The problem of dementia
Dementia is a neurodegenerative disorder with global impact, with the largest proportion of cases occurring in low and middle-income countries. It is estimated that there are 46.8 million cases globally with approximately 10 million new cases each year or a new case occurring every 3 seconds (Prince et al., 2015). For comparison there are 36.7 million HIV cases with an estimated 2 million new cases each year (WHO, 2017). The rise in dementia prevalence is largely due to population ageing, with the oldest being at highest risk. To this date there are no disease modifying medications for Alzheimer’s disease or the other causes of dementia. Academics and research groups are increasingly focused on prevention or delay of dementia (Brayne and Miller, 2017) and a number of organisations now prioritise dementia indicating a strong and coherent international effort to address this problem. Examples include the World Health Organisation (WHO) which has established a Global Dementia Observatory; the World Dementia Council; the Organisation for Economic Co-operation and Development (OECD); the U.S. National Alzheimer’s Project Act (NAPA); and the Global Council on Brain Health.
Prevention and delay are urgent priorities
The high prevalence of dementia combined with the severe disability it incurs create huge societal and individual impact and cost. Delaying the onset of dementia even by 2 years would reduce prevalence and care costs and provide enormous economic and social benefits (Brookmeyer et al., 2007). Importantly, it is now well established that modifiable risk factors influence the likelihood of developing dementia and the age at which it is developed (Barnes and Yaffe, 2011; Norton et al., 2014; Livingston et al., 2017). This points to the potential for creation of large-scale preventive strategies and programs. Risk factors repeatedly demonstrated in observational studies include (but are not limited to) depression, diabetes, excess alcohol, low education, low physical activity, mid-life hypercholesterolaemia, mid-life hypertension, mid-life obesity, poor diet and smoking (Lautenschlager et al., 2010; Barnes and Yaffe, 2011; Dodge et al., 2011; Clare et al., 2012; Norton et al., 2014; Peters et al., 2014; Anstey et al., 2015; Prince et al., 2015; Livingston et al., 2017;). Risk reduction may work to reduce overall prevalence of dementia by delaying disease onset or preventing the onset of dementia symptomology altogether (Barnes and Yaffe, 2011; Norton et al., 2014). Data from observational studies estimate that reduction in exposure to risk factors through lifestyle and medical treatment may reduce rates significantly, up to 30%, but evidence from trials is limited and complicated by unresolved methodological issues (Prince et al., 2015). These include a lack of clarity about the age at which it is best to intervene, the risk factors for which modification will yield the biggest impact on brain health, sex and gender differences in risk profiles, dosage of treatments and ethnic differences. Compared to other areas of population health, dementia prevention research is relatively new, with a small evidence base in any individual domain.
The need for knowledge transfer and to link researchers together
Given the global significance of dementia, there is a need to ensure that research findings related to risk reduction and prevention are communicated among the scientific community and made accessible to policy makers. There has been no entity or organisation solely devoted to this purpose, and this has motivated the establishment of the International Research Network on Dementia Prevention (IRNDP; http://coghealth.net.au/). Whilst most existing consortia, for example, the Consortium of Cohorts for Alzheimer’s Prevention Action (CAPA) seek to combine data and or have a regional focus, the IRNDP is a multinational network aiming to link researchers globally to foster new research and accelerate knowledge translation that will prevent dementia worldwide, figure 1. IRNDP has an interactive online hub and works to generate collaborative grant applications, studies, publications and recommendations for policy with input and regional expertise from high, medium and low income countries and key stakeholders including the WHO. The approach by the IRNDP places it in a uniquely beneficial position in terms of cutting edge and innovative work but with clear relevance and opportunities for translation. This collaborative approach facilitates horizon scanning, adaptation and innovation towards future areas of research. In particular there is a need to link researchers from low, middle and high income countries to combine knowledge and to avoid wasting resources on unnecessary duplication of research efforts. Figure 1 depicts the overall mission and aim of the IRNDP and its linking of academics and stakeholders.
Figure 1.
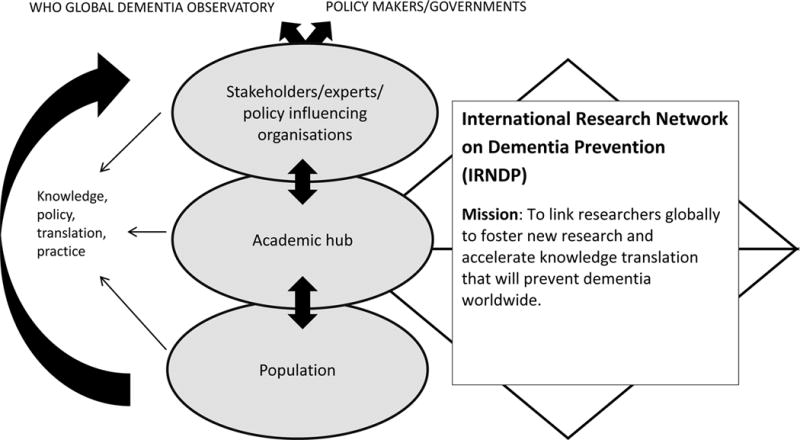
Role of the IRNDP
What about studies showing incidence is decreasing?
Some may ask whether we need to be concerned about dementia given the recent reports of reducing incidence, for example, in the United Kingdom (Matthews et al., 2016) and the United States (Satizabal et al., 2016). It is critical to note that these studies adjusted for age. Thus, although it appears that an older adult at a given age is less likely to develop dementia today compared to several decades ago, the total number of older adults is still rising, and the total number of dementia cases is still projected to increase dramatically over the coming years. In addition, authors have speculated that higher levels of education and better risk factor management are the reason for these findings. This is very positive, and supports the view that risk factor modification has the potential to reduce dementia incidence. However, these studies occurred in countries with well-developed health systems and chronic disease management as well as relatively high levels of education. Whilst giving reason for hope in relation to risk reduction strategies, these findings do not diminish the overall need for further research in dementia prevention for the following reasons: i) the evidence base is not consistent with at least one study finding no evidence for a decline in incidence (van Bussel et al., 2017), ii) the problem of dementia is largest in countries that do not have the high levels of education or health systems observed in the publications reporting a reduced incidence, iii) the populations on whom the studies were conducted were born prior to the cohorts who are currently experiencing the obesity and diabetes epidemics, and iv) new risk factors are emerging such as air pollution (Power et al.,, 2016), for which we lack information on population attributable risk but which may be having a major impact globally. The problem is so large that the need to act remains urgent.
Value adding by working together on dementia prevention
Communication and networking provide the opportunity for research findings to be transmitted quickly among the academic community and to establish relationships that will facilitate collaboration and comparison of findings. Prevalence of risk factors and patterns of exposure vary from one population to the next, in particular, between high and low or middle income countries and high and low socioeconomic groups. This variation also provides natural experiments that will inform understanding of the association between risk and protective factors and dementia.
Clinical trials of interventions in multiple risk factors to date have had some success (Ngandu et al., 2015) and similar studies in different populations are underway. However, despite subgroup analyses indicating positive future directions for further research, the overall results for other such trials have been disappointing, (Moll van Charante et al., 2016; Andrieu et al., 2017).This may be in part due to lack of sensitivity in sample selection, attrition, length of follow-up, insufficient statistical power or lack of sensitivity in outcome measures. Many outstanding questions have been raised relating to risk factor exposure and potential intervention. To gain the nuanced understanding of risk factors needed in order to develop appropriately targeted public health messaging and resource we need targeted systematic reviews and meta-analyses, epidemiological studies and clinical trials relevant to and with expertise and local knowledge from both high and low and middle income countries. Research is required to evaluate interventions that address multiple risk factors, to evaluate age, dosage and length of treatment and determine whether results from developed countries generalize to less developed countries.
What the IRNDP will achieve
The IRNDP will form a focal point for multicountry grant applications, meetings and collaborations to share knowledge and ideas about dementia prevention. Bringing researchers together provides opportunities for collaborative studies and publications, data sharing, data pooling and use of big-data, sharing of expertise, development of consensus statements, and guidelines. These outputs will provide much needed resources to inform governments and private organisations on strategies to reduce risk factors for dementia and to develop international and nation-specific guidelines. Working together, members of the IRNDP will play an important role in supporting emerging researchers in the field of dementia risk reduction by providing them with a community of scholars, opportunities for sharing information and mentorship.
The IRNDP aims to facilitate the integration of dementia risk reduction into more general chronic disease management and prevention strategies, to offer an efficient and effective approach to population level dementia prevention. This approach is congruent with views emerging in the US and in Europe (Prince et al., 2015), for example, as seen in the latest National Institute for Health and Care Excellence (NICE) resources (NICE Guideline Dementia, disability and frailty in later life – mid-life approaches to delay or prevent onset NG16), by providing the most current evidence for use in traditional outlets and social media. Dementia risk factors draw from many domains of influence. Linking experts across many disciplines and sectors is a key opportunity provided by working together in this network. Translating the findings from research into policies and guidelines at the population level as well as for clinicians in practice is a core goal of the network.
Acknowledgments
The development of the IRNDP has been supported by the Dementia Centre for Research Collaboration part of the NHMRC National Institute for Dementia Research (NNIDR), An Australian Government initiative.
Footnotes
Conflict of interest
Nicola T Lautenschlager was at the time of submission Editor-in-Chief of International Psychogeriatrics. Therefore, this editorial was reviewed by another member of the editorial team.
Deborah Barnes is co-founder and board director for Together Senior Health, which is dedicated to helping individuals living with dementia and their caregivers maintain function and quality of life.
All authors have formal roles in the development of the IRNDP. Kaarin J. Anstey, Ruth Peters, Linda Clare, Nicola T. Lautenschlager, Hiroko H. Dodge, Deborah Barnes and Suzana Shahar are members of the IRNDP Leadership Committee. Henry Brodaty and Glenn Rees are members of the IRNDP International Advisory Group.
Contributor Information
Kaarin J. Anstey, Centre for Research on Ageing, Health & Wellbeing, ANU College of Medicine, Biology & Environment, Australian National University, Australia
Ruth Peters, Centre for Research on Ageing, Health & Wellbeing, ANU College of Medicine, Biology & Environment, Australian National University Australia.
Linda Clare, Centre for Research in Ageing and Cognitive Health (REACH), School of Psychology, the University of Exeter Medical School, University of Exeter UK.
Nicola T. Lautenschlager, Academic Unit for Psychiatry of Old Age, Department of Psychiatry, The University of Melbourne & North Western Mental Health, Melbourne Health, Melbourne, Australia
Hiroko H. Dodge, Department of Neurology, Layton Aging and Alzheimer’s Disease Center, Oregon Health and Science University, Department of Neurology, Michigan Alzheimer’s Disease Center, University of Michigan, USA
Deborah Barnes, School of Medicine, University of California San Francisco, USA and San Francisco Veterans Affairs Health Care System, USA.
Suzana Shahar, Centre of Community Rehabilitation and Aging, Universiti Kebangsaan Malaysia.
Henry Brodaty, Centre for Healthy Brain Ageing, Medicine, University of New South Wales, Australia.
Glenn Rees, Alzheimer’s Disease International.
References
- Andrieu S, et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet neurology. 2017;16:377–389. doi: 10.1016/S1474-4422(17)30040-6. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Eramudugolla R, Hosking DE, Lautenschlager NT, Dixon RA. Bridging the Translation Gap: From Dementia Risk Assessment to Advice on Risk Reduction. Journal of Prevention of Alzheimers Disease. 2015;2:189–198. doi: 10.14283/jpad.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurology. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayne C, Miller B. Dementia and aging populations—A global priority for contextualized research and health policy. PLoS Medicine. 2017;14:e1002275. doi: 10.1371/journal.pmed.1002275. https://doi.org/10.1371/journal.pmed.1002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Johnson E, Zeigler-Graham K, Arrighi M. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s & Dementia. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- Clare L, et al. The AgeWell study of behavior change to promote health and wellbeing in later life: study protocol for a randomized controlled trial. Trials. 2012;13:115. doi: 10.1186/1745-6215-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge HH, Chang CC, Kamboh IM, Ganguli M. Risk of Alzheimer’s disease incidence attributable to vascular disease in the population. Alzheimers and Dementia. 2011;7:356–60. doi: 10.1016/j.jalz.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager NT, Cox K, Kurz AF. Physical activity and mild cognitive impairment and Alzheimer’s disease. Current Neurology and Neuroscience Reports. 2010;10:352–358. doi: 10.1007/s11910-010-0121-7. [DOI] [PubMed] [Google Scholar]
- Livingston G, et al. Dementia prevention, intervention and care. Lancet The Lancet Commissions. 2017 doi: 10.1016/S0140-6736(17)31363-6. doi. org/10.1016/S0140-6736(17)31363-6. [DOI] [PubMed]
- Matthews FE, et al. Ageing Studies, C. A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nature Communications. 2016;7:11398. doi: 10.1038/ncomms11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll van Charante EP, et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet. 2016;388:797–805. doi: 10.1016/S0140-6736(16)30950-3. [DOI] [PubMed] [Google Scholar]
- Ngandu T, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet neurology. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- Peters R, Booth A, Peters J. A systematic review of calcium channel blocker use and cognitive decline/dementia in the elderly. Journal of Hypertension. 2014;32:1945–1957. doi: 10.1097/HJH.0000000000000273. [DOI] [PubMed] [Google Scholar]
- Power MC, et al. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: A systematic review of epidemiologic research. Neurotoxicology. 2016;56:235–253. doi: 10.1016/j.neuro.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M, et al. The global impact of dementia. World Alzheimer’s Report. 2015 https://www.alz.co.uk/research/world-report-2015.
- Satizabal CL, et al. Incidence of Dementia over Three Decades in the Framingham Heart Study. The New England journal of medicine. 2016;374:523–532. doi: 10.1056/NEJMoa1504327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bussel EF, et al. Dementia incidence trend over 1992-2014 in the Netherlands: Analysis of primary care data. PLoS Medicine. 2017;14:e1002235. doi: 10.1371/journal.pmed.1002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Global Health Observatory Data. 2017 http://www.who.int/gho/hiv/en/


