging studies, pattern separation may depend largely on the integrity of the hippocampus, and specifically, the dentate gyrus (Wesnes, et al., 2014) and CA3 subfields (for review see Gilbert and Brushfield (2009); (Gilbert & Kesner, 2006; Kesner & Rolls, 2015; Yassa & Stark, 2011). Therefore, it is possible that disrupted pattern separation may contribute to episodic memory deficits in patients with known hippocampal dysfunction, including TLE.
To date, most studies of pattern separation have focused on impairments associated with normal or pathological aging (for review see Gilbert, et al., 2016). In particular, several studies have demonstrated that pattern separation performance declines with typical aging (Holden, et al., 2012; Stark, et al., 2010) and that this age-related decline reflects hippocampal structural and functional changes (Doxey & Kirwan, 2015; Yassa, Lacy, et al., 2011; Yassa, Mattfeld, et al., 2011). Although spatial memory has been shown to decline with age across different species (Light & Zelinski, 1983; Park, et al., 1982; Perlmutter, et al., 1981), only a few studies have examined age-related declines in spatial pattern separation performance (Holden, et al., 2012; Reagh, et al., 2016; Reagh, et al., 2014; Stark, et al., 2010). Deficits in pattern separation performance have also been reported in patients with Alzheimer’s disease (AD) and mild cognitive impairment (MCI) (Ally, et al., 2013; Stark, et al., 2013; Yassa, et al., 2010) and these deficits have been linked directly to hippocampal dysfunction in both patient groups (Wesnes, et al., 2014; Yassa, et al., 2010). Overall, these studies demonstrate that disrupted pattern separation may contribute to visuospatial memory impairments, and that these deficits are likely associated with hippocampal dysfunction.
Despite increasing evidence that poor pattern separation contributes to episodic memory impairments in older adults and other patient populations with known hippocampal damage, and the high incidence of episodic memory impairment and hippocampal atrophy in TLE (de Lanerolle, et al., 1989; Hermann, et al., 1997), there are no current studies examining pattern separation performance in patients with TLE. In the present study, we examine the performance of patients with TLE on a previously published task hypothesized to place high demands upon spatial pattern separation (Holden, et al., 2012; Sheppard, et al., 2016). The test was based on a task developed in the mid 1990s for use in animal models (Gilbert, et al., 1998; Gilbert, et al., 2001). This was one of the first tests developed to assess pattern separation on a behavioral level. The test was found to be dependent on functioning of the hippocampus (Gilbert, et al., 1998) and specifically the DG (Gilbert, et al. (2001) and CA3 subregions (Gilbert & Kesner, 2006).
We compare the performance of patients with medically-refractory TLE to healthy, age- and education-matched adults on a delayed match-to sample task for spatial location, with graded levels of interference. We hypothesized that our patients would perform more poorly than healthy adults. In addition, we hypothesized that poorer performance on the task would be associated with hippocampal atrophy, particularly in task conditions that place greater demands on pattern separation (i.e., high spatial interference).
1.2 Methods
1.2.1 Participants
This study was approved by the Institutional Review Board at the University of California, San Diego (UC San Diego) and San Diego State University. Informed consent was collected from all participants in accordance to the Declaration of Helsinki. Twenty-two patients with medically-refractory TLE met inclusion/exclusion criteria for the study. Inclusion criterion included a TLE diagnosis, unilateral seizure onset, and age between 18–65 years of age. Patients were excluded if they had any MRI-visible lesions besides mesial temporal sclerosis (MTS) or if there was evidence on video-EEG of multifocal seizure onset. No patients showed evidence of bilateral seizure onset. All TLE patients were recruited through referral from the UC San Diego Epilepsy Center. All patients were under surgical evaluation and were diagnosed by board-certified neurologists with expertise in epileptology in accordance with the criteria defined by the International League Against Epilepsy (Kwan, et al., 2010). Primary seizure onset was determined by video-EEG telemetry, seizure semiology, and neuroimaging evaluation. The presence of MTS was determined by inspection of MRI images by a board-certified neuroradiologist. Of the 22 patients, 13 were diagnosed with right TLE (RLTE; 5 with MTS) and 9 were diagnosed with left TLE (LTLE; 6 with MTS). Twenty healthy adults with no reported history of neurological or psychiatric disorder and who were age and education matched to the patient group were included.
1.2.2 Spatial Pattern Separation Task
All participants were administered a delayed match-to-sample task for spatial location involving four degrees of spatial interference to tax spatial pattern separation (Holden, et al., 2012; Sheppard, et al., 2016). In the current study, the participant was seated approximately 40 cm in front of a computer monitor. A 15 cm black border was affixed around the perimeter of the screen to eliminate the possibility of using visual cues to remember the spatial location of the stimuli. Each trial consisted of a sample phase followed by a choice phase. During the sample phase, a gray circle measuring 1.7 cm in diameter appeared on the computer screen for 5 s. The circle appeared in one of 18 possible locations within a fixed non-visible horizontal line across the middle of the screen. The participant was instructed in advance to remember the location of the gray circle on the screen. There was a 10 s delay between the sample phase and choice phase during which participants were required to look away from the screen and read a string of random letters to prevent the participant from fixating the eyes on the location of the sample phase circle. After the 10 s delay, a tone was presented to signal the start of the choice phase. During the choice phase, two circles were displayed, one red and one blue. One of the colored circles (target) was in the same location as the original gray circle from the sample phase (correct choice). The foil circle was in a location that was either to the left or the right of the target circle (incorrect choice). There were four possible spatial separations that were used to separate the target and foil circles during the choice phase: 0 cm (edges of each circle were touching), 0.5 cm, 1.0 cm, and 1.5 cm. During the choice phase, the participant was asked to indicate which colored circle (red or blue) was in the same location as the gray circle from the sample phase by stating its color.
There were a total of 48 trials for the task, consisting of 12 trials for each of the four spatial separations. Each group of 12 trials was balanced across the entire width of the screen to ensure that there was not an unintentional bias toward one particular area on the screen. To minimize fatigue effects, the 48 trials were split into two sets of 24 trials, with a break in between. The two sets were identical in design. The order of each set of 24 trials was pseudo-randomized, with the restriction that there were no more than two consecutive trials of the following: (1) spatial separation, (2) correct circle on a particular side, or (3) correct circle of a particular color.
1.2.3 MRI Acquisition
Structural data were available for all TLE patients. MRI data were collected on a General Electric Discovery MR750 3T scanner with an 8-channel phased-array head coil at the Center for Functional MRI at UC San Diego. Image acquisition included a conventional three-plane localizer, GE calibration scan, a T1-weighted 3D customized FSPGR structural sequence (TR = 8.08 ms, TE = 3.16 ms, TI = 600 ms, flip angle = 8°, voxel size 1 × 1.33 × 1.2, FOV = 256 mm, matrix = 256 × 192, slice thickness = 1.2 mm, 172 slices).
1.2.3.1 Hippocampal segmentation
Individual T1-weighted images were processed with the FreeSurfer (v5.3.0) software package (http://surfer.nmr.mgh.harvard.edu) for semi-automated segmentation and labeling of the hippocampus (Fischl, et al., 2002). Quality control of all images was performed in concordance with the standardized ENIGMA subcortical segmentation protocol (http://enigma.ini.usc.edu/ongoing/enigma-epilepsy/enigma-epilepsy-protocols/). In addition, for this project, careful inspection of all hippocampi was performed by a trained image analyst by overlaying the subcortical segmentation on their T1-weighted images and visually checking for evidence of inaccurate segmentation. In no case were errors in segmentation identified by visual inspection and all patient MRIs were retained in the analysis. In order to control for differences in brain size, we regressed out intracranial volume (ICV), sex, and age from both left and right hippocampal volume.
1.2.4 Neuropsychological Measures
Patients with TLE and healthy adults completed the Brief Visuospatial Memory Test-Revised (BVMT-R; Benedict, 1997) to evaluate visuospatial episodic memory and the Benton Judgment of Line Orientation Test (JoLO; Benton, et al., 1994) to evaluate visuospatial perceptual skills. The BVMT-R consists of six geometric designs that are shown for a period of 10 seconds (Benedict, 1997). Subjects are asked to reproduce the designs immediately after each trial, and scores are summed across three trials to obtain an immediate recall score. Subjects are asked to reproduce the designs following a 25–30 minute delay in order to obtain a “delayed recall” score. Immediate recall and delayed recall scores from the BVMT-R were used in the analysis given their sensitivity to memory dysfunction and hippocampal atrophy (Benedict & Groninger, 1995; Bouchard, et al., 2008; Troyer, et al., 2008). In addition, both groups completed the Wechsler Memory Scale-III Spatial Span subtest (WMS-III Spatial Span; Wechsler, 1987) to evaluate spatial working memory; the WMS-III Logical Memory subtest to evaluate verbal episodic memory, and the Wechsler Test of Adult Reading (WTAR) to estimate premorbid intellectual functioning,
1.2.5 Statistical Analysis
A 3 × 4 mixed analysis of covariance (ANCOVA) model with group (TLE, healthy adults) as the between-subject factor and spatial separation (0 cm, 0.5 cm, 1 cm, 1.5 cm) as the within-subject factor was used to analyze percent of correct responses on the spatial pattern separation task. Given reported sex (Moffat, et al., 1998; Yagi, et al., 2016) and age (Holden & Gilbert, 2012) differences in spatial memory and pattern separation, we included sex and age as covariates. Analysis of variance (ANOVA), independent t-tests and Fisher’s Exact test were used to test differences in demographic variables. Effect sizes were calculated using Cohen’s d. Correction for false discovery rate (FDR) was used to control for multiple comparisons.
1.3 Results
1.3.1 Participant demographic and neuropsychological data
There were no differences in age or education between TLE patients and healthy adults [Age: t (40) = 1.75, p = 0.088; Education: t (40) = −1.05, p = 0.300] (See Table 1). There were no differences in the distribution of sex (χ2 = 0.287, Fisher’s Exact p = 0.730). Impairment was defined using two clinically-relevant cut-offs - 1.0 and 1.5 standard deviation below the normative mean. Based on the 1.5 standard deviation cut-off, seven TLE patients were impaired on the BVMT -R immediate recall and three patients were impaired on the BVMT- delayed recall. Based on the 1.0 standard deviation cut-off, 11 patients were impaired on the BVMT -R immediate recall and four patients were impaired on the BVMT- delayed recall (Table 2).
Table 1.
Demographics and clinical variables
| TLE Patients | Healthy Controls | |||
|
|
||||
| n | 22 | 20 | ||
| Sex: M/F | 5/17 | 6/14 | ||
| Onset side: L/R | 9/13 | |||
| MTS: Yes/No | 11/11 | |||
|
|
||||
| Range | Mean (SD) | Range | Mean (SD) | |
|
|
||||
| Age | 19–64 | 39.4 (13.31) | 19–60 | 32.85 (10.69) |
| Education | 8–20 | 14.63 (2.63) | 12–20 | 15.4 (2.01) |
| Disease duration | 2–48 | 17.36 (16.67) | ||
| Age of seizure onset | 2–61 | 21.95 (15.57) | ||
TLE: temporal lobe epilepsy; M: males; F: females; L: left; R: right; MTS: mesial temporal sclerosis; SD: standard deviation; means and standard deviations are represented in years
Table 2.
Neuropsychological variables
| TLE | Healthy adults | |
|---|---|---|
|
|
||
| Mean (SD) | Mean (SD) | |
|
|
||
| BVMT-R Immediate* | 22.40 (4.69) | 26.2 (5.88) |
| BVMT-R Delayed | 9.18 (2.06) | 9.6 (2.04) |
| JoLO* | 21.3 (4.59) | 25.16 (3.78) |
| Spatial Span backwards | 8.09 (1.66) | 8.75 (1.98) |
| Logical Memory I* | 34.65 (9.42) | 43.09 (4.59) |
| Logical Memory II* | 20.55 (6.76) | 27.45 (5.87) |
| WTAR | 33.14 (8.17) | 39.27 (13.35) |
Indicates significant difference between patients and healthy controls
BVMT-R: Brief Visuospatial Memory Test-Revised; JoLO: Benton Judgment of Line Orientation Test total correct; WTAR: Wechsler Test of Adult Reading TLE: temporal lobe epilepsy
1.3.2 Group differences in spatial pattern separation performance
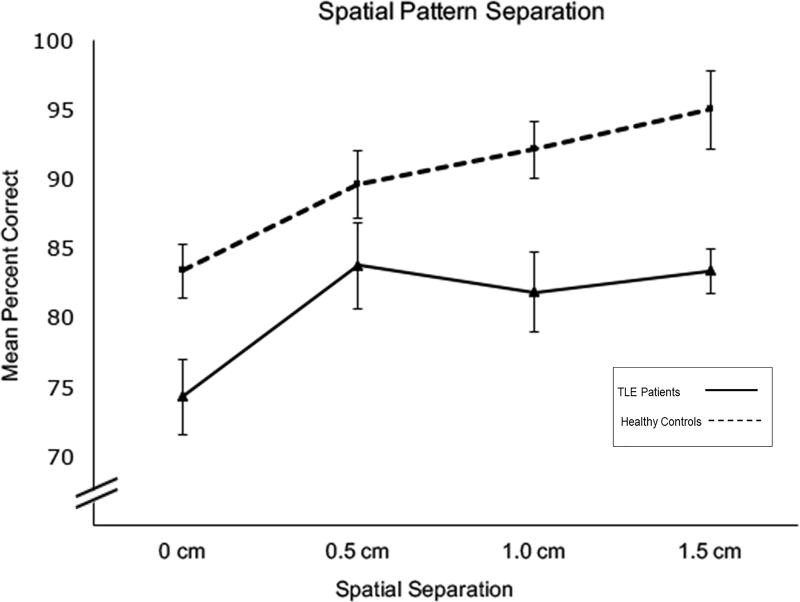
The ANCOVA revealed a significant main effect of group, F (1, 38) = 7.59, p = 0.009, with TLE patients performing worse than the healthy control group (see Fig. 1). There was also a significant main effect of spatial separation, Greenhouse-Geisser F (2.87, 109.112) = 3.022, p = 0.033. There was no significant spatial separation by group interaction F (2.87, 109.112) = 1.232, p = 0.301, nor was there a spatial separation by sex interaction F (2.87, 109.112) = 0.446, p = 0.712 or a spatial separation by age interaction, F (2.87, 109.112) = 1.221, p = 0.309. Planned polynomial contrasts revealed a significant linear effect of spatial separation, F (1, 38) = 4.639, p =0.038. Neither sex (p = 0.753) nor age (p = 0.157) were significant covariates in the model. Smaller spatial separations between stimuli have been hypothesized to result in greater interference and demand for pattern separation than larger separations. As shown in Fig. 1, as the spatial separation increased, performance improved for both groups. Although the pattern in Fig. 1 appears to reveal a slight increase in performance for the TLE group at the 0.5 cm separation, a pair-wise t-test revealed no significant differences between percent correct at the 0.5 cm and 1 cm separations (t (22) = 0.865, p = 0.397). However, in the TLE group there was a significant difference between performance on the 0 cm and 1.5 cm separations (t (22) = −3.716, p = 0.001). The group performed significantly better on 1.5 cm separation (lowest pattern separation demands) compared to the 0 cm separation (highest pattern separation demands). Effect sizes differences between the TLE group and healthy controls at each spatial separation were the following: 0 cm: d =0.85; 0.5 cm: d =0.45; 1.0 cm: d =0.88; 1.5 cm: d =1.07.
Figure 1.
Mean percent correct performance across four spatial separation conditions. Error bars represent standard error.
To facilitate comparisons with neuropsychological and neuroimaging data, we created “high” and “low” spatial interference conditions by collapsing across the 0 and 0.5 cm spatial separation trials (high interference) and collapsing across the 1.0 and 1.5 cm spatial separation trials (low interference), an approach used in prior studies (e.g., Rotblatt, et al., 2015). This resulted in an increase in the number of trials per interference condition, providing a more robust measure. Effect sizes for group differences at the high and low interference conditions were calculated using Cohen’s d. In the high interference condition, there was a medium effect size between TLE patients and healthy adults (d = 0.72) and a large effect size in the low interference condition (d =1.17).
1.3.3 Relationship between TLE-related clinical variables and pattern separation performance
Differences in task performance between RTLE and LTLE and MTS and non-MTS patients were assessed using Mann-Whitney U tests due to small sample size. There were no differences between RTLE and LTLE patients in any of the four spatial separations (0 cm: U = 49.0, p = 1.0; 0.5 cm: U = 43.0, p = 0.609; 1.0 cm: U = 41.5, p = 0.55; 1.5 cm: U = 37.5, p = 0.37). There were no differences between patients with MTS and those without MTS in any of the four spatial separations (0 cm: U = 49.5, p = 0.478; 0.5 cm: U = 58.0, p = 0.898; 1.0 cm: U = 53.5, p = 0.652; 1.5 cm: U = 41.0, p = 0.227). To determine the unique contribution of epilepsy-related variables (e.g., disease duration, age of seizure onset) on task performance in the high and low interference conditions, multiple linear regressions were conducted. Neither disease duration nor age of seizure onset significantly contributed to performance on the high interference condition (age of onset = −0.010, p = 0.854; duration = −0.061, p = 0.259) or low interference condition (age of onset = −0.003, p = 0.956; duration = −0.043, p = 0.397).
1.3.4 Relationship between Neuropsychological Measures and Spatial Pattern Separation in Patients with TLE and Healthy Controls
We conducted partial correlations controlling for age, to examine relationships between raw scores on neuropsychological measures and performance in the high and low interference conditions in TLE patients and healthy adults. In TLE patients, poorer performance in the high spatial interference condition was associated with lower scores on BVMT-R delayed recall and with lower scores on Spatial Span backwards (see Table 3). Given that Spatial Span backwards was correlated with performance on the high interference condition, we also ran a partial correlation analysis between scores on the BVMT-R delayed recall and the high interference condition controlling for Spatial Span backwards, and this association remained significant (Partial r = 0.496, p = 0.026). In healthy adults, poorer performance in the high spatial interference condition was associated with lower scores on the BVMT-R delayed recall.
Table 3.
Partial Pearson’s r correlations between highest and lowest interference conditions and neuropsychological measures
| TLE | Healthy Adults | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| High Interference | Low Interference | High Interference | Low Interference | |||||
|
|
|
|||||||
| r | p-value | r | p-value | r | p-value | r | p-value | |
|
|
|
|||||||
| BVMT-R Immediate | 0.256 | 0.263 | 0.232 | 0.311 | 0.425 | 0.070 | 0.021 | 0.933 |
| BVMT-R Delayed | 0.509 | 0.018* | 0.271 | 0.234 | 0.532 | 0.019* | −0.099 | 0.688 |
| JoLO | 0.410 | 0.081 | 0.269 | 0.265 | 0.166 | 0.511 | 0.464 | 0.052 |
| Spatial Span backwards | 0.564 | 0.008* | 0.415 | 0.061 | 0.257 | 0.445 | 0.301 | 0.369 |
Partial Pearson’s r correlations controlling for age
Indicates statistical significant with FDR correction at α = 0.05;
High Interference indicates 0cm and 0.5cm spatial separation conditions and Low Interference indicates 1.0cm and 1.5cm spatial separation condition; BVMT-R: Brief Visuospatial Memory Test-Revised; JoLO: Benton Judgment of Line Orientation Test total correct; TLE: temporal lobe epilepsy
1.3.5 Relationship between Hippocampal Volume and Spatial Pattern Separation
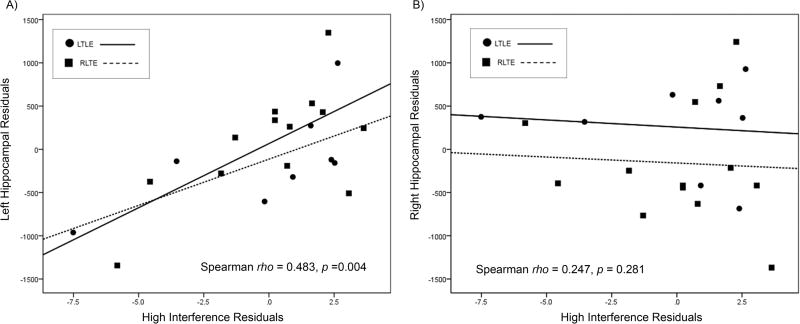
Hippocampal volumes were available for all TLE patients. No participants were excluded due to poor hippocampal segmentation. One patient was identified as a significant outlier (left hippocampal volume was greater than three standard deviations below the mean of the entire group) and was removed from the correlation analysis. Intracranial volume, sex and age were regressed out from hippocampal volumes; age and sex were regressed out from the spatial pattern separation task scores. These residuals were then used to examine the relationship between left and right hippocampal volumes and performance in both the high and the low interference conditions. Lower left hippocampal volume was associated with poorer performance on the high interference condition (Spearman rho = 0.483, p = 0.004) (Fig. 2). There was no significant association between left hippocampal volume and the low interference condition (Spearman rho = 0.247 p = 0.281); right hippocampal volume was not associated with performance on the high interference condition (Spearman rho = −0.01, p = 0.964) or low interference condition (Spearman rho = −0.113, p = 0.626).
Figure 2.
Relationship between left and right hippocampal volume and performance in the high interference condition in TLE patients. The y-axis represents residuals from hippocampal volumes with covariates of intracranial volume, age and sex. The x-axis represents residuals from pattern separation task performance with covariates of age and sex. Better performance in the high interference condition was associated with higher left hippocampal volume.
To determine the impact of side of seizure onset and MTS status on the association between hippocampal volume and performance on the high interference condition, we ran partial correlations on the residuals controlling for MTS status and side of seizure onset. Lower left hippocampal atrophy was still associated with lower performance on the high interference condition (partial r = 0.668, p = 0.002). Right hippocampal volume was not associated with performance on the high interference condition (partial r = 0.075, p = 0.760).
1.4 Discussion
We present the first data on pattern separation performance in patients with TLE using a previously published behavioral spatial pattern separation task that has been linked to episodic memory decline in older adults (Holden, et al., 2012; Sheppard, et al., 2016). Our data revealed three novel findings: (1) individuals with TLE show impaired spatial pattern separation performance relative to healthy age- and education-matched adults (2) poor spatial pattern separation performance in TLE is associated with visuospatial episodic memory impairment; and (3) under high spatial interference conditions, pattern separation performance is associated with the extent of left hippocampal atrophy in TLE. These findings reinforce the idea that episodic memory deficits in TLE may, in part, reflect less efficient hippocampal-dependent pattern separation processes.
Episodic memory deficits are among the most common cognitive impairments associated with TLE (Helmstaedter, et al., 2003). As noted above, the most striking finding in this study is that on a task hypothesized to assess spatial pattern separation, patients with TLE performed significantly worse than a demographically similar group of healthy adults. Like the healthy controls, patients with TLE showed increased accuracy as the level of interference decreased. In addition, associations between pattern separation performance and delayed recall on the BVMT remained significant even after controlling for spatial attention/working memory performance. These data suggest that poor pattern separation in TLE, may help explain the episodic memory impairments commonly found in this clinical population (Hermann, et al., 1997).
Our current findings in TLE are highly consistent with those of Holden and colleagues (2012), which utilized the same delayed-match-to-sample task in older adults. In both studies, performance improved as the level of spatial separation increased, thus decreasing the demand for pattern separation. In addition, both groups exhibited poorer accuracy relative to the healthy young control group of comparison. As previously mentioned, in our study effect sizes for group differences were medium to large between TLE patients and healthy adults for both high and low interference conditions, respectively. In fact, these effect sizes appear larger than the effect sizes between young and older adults reported by Holden et al. (2012). In another study using the same task, Sheppard et al. (2016) found that a group of cognitively intact older adult carriers of the apolipoprotein epsilon E4 allele (APOE-ε4) performed worse than APOE-ε4 non-carriers and younger adults. These studies provide further evidence that this task is sensitive to spatial pattern separation deficits in patients with known hippocampal injury, as well as those at risk for hippocampal dysfunction and subsequent memory decline. Thus, spatial pattern separation tasks could be used in conjunction with standard neuropsychological measures to improve the detection of memory impairment and/or identify individuals at highest risk for future memory decline.
A second key finding in this study is that poorer pattern separation performance was associated with lower visuospatial memory scores in both patients with TLE and healthy adults. Specifically, in patients with TLE poorer pattern separation under high levels of interference was associated with poorer spatial working memory and delayed visuospatial memory. However, the association between poor spatial pattern separation and delayed visuospatial memory in patients with TLE does not seen to be accounted for by deficits in spatial attention/working memory. In healthy adults, poorer performance in the high interference condition was also associated with poorer performance on delayed visuospatial memory. It is of interest that only the TLE group seemed to rely partially on spatial working memory abilities to complete the task. Previous studies have shown an association between poorer spatial working memory and poorer pattern separation performance under high levels of interference in older adults (DeFord, et al., 2016). Notably, although there were trends between pattern separation performance and a test of basic visuospatial processing, these associations were not significant, therefore, visuospatial processing was not driving pattern separation performance. These data suggest that the association between spatial pattern separation performance and visuospatial memory is not unique to TLE, but may also capture some of the individual variability in visuospatial memory observed in healthy populations.
A third novel finding of this study was an association between poorer pattern separation and left hippocampal volume loss in TLE. Although this may seem counterintuitive given the data implicating the right hippocampus in visuospatial memory (Abrahams, et al., 1999; Bohbot, et al., 1998; Maguire, et al., 1997; Milner, 1965), the relationship between hippocampal involvement and pattern separation performance has been inconsistent. Some studies have reported an association between pattern separation and the right hippocampus (Motley & Kirwan, 2012), whereas others have reported an association with the left hippocampus (Doxey & Kirwan, 2015). In a recent resting-state fMRI study, Doxey and Kirwan (2015) found that greater left CA3/DG hippocampal subfield activations were associated with better pattern separation performance on an object mnemonic discrimination task. The authors suggested that this association could be attributed to the type of mnemonic strategy the participants used during the task. In addition, although verbal and visuospatial memory were classically thought to be segregated and rely on the left and right hippocampus, respectively, there is now sufficient evidence from both clinical and experimental studies that the concept of “material-specificity” is oversimplified and that visuospatial memory may rely on both hippocampi (Saling, 2009). In fact, Kumaran and Maguire (2007) found that the left hippocampus was primarily recruited during a visuospatial memory task that involved either a match or mismatch of spatial and temporal components. In addition, in an fMRI study, Igloi, et al. (2010) found greater activation of the left hippocampus when subjects used egocentric strategies to solve a spatial navigation task, whereas the right hippocampi was involved when allocentric or map-based navigation strategies were employed. Although the reasons that we found an association with left hippocampal volume in our study is not clear, it is possible that one or more processes involved in visuospatial pattern separation or the strategies employed to perform the task, place greater demands on the left hippocampus than the right.
In this study, we calculated total right and left hippocampal volumes. Despite the association with left hippocampal volume, we did not find an association between right hippocampal volume and spatial pattern separation performance. However, given increasing evidence that pattern separation depends largely on the integrity of CA3/DG (Doxey & Kirwan, 2015; Gilbert & Brushfield, 2009; Gilbert & Kesner, 2006; Yassa & Stark, 2011), a more enriched analysis would focus on the relationship between pattern separation and volume loss or loss of dendritic microstructure within hippocampal subfields, as well as their afferent and efferent connections. Recent data using ultrahigh-resolution resting-state fMRI and DTI in older adults has shown that altered functional activation within CA3/DG during pattern separation (i.e., “behavioral rigidity”) was associated with reduced integrity of CA3/DG coupled with compromise to the primary afferent input to these subfields from the entorhinal cortex (i.e., the perforant path) (Yassa, Mattfeld, et al., 2011). Thus, an analysis that focuses on structural and microstructural changes within a broader hippocampal network in TLE, including the entorhinal cortex and perforant path, may further explain pattern separation deficits in TLE and other neurological disorders. This type of subfield analysis may also unveil an association between one or more right hippocampal subfields and spatial pattern separation performance that was not revealed when examining whole right hippocampal volume. In addition, although the association between left hippocampal volume and pattern separation performance was significant while controlling for MTS status and side of seizure onset, a subfield analysis may reveal subtler differences among patients with distinctive TLE clinical characteristics (i.e., RTLE vs. LTLE, MTS positive vs MTS negative).
Several limitations of this study should be addressed. First, it is important to note that pattern separation is a process that occurs at a neural level. Although there are a growing number of studies that examine pattern separation on a behavioral level, we must be cautious when attributing results to deficits in pattern separation as this cannot be fully validated at the behavioral level with any task. Second, we did not find a significant group by spatial separation interaction; instead we found that patients with TLE show a similar increase in performance across separation relative to the healthy adult group, but with poorer accuracy across all conditions. In fact, the greatest group differences were found on the largest separation condition. It is of note that this exact same pattern of performance has been demonstrated in previous studies examining pattern separation performance in normal and pathological aging (Holden, et al., 2012; Sheppard, et al., 2016; Stark, et al., 2010). Thus, although a group by level of interference interaction would appear to provide the most robust evidence of impaired pattern separation in TLE, this finding may not be necessary and/or the relationship may be more general. That is, the association between pattern separation performance and memory performance may capture individual variability present in healthy populations, which is exacerbated in the face of certain disease pathologies. Third, although our study focuses on the potential contribution of poor pattern separation to impaired visuospatial memory in TLE, impairments in other processes such as pattern completion may also have contributed to lower visuospatial memory performance. Fourth, our sample size was too small to divide the patients into LTLE and RTLE groups or between MTS negative and MTS positive. However, there were no significant differences in performance across levels of interference between patients with LTLE and RTLE and between patients with and without MTS and therefore, we combined our patients into a single group. In addition, given the small sample size we were not able to run volumetric analyses with these subgroups. Future studies with a larger sample are needed in order to examine subtle differences among these groups, particularly differences in patients with and without MTS. Fifth as noted above, we did not analyze volume loss within specific hippocampal subfields. Because there is mounting evidence demonstrating that CA3 and DG subfields are essential for pattern separation, future studies evaluating the association between hippocampal subfields and spatial pattern separation in TLE would be of great interest. Finally, we did not have neuroimaging data in the healthy control group, and therefore, we are unable to determine whether poor pattern separation using the task described in our study is associated with hippocampal volume loss outside of patients with medically-refractory TLE.
1.5 Conclusion
We demonstrate that patients with chronic, refractory TLE show impairments on a task hypothesized to measure spatial pattern separation, and that poor pattern separation may be associated with hippocampal pathology and contribute to spatial episodic memory impairments observed in many patients with TLE.
Highlights.
Individuals with TLE showed less efficient spatial pattern separation performance
Poorer spatial pattern separation performance was associated with visuospatial memory
Left hippocampal atrophy was associated with poor spatial pattern separation
Acknowledgments
This work was supported by the National Institutes of Health [R01 NS065838 to C.R.M; 5R01AG034202 to P.E.G.; 5R25GM058906 to S.Y.D.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams S, Morris RG, Polkey CE, Jarosz JM, Cox TC, Graves M, Pickering A. Hippocampal involvement in spatial and working memory: a structural MRI analysis of patients with unilateral mesial temporal lobe sclerosis. Brain Cogn. 1999;41:39–65. doi: 10.1006/brcg.1999.1095. [DOI] [PubMed] [Google Scholar]
- Ally BA, Hussey EP, Ko PC, Molitor RJ. Pattern separation and pattern completion in Alzheimer's disease: evidence of rapid forgetting in amnestic mild cognitive impairment. Hippocampus. 2013;23:1246–1258. doi: 10.1002/hipo.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RH. The brief visuospatial memory test – revised: Professional manual. Odessa: Psychological Assessment Resources; 1997. [Google Scholar]
- Benedict RH, Groninger L. Preliminary standardization and validation of a new visuospatial memory test with six alternate forms. The Clinical Neuropsychologist. 1995;9:11–16. [Google Scholar]
- Benton A, Sivan A, Hamsher K, Varney N, Spreen O. Contributions to neuropsychological assessment: A clinical manual. New York: Oxford University Press; 1994. [Google Scholar]
- Bohbot VD, Kalina M, Stepankova K, Spackova N, Petrides M, Nadel L. Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia. 1998;36:1217–1238. doi: 10.1016/s0028-3932(97)00161-9. [DOI] [PubMed] [Google Scholar]
- Bouchard TP, Malykhin N, Martin WR, Hanstock CC, Emery DJ, Fisher NJ, Camicioli RM. Age and dementia-associated atrophy predominates in the hippocampal head and amygdala in Parkinson's disease. Neurobiol Aging. 2008;29:1027–1039. doi: 10.1016/j.neurobiolaging.2007.02.002. [DOI] [PubMed] [Google Scholar]
- de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- DeFord NE, Landy KM, Pirogovsky-Turk E, Van Etten EJ, Graves LV, Salmon DP, Filoteo JV, Gilbert PE. The effect of interference on temporal order memory in individuals with Parkinson's disease. Brain Cogn. 2016;107:30–36. doi: 10.1016/j.bandc.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxey CR, Kirwan CB. Structural and functional correlates of behavioral pattern separation in the hippocampus and medial temporal lobe. Hippocampus. 2015;25:524–533. doi: 10.1002/hipo.22389. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr Introduction to temporal lobe epilepsy. Epilepsy Res. 1996;26:141–150. doi: 10.1016/s0920-1211(96)00043-5. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Gilbert P, Holden H, Sheppard D, Morris A. The Neurobiological Basis of Memory. Springer International Publishing; 2016. Pattern Separation: A Key Processing Deficit Associated with Aging? pp. 115–135. [Google Scholar]
- Gilbert PE, Brushfield AM. The role of the CA3 hippocampal subregion in spatial memory: a process oriented behavioral assessment. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:774–781. doi: 10.1016/j.pnpbp.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. The role of the dorsal CA3 hippocampal subregion in spatial working memory and pattern separation. Behav Brain Res. 2006;169:142–149. doi: 10.1016/j.bbr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, DeCoteau WE. Memory for spatial location: role of the hippocampus in mediating spatial pattern separation. J Neurosci. 1998;18:804–810. doi: 10.1523/JNEUROSCI.18-02-00804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C. Cognitive outcomes of different surgical approaches in temporal lobe epilepsy. Epileptic Disord. 2013;15:221–239. doi: 10.1684/epd.2013.0587. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE. Chronic epilepsy and cognition: a longitudinal study in temporal lobe epilepsy. Ann Neurol. 2003;54:425–432. doi: 10.1002/ana.10692. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Schoenfeld J, Davies K. Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch Neurol. 1997;54:369–376. doi: 10.1001/archneur.1997.00550160019010. [DOI] [PubMed] [Google Scholar]
- Holden HM, Gilbert PE. Less efficient pattern separation may contribute to age-related spatial memory deficits. Front Aging Neurosci. 2012;4:9. doi: 10.3389/fnagi.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden HM, Hoebel C, Loftis K, Gilbert PE. Spatial pattern separation in cognitively normal young and older adults. Hippocampus. 2012;22:1826–1832. doi: 10.1002/hipo.22017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igloi K, Doeller CF, Berthoz A, Rondi-Reig L, Burgess N. Lateralized human hippocampal activity predicts navigation based on sequence or place memory. Proc Natl Acad Sci U S A. 2010;107:14466–14471. doi: 10.1073/pnas.1004243107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Rolls ET. A computational theory of hippocampal function, and tests of the theory: new developments. Neurosci Biobehav Rev. 2015;48:92–147. doi: 10.1016/j.neubiorev.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. Match mismatch processes underlie human hippocampal responses to associative novelty. J Neurosci. 2007;27:8517–8524. doi: 10.1523/JNEUROSCI.1677-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshe SL, Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- Lee TM, Yip JT, Jones-Gotman M. Memory deficits after resection from left or right anterior temporal lobe in humans: a meta-analytic review. Epilepsia. 2002;43:283–291. doi: 10.1046/j.1528-1157.2002.09901.x. [DOI] [PubMed] [Google Scholar]
- Light L, Zelinski E. Memory for spatial information in young and old adults. Developmental Psychology. 1983;19:901. [Google Scholar]
- Maguire EA, Frackowiak RS, Frith CD. Recalling routes around london: activation of the right hippocampus in taxi drivers. J Neurosci. 1997;17:7103–7110. doi: 10.1523/JNEUROSCI.17-18-07103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B. Visually-guided maze learning in man: Effects of bilateral hippocampal, bilateral frontal, and unilateral cerebral lesions. Neuropsychologia. 1965;3:317–338. [Google Scholar]
- Moffat SD, Hampson E, Hatzipantelis M. Navigation in a “Virtual” Maze: Sex Differences and Correlation With Psychometric Measures of Spatial Ability in Humans. Evolution and Human Behavior. 1998;19:73–87. [Google Scholar]
- Motley SE, Kirwan CB. A parametric investigation of pattern separation processes in the medial temporal lobe. J Neurosci. 2012;32:13076–13085. doi: 10.1523/JNEUROSCI.5920-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Norman KA. Hippocampal and neocortical contributions to memory: advances in the complementary learning systems framework. Trends Cogn Sci. 2002;6:505–510. doi: 10.1016/s1364-6613(02)02005-3. [DOI] [PubMed] [Google Scholar]
- Park DC, Puglisi JT, Lutz R. Spatial memory in older adults: effects of intentionality. J Gerontol. 1982;37:330–335. doi: 10.1093/geronj/37.3.330. [DOI] [PubMed] [Google Scholar]
- Perlmutter M, Metzger R, Nezworski T, Miller K. Spatial and temporal memory in 20 to 60 year olds. J Gerontol. 1981;36:59–65. doi: 10.1093/geronj/36.1.59. [DOI] [PubMed] [Google Scholar]
- Reagh ZM, Ho HD, Leal SL, Noche JA, Chun A, Murray EA, Yassa MA. Greater loss of object than spatial mnemonic discrimination in aged adults. Hippocampus. 2016;26:417–422. doi: 10.1002/hipo.22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagh ZM, Roberts JM, Ly M, DiProspero N, Murray E, Yassa MA. Spatial discrimination deficits as a function of mnemonic interference in aged adults with and without memory impairment. Hippocampus. 2014;24:303–314. doi: 10.1002/hipo.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotblatt LJ, Sumida CA, Van Etten EJ, Turk EP, Tolentino JC, Gilbert PE. Differences in temporal order memory among young, middle-aged, and older adults may depend on the level of interference. Front Aging Neurosci. 2015;7:28. doi: 10.3389/fnagi.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saling MM. Verbal memory in mesial temporal lobe epilepsy: beyond material specificity. Brain. 2009;132:570–582. doi: 10.1093/brain/awp012. [DOI] [PubMed] [Google Scholar]
- Sheppard DP, Graves LV, Holden HM, Delano-Wood L, Bondi MW, Gilbert PE. Spatial pattern separation differences in older adult carriers and non-carriers for the apolipoprotein E epsilon 4 allele. Neurobiol Learn Mem. 2016;129:113–119. doi: 10.1016/j.nlm.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, Stark CE. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013;51:2442–2449. doi: 10.1016/j.neuropsychologia.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Stark CE. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learn Mem. 2010;17:284–288. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer AK, Murphy KJ, Anderson ND, Hayman-Abello BA, Craik FI, Moscovitch M. Item and associative memory in amnestic mild cognitive impairment: performance on standardized memory tests. Neuropsychology. 2008;22:10–16. doi: 10.1037/0894-4105.22.1.10. [DOI] [PubMed] [Google Scholar]
- Viskontas IV, McAndrews MP, Moscovitch M. Remote episodic memory deficits in patients with unilateral temporal lobe epilepsy and excisions. J Neurosci. 2000;20:5853–5857. doi: 10.1523/JNEUROSCI.20-15-05853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale—Revised Manual. New York: Psychological Corporation, Harcourt Brace Jovanovich Inc.; 1987. [Google Scholar]
- Wesnes KA, Annas P, Basun H, Edgar C, Blennow K. Performance on a pattern separation task by Alzheimer's patients shows possible links between disrupted dentate gyrus activity and apolipoprotein E in4 status and cerebrospinal fluid amyloid-beta42 levels. Alzheimers Res Ther. 2014;6:20. doi: 10.1186/alzrt250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi S, Chow C, Lieblich SE, Galea LA. Sex and strategy use matters for pattern separation, adult neurogenesis, and immediate early gene expression in the hippocampus. Hippocampus. 2016;26:87–101. doi: 10.1002/hipo.22493. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CE. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CE. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci U S A. 2011;108:8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage. 2010;51:1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]