Abstract
Objective
Impaired wound healing is a major complication of diabetes mellitus. The mechanisms that govern wound healing, however, are complex and incompletely understood. In the present study, we determined the inhibitory role of protein tyrosine phosphatase 1B (PTP1B) in the process of diabetic wound healing.
Approach and Results
First, by comparing the wound healing process in PTP1B knockout (PTP1B−/−) mice, ob/ob mice and their wild-type littermates in the presence or absence of streptozotocin treatment, we showed that the inhibition of mouse wound healing in streptozotocin-induced diabetic conditions is because of the upregulation and activation of PTP1B. Second, the impaired wound healing in ob/ob mice and streptozotocin-treated wild-type mice was rescued by a PTP1B inhibitor. Third, PTP1B, which is upregulated under hyperglycemic condition, inhibited the tube formation, proliferation, and migration of human microvascular endothelial cells induced by vascular endothelial growth factor, whereas this inhibition was largely abolished by the PTP1B inhibitor. Finally, mechanism study further indicated that PTP1B likely suppressed the proliferation, migration, and tube formation of vascular endothelial cells through dephosphorylation of vascular endothelial growth factor receptor 2.
Conclusions
Our study demonstrated that PTP1B negatively modulated the diabetic wound healing process by dephosphorylating the endothelial cell vascular endothelial growth factor receptor 2 and that the specific inhibitor of PTP1B might serve as a potential novel therapeutic tool for diabetic wound healing.
Keywords: angiogenesis effect; hyperglycemia; PTP1B protein, human; reactive oxygen species; vascular endothelial growth factor receptor-2, human; wound healing
Delayed wound healing is a major complication of diabetes mellitus.1–3 Ulcer wounds with impaired healing and repeated infection lead to the risk of lower limb amputation. According to the American Diabetes Association, the incidence of foot ulceration in diabetic patients may reach 40%, making it a large healthcare burden.4 Although previous studies have shown that increased apoptosis, impaired cellular infiltration, reduced angiogenesis, and decreased formation and organization of collagen fibers5–7 can contribute to delayed wound healing under diabetic conditions, wound healing is a complex process, and the mechanism underlying impaired diabetic wound healing remains incompletely understood.
Protein tyrosine phosphatase 1B (PTP1B), a negative regulator of metabolic signaling pathways, such as the insulin and leptin signaling pathways, is the prototype of the superfamily of PTPs and belongs to the nontransmembrane subfamily 1 of intracellular PTPs.8,9 PTP1B has been reported to be upregulated under hyperglycemia and diabetic conditions.8–10 PTP1B is involved in multiple cellular processes, such as glucose uptake, proliferation, differentiation, apoptosis, cell–cell adhesion, extracellular matrix attachment, motility, and invasion.11 Dinh et al12 reported that PTP1B served as a key factor associated with the failure of diabetic foot ulcers to heal. Nakamura et al13 also found that PTP1B played an important role in modulating VEGF-mediated angiogenesis. In fact, PTP1B has been show to target various substrates to dephosphorylate these phosphorylated molecules. Recent studies by us14 and others15 showed that PTP1B could dephosphorylate the N-ethylmaleimide-sensitive fusion protein to elicit soluble N-ethylmaleimide-sensitive-factor attachment protein receptor complex disassembly during human sperm exocytosis. In insulin signaling, PTP1B dephosphorylated the insulin receptor and its substrates, particularly the insulin receptor substrate proteins, to control the insulin signaling pathway negatively.16–19 PTP1B also negatively controlled the leptin signaling pathway by dephosphorylating Janus kinase 2, a downstream mediator of leptin signaling.20–23 Several research groups have reported that PTP1B can dephosphorylate Src at the Y529 site to activate the kinase and promote cell proliferation.24–27 In contrast, PTP1B can also serve as a cell growth inhibitor by dephosphorylating other receptor tyrosine protein kinase, including the epidermal growth factor and platelet-derived growth factor receptors. A recent study by Nakamura et al13 showed that the vascular endothelial growth factor receptor 2 (VEGFR2), a critical receptor modulating endothelial cell proliferation, migration, and tube formation, was dephosphorylated by PTP1B, suggesting that upregulated PTP1B in endothelial cells may contribute to the impairment of VEGFR2-associated angiogenesis and diabetic wound healing.
In the present study, using PTP1B knockout (PTP1B−/−) mice, ob/ob mice and their wild-type littermates, we defined the role of PTP1B in diabetic wound healing. The mechanism underlying hyperglycemia-induced PTP1B expression and the potential VEGFR2-mediated signal downstream of PTP1B during vascular endothelial cell proliferation, migration, and tube formation was also explored.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement
Results
PTP1B Impairs Wound Healing in Diabetic Mice
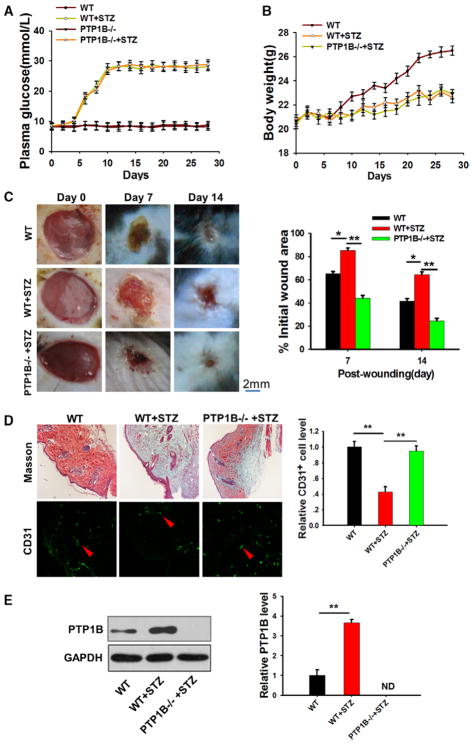
As a critical modulator of metabolism, PTP1B protein expression and its activity have been reported to be upregulated under hyperglycemic and diabetic conditions.8–10 To test the role of PTP1B in the modulation of diabetic wound healing, we conducted a wound healing experiment using PTP1B−/− mice and their wild-type (WT) littermates. First, we established diabetic model by injecting streptozotocin (40 mg/kg, dissolved in a citrate buffer just before the injection) once a day for 5 consecutive days.28 Daily monitoring of plasma glucose levels indicated that both PTP1B−/− and WT mice were hyperglycemic after streptozotocin treatment (Figure 1A). Streptozotocin treatment also resulted in a similar body weight loss in PTP1B−/− and WT mice (Figure 1B). Next, a full-thickness skin wound was created.29 The wound was protected with sterilized gauze to prevent infection. The wound healing process was monitored every day.29 As shown in Figure 1C, WT mice treated with streptozotocin showed significantly delayed wound healing when compared with mice lacking streptozotocin treatment. To our surprise, streptozotocin treatment did not slow wound healing in PTP1B−/− mice even though these mice had a similar hyperglycemic condition, suggesting that the effects of streptozotocin-induced diabetic conditions on mouse wound healing may be mediated through PTP1B elevation or activation. Immunofluorescence staining and analysis (Figure 1D) confirmed such differential wound healing. Moreover, an ordered collagen structure and active neovascularization were revealed in streptozotocin-treated PTP1B−/− mice, whereas streptozotocin-treated WT mice showed a disruption of collagen structure and slow neovascularization. Western blot results confirmed that PTP1B levels were upregulated in streptozotocin-treated WT mice when compared with WT littermates (Figure 1E).
Figure 1.
Protein tyrosine phosphatase 1B (PTP1B)−/− mice showed an accelerated diabetic wound healing rate. A, Plasma glucose level in wild-type (WT) and PTP1B−/− mice treated with or without streptozotocin (STZ). B, Body weight of WT and PTP1B−/− mice treated with or without STZ. C, Left, Images of the skin wound in WT, STZ-treated, and STZ-treated PTP1B−/− mice from 0, 7, and 14 days post wounding. Right, Quantitative analysis of wound healing in WT and PTP1B−/− mice treated with or without STZ. The percentage of the wound area at different time point post injury vs the initial wound area was used as an indicator for wound healing measurement. D, Left, Masson trichrome staining and CD31 labeling assay of skin tissues of WT and PTP1B−/− mice treated with or without STZ on day 7 post wounding. Right, Quantitative analysis of the number of CD31+ cells. E, Western blot results showing the PTP1B protein levels of skin tissues in WT and PTP1B−/− mice treated with or without STZ. Images of the Western blot assay were analyzed using the Bandscan software. Data are presented as the mean±SD of ≥3 independent experiments. There were 6 mice in each group per condition. *P<0.05 and **P<0.01.
PTP1B Inhibition Rescues Impaired Wound Healing in Diabetic Mice
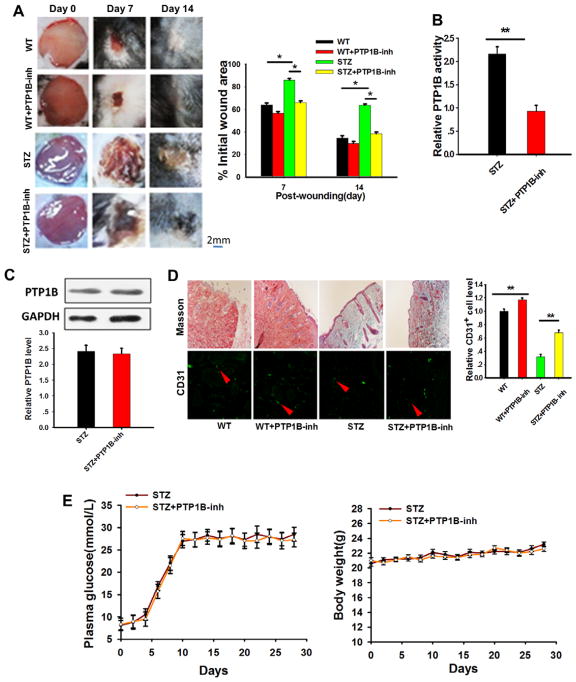
To validate the role of PTP1B in delaying diabetic wound healing, a specific PTP1B inhibitor, FRJ,14,30 was used. In this experiment, the PTP1B inhibitor was added to the wound every other day. Wound healing was imaged, and the skin tissues were obtained and analyzed. As shown in Figure 2A, administration of the FRJ significantly accelerated the wound healing process in streptozotocin-treated WT mice. FRJ treatment slightly enhanced the wound healing process in WT mice, but the difference was not significantly. Enzyme activity and protein expression analysis revealed that PTP1B activity (Figure 2B) but not PTP1B protein expression (Figure 2C) were suppressed in skin tissues by the PTP1B inhibitor. Masson staining and immunofluorescence labeling with an anti-CD31 antibody31 showed that skin tissues treated with the PTP1B inhibitor maintained a better collagen structure and stronger angiogenesis (Figure 2D). The treatment with PTP1B inhibitor did not change the plasma glucose level and body weight in streptozotocin-treated mice (Figure 2E). To test the specificity of FRJ, we also treated PTP1B−/− mice with FRJ during the course of wound healing. As shown in Figure I in the online-only Data Supplement, FRJ treatment did not further alter the wound healing (A and B), plasma glucose level (C), and body weight loss in streptozotocin-treated PTP1B−/− mice.
Figure 2.
Protein tyrosine phosphatase 1B (PTP1B) inhibition ameliorated diabetic wound healing. A, Left, Images of wound skin in wild-type (WT) mice and streptozotocin (STZ)–treated mice in the presence or absence of PTP1B inhibitor (PTP1B-inh) on 0, 7, and 14 days post wounding. Right, Quantitative analysis of wound healing in WT mice and STZ-treated mice in the presence or absence of FRJ. The percentage of the wound area at different time point post injury vs the initial wound area was used as an indicator for wound healing measurement. B, PTP1B activity was assessed by a specific kit in STZ-treated mice in the presence or absence of FRJ. C, Western blot results of the PTP1B protein level of STZ-treated mice in the presence or absence of PTP1B inhibitor. Images of the Western blot assay were analyzed using the Bandscan software, and statistical analysis is presented below. D, Left, Masson trichrome staining and anti-CD31 antibody labeling of skin tissues of STZ-treated mice in the presence or absence of FRJ 7 days post injury. Right, Quantitative analysis of the number of CD31+ cells. E, Plasma glucose level and body weight of STZ-treated mice in the presence or absence of PTP1B inhibitor. Data are presented as the mean±SD of 3 independent experiments. There were 5 to 7 mice in each group per condition. *P<0.05 and **P<0.01.
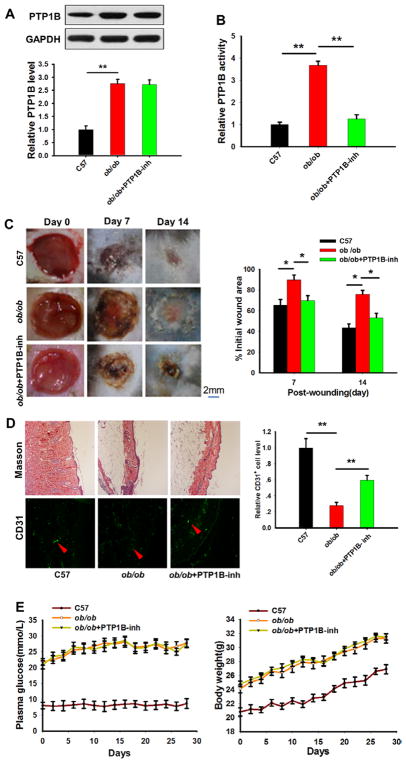
The ob/ob mice displayed typical diabetic symptoms, such as pronounced hyperglycemia, insulin resistance, and marked obesity, and thus have been widely used to establish an animal model for type 2 diabetes mellitus.32,33 Western blotting revealed that PTP1B expression levels were significantly higher in ob/ob mice compared with WT mice (Figure 3A). Similar to the observations above, the PTP1B inhibitor did not affect PTP1B expression levels (Figure 3A) but significantly suppressed PTP1B activity (Figure 3B). Next, we compared the wound healing process in ob/ob and WT mice and monitored the effects of the PTP1B inhibitor. As shown in Figure 3C, a striking difference in the wound healing process between ob/ob and WT mice was observed. WT mice displayed an accelerated resurfacing rate versus ob/ob mice, but the delayed wound healing in ob/ob mice could be markedly accelerated by the PTP1B inhibitor. Further analysis of the wound skin structure by Masson staining and neovascularization by counting the number of CD31-positive cells supported that ob/ob mice had a delayed wound healing compared with WT control mice, whereas PTP1B inhibitor administration strongly reversed the impaired wound healing in ob/ob mice (Figure 3D). When compared with ob/ob mice, WT mice had a more compact and orderly arranged collagen structure in the wound tissues. The treatment with PTP1B inhibitor did not change the hyperglycemia and overweight condition in ob/ob mice (Figure 3E).
Figure 3.
The ob/ob mice displayed impaired wound healing while protein tyrosine phosphatase 1B (PTP1B) inhibition relieved the symptoms. A, PTP1B protein levels in wild-type (WT), ob/ob, and PTP1B inhibitor-treated ob/ob mice assessed by Western blot. Western blot images were analyzed using the Bandscan Software, and statistical analysis is presented below. B, Relative PTP1B activity was assessed by a specific kit in WT, ob/ob, and PTP1B inhibitor-treated ob/ob mice. C, Left, Images of the skin wound from WT, ob/ob, and PTP1B inhibitor-treated ob/ob mice at 0, 7, and 14 days post wounding. Right, The ratio of the wound area to the initial wound area at each time point after injury in WT, ob/ob, and PTP1B inhibitor-treated ob/ob mice. D, Left, Masson trichrome staining and anti-CD31 labeling assay of skin tissues in WT, ob/ob, and PTP1B inhibitor-treated ob/ob mice 7 days post wounding. Right, Quantitative analysis of the number of CD31+ cells. E, Plasma glucose level and body weight of WT mice and ob/ob mice in the presence or absence of PTP1B inhibitor. Data are presented as the mean±SD of 3 independent experiments. There were 6 mice in each group per condition. *P<0.05 and **P<0.01.
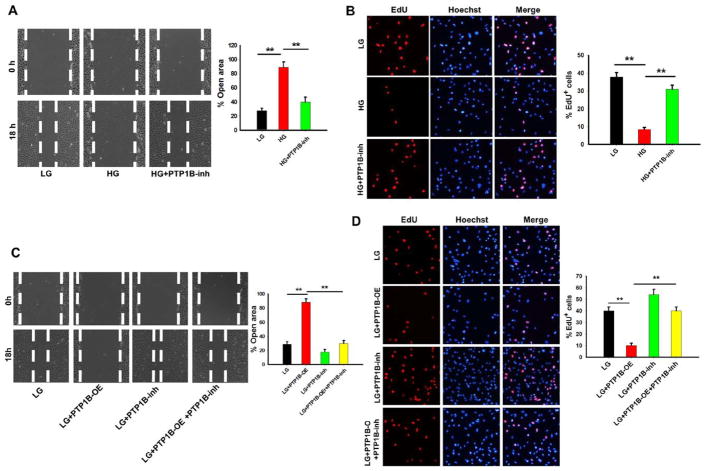
PTP1B inhibition rescues the impairment of VEGF-mediated human microvascular endothelial cell (HMEC)-1 tube formation under high glucose concentration (HG).
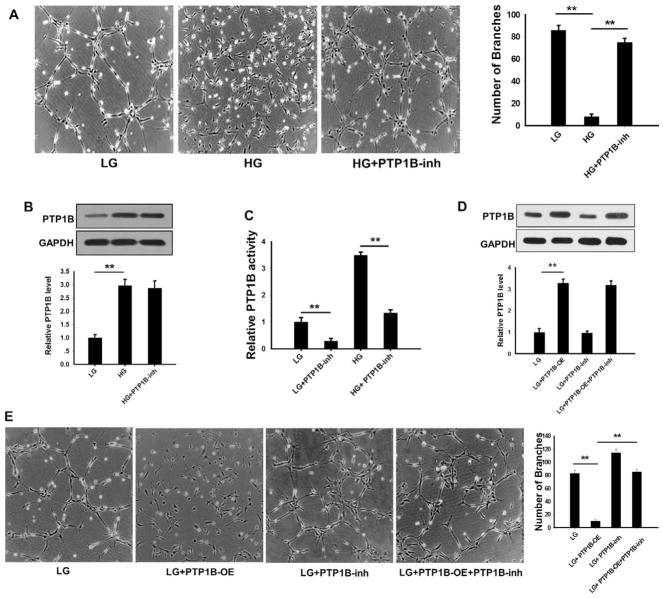
Previous studies have reported that the hyperglycemia induces PTP1B expression and activates its activity.11,24,34,35 Thus, we tested the effects of HG on PTP1B expression and activity. In this experiment, HMEC-1 cells were treated with 30 mmol/L (HG) or 5 mmol/L (low glucose concentration [LG]) glucose for 12 hours and then placed onto a Matrigel-coated culture dish. The PTP1B inhibitor was added during this incubation to test the effects of PTP1B activity on VEGF-stimulated HMEC-1 tube formation. HMEC-1 cell branching was monitored as previously described.36 As shown in Figure 4A, VEGF-stimulated tube formation in HMEC-1 cells was markedly inhibited by HG but not by LG. The impairment of HMEC-1 cell tube formation in HG, however, was largely rescued by the administration of FRJ. In agreement with previous reports,11,24,35 we also found that PTP1B expression level was increased by HG (Figure 4B). The activity of PTP1B was strongly suppressed by FRJ under both high and LG (Figure 4C). To confirm the direct effects of PTP1B on HMEC-1 cell tube formation, we overexpressed PTP1B in HMEC-1 cells (PTP1B-overexpress) by transfection with a PTP1B-expressing plasmid and then assessed the tube formation in HMEC-1 cells. Overexpression of PTP1B significantly increased PTP1B protein levels in HMEC-1 cells (Figure 4D). As shown in Figure 4E, increased PTP1B activity in HEMC-1 cells by overexpressing PTP1B markedly suppressed VEGF-induced tube formation in HMEC-1 cells. The administration of FRJ, however, promoted tube formation in HMEC-1 cells.
Figure 4.
High concentration of glucose (HG) reduced but protein tyrosine phosphatase 1B (PTP1B) inhibition increased vascular endothelial growth factor -stimulated human microvascular endothelial cell (HMEC)-1 cell tube formation. A, Left, Representative image of HMEC-1 cells on Matrigel. Right, Quantitative analysis of tube formation by counting the number of branches from 5 randomly selected fields per well. B, Western blot results of PTP1B protein expression in HMEC-1 cells cultured in low glucose (LG) or HG conditions in the presence or absence of FRJ. Western blotting was analyzed using the Bandscan software, and statistical analysis is presented below. C, Relative PTP1B activity was assessed by a specific kit in HMEC-1 cells cultured in LG or HG conditions in the presence or absence of FRJ. D, Western blot results of PTP1B protein levels in HMEC-1 cells transfected with a PTP1B-vector or treated with FRJ. E, Left, Representative image of HMEC-1 cells on Matrigel that were transfected with the PTP1B-vector or treated with FRJ. Right, Quantitative analysis of tube formation by counting the number of branches from 5 randomly selected fields per well. Data are presented as the mean±SD of 3 independent experiments. **P<0.01. OE indicates overexpress; and PTP1B-inh, PTP1B inhibitor.
HMEC-1 Proliferation and Migration Is Reduced by PTP1B Overexpression but Enhanced by PTP1B Inhibition
Proliferation and migration of vascular endothelial cells represent important steps in the formation of new blood vessels.33 To test the role of PTP1B in HMEC-1 cell proliferation and migration, a scratch wound was created on HMEC-1 monolayers cultured in medium containing LG or HG or HMEC-1 monolayers in the presence or absence of FRJ. As shown in Figure 5A, HG inhibited migration, but FRJ treatment promoted HMEC-1 cell proliferation. The percentage of open wound area at 18 hours was 25±2% for LG and 85±4% for HG. Treatment with FRJ significantly decreased the open wound area of HMEC-1 monolayers cultured in HG. An EdU assay further showed that high concentration of glucose suppressed VEGF-stimulated HMEC-1 proliferation (Figure 5B). The number of EdU-positive cells was 9.1±1.2% for HG versus 38.3±3.4% for LG. Furthermore, FRJ administration abolished the antiproliferative effects of HG on HMEC-1 cells (the EdU-positive number increased to 32.3±4.1%). To verify the direct effects of PTP1B on HMEC-1 proliferation and migration, we overexpressed PTP1B in HMEC-1 cells and monitored cell proliferation and migration. As shown in Figure 5C, the wound healing assay revealed a delayed wound resurfacing after PTP1B overexpression. The percentage of open wound area at 18 hours was 85.1±3.7% for PTP1B overexpression with LG versus 24.0±2.1% for LG alone. In contrast, FRJ increased the rate of wound closure in HMEC-1 cells cultured in LG with the percentage of open wound area reduced to 18.2±4.1%. The EdU assay also showed that PTP1B overexpression decreased HMEC-1 proliferation but that PTP1B inhibition promoted HMEC-1 proliferation (Figure 5D). Moreover, the EdU-positive number was 39.4±3.1% for LG, 11.2±2.1% for HG, and 54.7 ± 4.2 % for LG plus the PTP1B inhibitor.
Figure 5.
Protein tyrosine phosphatase 1B (PTP1B) inhibition increased, but PTP1B overexpression decreased human microvascular endothelial cell (HMEC)-1 cell proliferation and migration. A, Left, Representative images of wound closure in HMEC-1 monolayers that were cultured in low or high glucose (LG or HG) conditions in the presence or absence of FRJ, respectively. Phase contrast microscopy (original magnification, ×20) after wounding at 0 and 18 hours. Right, The percentage of wound closure in LG or HG conditions in the presence or absence of FRJ at 18 hours, relative to the initial width of the wound gaps. B, EdU labeling assay of HMEC-1 cells with the same treatments as in A. The percentage of EdU-positive cells in these groups was calculated from the total cell numbers. C, Representative images of the wound closure of HMEC-1 monolayers that were cultured in LG concentration and transfected with a PTP1B-vector or treated with FRJ. D, EdU labeling assay of HMEC-1 cells with the same treatment as in C. The percentage of EdU-positive cells was counted relatively to the total number of HMEC-1 cells. Data are presented as the mean±SD of 3 independent experiments. **P<0.01. OE indicates overexpress; and PTP1B-inh, PTP1B inhibitor.
Hyperglycemia Induces PTP1B Level Through Reactive Oxygen Species-Nuclear Factor-κB Axis
To confirm hyperglycemia as an important factor for inducing PTP1B expression in vascular endothelial cells, we initially incubated HMEC-1 cells in HG medium for 24 hours and then switched to LG medium and continuously cultured for another 24 hours. As shown in Figure II in the online-only Data Supplement, HG induced PTP1B expression, whereas LG medium reversed the expression level of PTP1B. In vivo study also showed that hyperglycemia is a key factor for PTP1B upregulation. In this experiment, WT mice were initially treated with streptozotocin to achieve hyperglycemia and then injected with insulin twice per day during the whole course of wound healing. As shown in Figure III in the online-only Data Supplement, insulin injection restored the plasma glucose level and body weight of streptozotocin-treated mice (A). The increased PTP1B level in streptozotocin-treated mice was also abolished by insulin injection (B). Lowering the plasma glucose level through insulin injection also significantly improved the wound healing (C), and migration of CD31 endothelial cells (D) in streptozotocin-treated mice. These results collectively suggest that hyperglycemia plays a critical role in upregulating PTP1B in vascular endothelial cells.
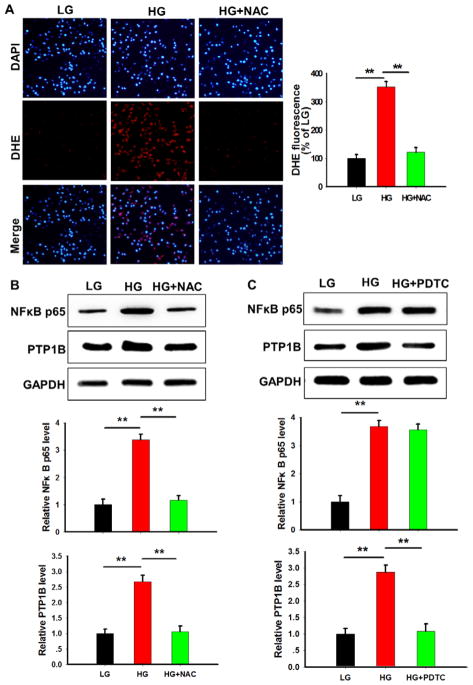
Hyperglycemia has been reported to increase the cellular level of tumor necrosis factor (TNF)-α and reactive oxygen species (ROS), 2 major mechanisms for upregulating the level of PTP1B.37,38 We next tested whether TNF-α or ROS was involved in the regulation of PTP1B upregulation under hyperglycemia condition. We detected a low level of TNF-α in HMEC-1 cells, and the TNF-α level was not altered after the incubation with HG (data not shown), suggesting that TNF-α did not play a major role in the upregulation of PTP1B by hyperglycemia. However, we found that ROS level (detected by DHE staining) was significantly increased by hyperglycemia (Figure 6A), suggesting that ROS is likely involved in regulation of PTP1B level. In agreement with previous finding that ROS can increase PTP1B level via activating nuclear factor (NF)-κB pathway,38 our data showed that the level of NF-κB in HMEC-1 cells was increased under the hyperglycemia condition. As shown in Figure 6B, depletion of hyperglycemia-induced ROS by 10 mmol/L N-acetylcysteine (NAC) abolished the upregulation of NF-κB level. To confirm that hyperglycemia-induced NF-κB is a major reason for PTP1B upregulation, we also inhibited NF-κB activity by 100 mmol/L pyrrolidine dithiocarbamate39 and found that pyrrolidine dithiocarbamate treatment completely abolished the hyperglycemia-induced upregulation of PTP1B (Figure 6C). Taken together, these results suggest that the overexpression of PTP1B in HMEC-1 cells under hyperglycemia condition is likely through the activation of the ROS-NF-κB axis.
Figure 6.
High concentration of glucose (HG) induced protein tyrosine phosphatase 1B (PTP1B) via activation of reactive oxygen species (ROS)-nuclear factor-κB (NF-κB) axis. A, ROS production in human microvascular endothelial cell-1 under hyperglycemia condition. B, The effect of ROS depletion by N-acetylcysteine (NAC) on the NF-κB level induced by HG. C, The effect of NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC) on the PTP1B level induced by HG. Data are presented as the mean±SD of 3 independent experiments. **P<0.01. LG indicates low concentration of glucose.
PTP1B Dephosphorylates the Endothelial VEGFR2 During Angiogenesis
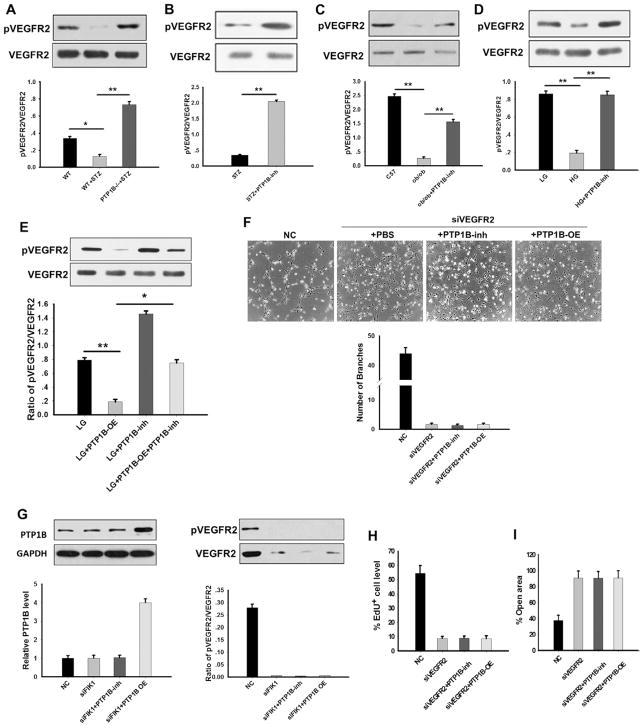
A recent study by Nakamura et al13 has shown that VEGFR2 may be a substrate for PTP1B. Because VEGFR2 is vital for VEGF-induced vascular endothelial cell tube formation and proliferation,40,41 we tested whether PTP1B impairs wound healing in diabetic mice or HMEC-1 cells under hyperglycemic conditions by dephosphorylating VEGFR2. As shown in Figure 7A, we found that VEGFR2 phosphorylation was significantly suppressed in streptozotocin-treated mice when compared with WT mice or streptozotocin-treated PTP1B−/− mice. In contrast, PTP1B inhibition remarkably increased the level of phosphorylated VEGFR2 in WT mice treated with streptozotocin (Figure 7B) and ob/ob mice (Figure 7C). These results indicated that VEGFR2 was dephosphorylated by PTP1B, which activity was enhanced under hyperglycemia condition. To verify whether PTP1B inhibits the proliferation, migration, and tube formation of HMEC-1 cells by dephosphorylating VEGFR2, we monitored the effect of glucose and PTP1B inhibitor on the phosphorylation of VEGFR2 in VEGF-stimulated HEMC-1 cells. The results revealed that VEGFR2 phosphorylation was reduced by HG but enhanced when cells were treated with PTP1B inhibitor (Figure 7D). To show dephosphorylation of VEGFR2 by PTP1B directly, we overexpressed PTP1B in HMEC-1 cells. As shown in Figure 7E, VEGFR2 phosphorylation in HMEC-1 cells was strongly suppressed by PTP1B over-expression but enhanced by the treatment with PTP1B inhibitor.
Figure 7.
Protein tyrosine phosphatase 1B (PTP1B) modified human microvascular endothelial cell (HMEC)-1 cell proliferation, migration, and tube formation by regulating vascular endothelial growth factor receptor 2 (VEGFR2) phosphorylation. A, Western blotting of phosphorylated VEGFR2 (pVEGFR2) and total VEGFR2 in wild-type (WT) and PTP1B−/− mice treated with or without streptozotocin (STZ). B, Western blot analysis of pVEGFR2 and VEGFR2 levels in STZ-treated mice in the presence or absence of PTP1B inhibitor (PTP1B-inh). C, Western blot results of pVEGFR2 and VEGFR2 protein levels in WT and ob/ob mice in the presence or absence of PTP1B inhibitor. D, Western blot results of pVEGFR2 and VEGFR2 protein levels in HMEC-1 cells cultured in LG or HG in the presence or absence of PTP1B inhibitor. E, Western blot results of pVEGFR2 and VEGFR2 levels in HMEC-1 cells overexpressed with PTP1B or treated with PTP1B inhibitor in LG conditions. F, Top, Representative image of HMEC-1 cells on Matrigel. Bottom, Quantitative analysis of the formed tubes was obtained by counting the number of branches from 5 fields per well. G, Knockdown of HMEC-1 cell pVEGFR2 and total VEGFR2 with VEGFR2 siRNA. H, EdU labeling assay of HMEC-1 cells in the control and VEGFR2 siRNA groups. The percentage of EdU-positive cells was calculated relative to the total numbers of HMEC-1 cells. I, Wound closure of HMEC-1 cells at 18 hours. Data are presented as the mean±SD of 3 independent experiments. *P<0.05 and **P<0.01.
We next tested the effects of VEGFR2 dephosphorylation on the tube formation, proliferation, and migration of HMEC-1 cells. In this experiment, VEGFR2 expression in HMEC-1 cells was knocked down by transfection with VEGFR2 siRNA (siVEGFR2). As shown in Figure 7F, VEGF-induced tube formation of HMEC-1 cells was strongly blocked by VEGFR2 knockdown. Interestingly, in VEGFR2 knockdown HMEC-1 cells, either the promotion of HMEC-1 tube formation by PTP1B inhibition or the inhibition of HMEC-1 tube formation by PTP1B overexpression was abolished, suggesting that effect of PTP1B on the modulation of HMEC-1 cell tube formation is likely mediated through VEGFR2 dephosphorylation. Western blot analysis confirmed that the levels of both phosphorylated VEGFR2 and VEGFR2 were significantly reduced by transfection with siVEGFR2 (Figure 7G). In agreement with this observation, we found that the proliferation (Figure 7H; Figure IVA in the online-only Data Supplement) and wound closure rate of VEGFR2 knockdown HMEC-1 cells (Figure 7I; Figure IVB in the online-only Data Supplement) were significantly reduced even in the presence of PTP1B inhibitor.
Discussion
Although hyperglycemia caused an impaired wound closure and tube formation of vascular endothelial cells, the mechanism remains unclear. Various factors have been reported to contribute to the slow diabetic wound healing. These factors include growth factors, nitric oxide (NO), reactive oxygen species (ROS), matrix metalloproteinases, endothelial progenitor cells, and advanced glycation end products.42–46 Growth factors particularly the fibroblast growth factor family, epidermal growth factor, transforming growth factor β, VEGF, hepatocyte growth factor, insulin-like growth factor, and platelet-derived growth factor play important roles in directing the wound healing process. As a complicated process, wound healing involves different events such as inflammation resolution, re-epithelization, extracellular matrix formation, angiogenesis, and tissue remodeling.47
In the present study, we focused on the relationship between the level of PTP1B and vascular endothelial cell angiogenesis. Our data showed that, when compared with control mice without streptozotocin treatment, streptozotocin-treated mice displayed an impaired wound healing (Figure 1C). A delayed wound healing was also observed in ob/ob mice when compared with their WT littermates (Figure 3C). The wound tissue in ob/ob mice or streptozotocin-treated WT mice was composed of an irregular collagen structure and had an impaired neovascularization (Figures 1C and 1D and 3C and 3D). Importantly, streptozotocin-induced hyperglycemia had no effect on the wound healing in PTP1B−/− mice, suggesting that PTP1B plays an important role in the impaired wound healing by hyperglycemia in streptozotocin-treated mice or ob/ob mice. In support of this finding, we observed that HG in culture medium can greatly increase the expression level and activity of PTP1B in HMEC-1 cells (Figure 4B and 4C). Overexpression of PTP1B in HMEC-1 cells strongly inhibited endothelial cell proliferation, migration, and tube formation (Figures 4E and 5C and 5D). Interestingly, inhibition of PTP1B activity by FRJ significantly improved the wound healing in streptozotocin-treated mice (Figure 2A) and ob/ob mice (Figure 3C), as well as the tube formation, migration, and proliferation of HEMC-1 cells in the presence of HG (Figures 4A and 5A and 5B). Taken together, these results demonstrate that hyperglycemia impairs the wound healing in the presence of diabetes via increasing the expression level and activity of PTP1B, and that inhibition of PTP1B activity by specific PTP1B inhibitor may provide a potential effective therapeutic strategy for rescuing the impaired wound healing under diabetic condition. Our finding of PTP1B as an inhibitory modulator for wound healing is in agreement with the previous reports by Nakamura et al13 and Sugano et al.48 Using a mouse hindlimb ischemia model, Nakamura et al13 found that PTP1B expression and activity were markedly increased and PTP1B negatively regulated vascular endothelial cell adhesions and angiogenesis. In the study of wound healing in a rat model of hindlimb ischemia, Sugano et al48 also reported that PTP inhibitor could significantly accelerate angiogenesis process.
Recent studies have demonstrated that PTP1B was upregulated and activated by proinflammatory factors, such as TNF-α under a chronic, low-degree inflammatory condition.14,24 In the present study, we showed that hyperglycemia is a key factor in promoting PTP1B expression and activation. PTP1B level was significantly higher in streptozotocin-treated diabetic mice than in control mice without streptozotocin treatment (Figure 1E). Lowering plasma glucose level in streptozotocin-treated mice by injection insulin largely abolished the induction of PTP1B by hyperglycemia and rescued mouse wound healing (Figure IIIB and IIIC in the online-only Data Supplement). These results collectively suggest that the impaired wound healing in the presence of diabetes mellitus can be improved by restoring normal blood glucose level. Through determining the alteration of TNF-α and ROS levels in microvascular endothelial cells after the stimulation of HG in medium, we found that the level of ROS (Figure 6A) but not TNF-α (data not shown) was significantly increased by HG. Our data further show that the upregulation of PTP1B expression by hyperglycemia is likely through the activation of the ROS-NF-κB axis (Figure 6B and 6C).
PTP1B can target various phosphorylated molecules to execute its function,49,50 and VEGFR2 has been recently reported as a possible substrate of PTP1B.13,51 As a potent proangiogenic factor specific for vascular endothelial cells, VEGFR2 plays an essential role in promoting endothelial cell angiogenesis, including cell proliferation, migration, and tube formation.40,41 The activation of VEGFR2 by phosphorylation on its Tyr1157 will activate multiple signaling enzymes, including mitogen-activated protein kinase, Akt and protein kinase C, and initiate the downstream signaling pathways that lead to angiogenesis.40,47,52,53 In the present study, we confirmed that VEGFR2 is an essential component for directing vascular endothelial angiogenesis and that VEGFR2 may serve as a target of PTP1B in the presence of diabetes. Under hyperglycemic conditions, the levels of phosphorylated VEGFR2 were significantly reduced in streptozotocin-treated mice and ob/ob mice (Figure 7A and 7C). Decreased VEGFR2 activity in vascular endothelial cells was associated with disrupted collagen structure and impaired neovascularization and wound healing (Figure 1C and 1D). This finding was also supported by in vitro assays demonstrating that HG in culture significantly reduced VEGFR2 phosphorylation (Figure 7D) and inhibited the proliferation, migration, and tube formation of HMEC-1 cells (Figures 4A and 5A and 5B). Interestingly, after knocking down VEGFR2 with siVEGFR2 in HMEC-1 cells, endothelial cell proliferation, migration, and tube formation were suppressed (Figure 7F–7I). Because PTP1B inhibition largely restores the level of VEGFR2 phosphorylation in streptozotocin-treated diabetic mice (Figure 7B), ob/ob mice (Figure 7C), and HMEC-1 cells cultured in medium containing HG (Figure 7D), it is likely that the hyperglycemia-induced VEGFR2 dephosphorylation is mediated by PTP1B. Our results demonstrate that hyperglycemia can reduce phosphorylated VEGFR2 level in vascular endothelial cells and thus suppress endothelial cell angiogenesis and impair the wound healing.
In summary, our results revealed that PTP1B, which expression was upregulated under hyperglycemia conditions, impaired the diabetic wound healing process likely through dephosphorylating the endothelial cell VEGFR2. Moreover, PTP1B inhibited proliferation, migration, and tube formation of cultured microvascular endothelial cells induced by VEGF. Our study also provided PTP1B specific inhibitor as a potential novel therapeutic approach to improve diabetic wound healing.
Supplementary Material
Significance.
Impaired wound healing is a major complication of diabetic patients. The mechanisms that govern wound healing, however, are complicated and poorly understood. Here, we report that protein tyrosine phosphatase 1B (PTP1B), which is upregulated and activated under hyperglycemic condition, plays a critical role in inhibiting wound healing process. The impaired wound healing in diabetic mice or human microvascular endothelial cells cultured in the medium with high glucose concentration can be rescued by a PTP1B inhibitor. PTP1B inhibits vascular cell angiogenesis likely through dephosphorylation of vascular endothelial growth factor receptor 2. By demonstrating PTP1B as an important negative modulator in wound healing process, our study provides the specific inhibitor of PTP1B as a potential novel therapeutic tool for diabetic wound healing.
Acknowledgments
K. Zen, C. Zhang, and Y. Liu designed the research and analyzed data. J. Zhang and K. Zen drafted the article. J. Zhang, L. Li, J. Li, and Y. Zhang collected samples, performed experiments, and analyzed data.
Sources of Funding
This work was supported by grants from the National Basic Research Program of China (2012CB517603 and 2011CB504803), the National Natural Science Foundation of China (Nos 30988003, 30225037, 30471991, and 30570731) and the Natural Science Foundation of Jiangsu Province (No. BK2011013).
Nonstandard Abbreviations and Acronyms
- HG
high glucose concentration
- HMEC-1
human microvascular endothelial cells
- LG
low glucose concentration
- NF-κB
nuclear factor-κB
- PTP1B
protein tyrosine phosphatase 1B
- ROS
reactive oxygen species
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth factor receptor 2
- WT
wild-type
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.114.304705/-/DC1.
Disclosures
None.
References
- 1.Mendes JJ, Leandro C, Corte-Real S, Barbosa R, Cavaco-Silva P, Melo-Cristino J, Górski A, Garcia M. Wound healing potential of topical bacteriophage therapy on diabetic cutaneous wounds. Wound Repair Regen. 2013;21:595–603. doi: 10.1111/wrr.12056. [DOI] [PubMed] [Google Scholar]
- 2.Immonen JA, Zagon IS, Lewis GS, McLaughlin PJ. Topical treatment with the opioid antagonist naltrexone accelerates the remodeling phase of full-thickness wound healing in type 1 diabetic rats. Exp Biol Med (Maywood) 2013;238:1127–1135. doi: 10.1177/1535370213502632. [DOI] [PubMed] [Google Scholar]
- 3.Moura LI, Dias AM, Leal EC, Carvalho L, de Sousa HC, Carvalho E. Chitosan-based dressings loaded with neurotensin–an efficient strategy to improve early diabetic wound healing. Acta Biomater. 2014;10:843–857. doi: 10.1016/j.actbio.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 4.Yang W, Lu J, Weng J, et al. China National Diabetes and Metabolic Disorders Study Group. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 5.Jeffcoate WJ, Harding KG. Diabeticfootulcers. Lancet. 2003;361:1545–1551. doi: 10.1016/S0140-6736(03)13169-8. [DOI] [PubMed] [Google Scholar]
- 6.Lerman OZ, Galiano RD, Armour M, Levine JP, Gurtner GC. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol. 2003;162:303–312. doi: 10.1016/S0002-9440(10)63821-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fadini GP, Albiero M, Menegazzo L, Boscaro E, Pagnin E, Iori E, Cosma C, Lapolla A, Pengo V, Stendardo M, Agostini C, Pelicci PG, Giorgio M, Avogaro A. The redox enzyme p66Shc contributes to diabetes and ischemia-induced delay in cutaneous wound healing. Diabetes. 2010;59:2306–2314. doi: 10.2337/db09-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad F, Goldstein BJ. Increased abundance of specific skeletal muscle protein-tyrosine phosphatases in a genetic model of insulin-resistant obesity and diabetes mellitus. Metabolism. 1995;44:1175–1184. doi: 10.1016/0026-0495(95)90012-8. [DOI] [PubMed] [Google Scholar]
- 9.Dadke SS, Li HC, Kusari AB, Begum N, Kusari J. Elevated expression and activity of protein-tyrosine phosphatase 1B in skeletal muscle of insulin-resistant type II diabetic Goto-Kakizaki rats. Biochem Biophys Res Commun. 2000;274:583–589. doi: 10.1006/bbrc.2000.3188. [DOI] [PubMed] [Google Scholar]
- 10.Lam NT, Covey SD, Lewis JT, Oosman S, Webber T, Hsu EC, Cheung AT, Kieffer TJ. Leptin resistance following over-expression of protein tyrosine phosphatase 1B in liver. J Mol Endocrinol. 2006;36:163–174. doi: 10.1677/jme.1.01937. [DOI] [PubMed] [Google Scholar]
- 11.Stuible M, Doody KM, Tremblay ML. PTP1B and TC-PTP: regulators of transformation and tumorigenesis. Cancer Metastasis Rev. 2008;27:215–230. doi: 10.1007/s10555-008-9115-1. [DOI] [PubMed] [Google Scholar]
- 12.Dinh T, Tecilazich F, Kafanas A, Doupis J, Gnardellis C, Leal E, Tellechea A, Pradhan L, Lyons TE, Giurini JM, Veves A. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes. 2012;61:2937–2947. doi: 10.2337/db12-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura Y, Patrushev N, Inomata H, Mehta D, Urao N, Kim HW, Razvi M, Kini V, Mahadev K, Goldstein BJ, McKinney R, Fukai T, Ushio-Fukai M. Role of protein tyrosine phosphatase 1B in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells. Circ Res. 2008;102:1182–1191. doi: 10.1161/CIRCRESAHA.107.167080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi L, Zhang Q, Xu B, Jiang X, Dai Y, Zhang CY, Zen K. Sustained high protein-tyrosine phosphatase 1B activity in the sperm of obese males impairs the sperm acrosome reaction. J Biol Chem. 2014;289:8432–8441. doi: 10.1074/jbc.M113.517466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zarelli VE, Ruete MC, Roggero CM, Mayorga LS, Tomes CN. PTP1B dephosphorylates N-ethylmaleimide-sensitive factor and elicits SNARE complex disassembly during human sperm exocytosis. J Biol Chem. 2009;284:10491–10503. doi: 10.1074/jbc.M807614200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dadke S, Chernoff J. Protein-tyrosine phosphatase 1B mediates the effects of insulin on the actin cytoskeleton in immortalized fibroblasts. J Biol Chem. 2003;278:40607–40611. doi: 10.1074/jbc.M306772200. [DOI] [PubMed] [Google Scholar]
- 17.Bandyopadhyay D, Kusari A, Kenner KA, Liu F, Chernoff J, Gustafson TA, Kusari J. Protein-tyrosine phosphatase 1B complexes with the insulin receptor in vivo and is tyrosine-phosphorylated in the presence of insulin. J Biol Chem. 1997;272:1639–1645. doi: 10.1074/jbc.272.3.1639. [DOI] [PubMed] [Google Scholar]
- 18.Dadke S, Kusari J, Chernoff J. Down-regulation of insulin signaling by protein-tyrosine phosphatase 1B is mediated by an N-terminal binding region. J Biol Chem. 2000;275:23642–23647. doi: 10.1074/jbc.M001063200. [DOI] [PubMed] [Google Scholar]
- 19.Kenner KA, Anyanwu E, Olefsky JM, Kusari J. Protein-tyrosine phosphatase 1B is a negative regulator of insulin- and insulin-like growth factor-I-stimulated signaling. J Biol Chem. 1996;271:19810–19816. doi: 10.1074/jbc.271.33.19810. [DOI] [PubMed] [Google Scholar]
- 20.Choi JH, Kim HS, Kim SH, Yang YR, Bae YS, Chang JS, Kwon HM, Ryu SH, Suh PG. Phospholipase Cgamma1 negatively regulates growth hormone signalling by forming a ternary complex with Jak2 and protein tyrosine phosphatase-1B. Nat Cell Biol. 2006;8:1389–1397. doi: 10.1038/ncb1509. [DOI] [PubMed] [Google Scholar]
- 21.Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12:917–924. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- 22.Ahima RS, Osei SY. Leptin signaling. Physiol Behav. 2004;81:223–241. doi: 10.1016/j.physbeh.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Myers MP, Andersen JN, Cheng A, Tremblay ML, Horvath CM, Parisien JP, Salmeen A, Barford D, Tonks NK. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J Biol Chem. 2001;276:47771–47774. doi: 10.1074/jbc.C100583200. [DOI] [PubMed] [Google Scholar]
- 24.Bjorge JD, Pang A, Fujita DJ. Identification of protein-tyrosine phosphatase 1B as the major tyrosine phosphatase activity capable of dephosphorylating and activating c-Src in several human breast cancer cell lines. J Biol Chem. 2000;275:41439–41446. doi: 10.1074/jbc.M004852200. [DOI] [PubMed] [Google Scholar]
- 25.Woodford-Thomas TA, Rhodes JD, Dixon JE. Expression of a protein tyrosine phosphatase in normal and v-src-transformed mouse 3T3 fibroblasts. J Cell Biol. 1992;117:401–414. doi: 10.1083/jcb.117.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortesio CL, Chan KT, Perrin BJ, Burton NO, Zhang S, Zhang ZY, Huttenlocher A. Calpain 2 and PTP1B function in a novel pathway with Src to regulate invadopodia dynamics and breast cancer cell invasion. J Cell Biol. 2008;180:957–971. doi: 10.1083/jcb.200708048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arias-Romero LE, Saha S, Villamar-Cruz O, Yip SC, Ethier SP, Zhang ZY, Chernoff J. Activation of Src by protein tyrosine phosphatase 1B Is required for ErbB2 transformation of human breast epithelial cells. Cancer Res. 2009;69:4582–4588. doi: 10.1158/0008-5472.CAN-08-4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976;193:415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- 29.Galiano RD, Michaels JV, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12:485–492. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 30.Ding H, Zhang Y, Xu C, Hou D, Li J, Zhang Y, Peng W, Zen K, Zhang CY, Jiang X. Norathyriol reverses obesity- and high-fat-diet-induced insulin resistance in mice through inhibition of PTP1B. Diabetologia. 2014;57:2145–2154. doi: 10.1007/s00125-014-3315-8. [DOI] [PubMed] [Google Scholar]
- 31.Galeano M, Altavilla D, Cucinotta D, Russo GT, Calò M, Bitto A, Marini H, Marini R, Adamo EB, Seminara P, Minutoli L, Torre V, Squadrito F. Recombinant human erythropoietin stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetes. 2004;53:2509–2517. doi: 10.2337/diabetes.53.9.2509. [DOI] [PubMed] [Google Scholar]
- 32.Uchida K, Fujiwara H. Pancreatic lesions of small-obese mice (C57BL/6 J-ob/ob) with hyperglucagonemia. Jikken Dobutsu. 1988;37:421–428. doi: 10.1538/expanim1978.37.4_421. [DOI] [PubMed] [Google Scholar]
- 33.Hunt CE, Lindsey JR, Walkley SU. Animal models of diabetes and obesity, including the PBB/Ld mouse. Fed Proc. 1976;35:1206–1217. [PubMed] [Google Scholar]
- 34.Bourdeau A, Dubé N, Tremblay ML. Cytoplasmic protein tyrosine phosphatases, regulation and function: the roles of PTP1B and TC-PTP. Curr Opin Cell Biol. 2005;17:203–209. doi: 10.1016/j.ceb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Seely BL, Staubs PA, Reichart DR, Berhanu P, Milarski KL, Saltiel AR, Kusari J, Olefsky JM. Protein tyrosine phosphatase 1B interacts with the activated insulin receptor. Diabetes. 1996;45:1379–1385. doi: 10.2337/diab.45.10.1379. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Liu D, Chen X, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133–144. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem. 2008;283:14230–14241. doi: 10.1074/jbc.M800061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panzhinskiy E, Ren J, Nair S. Protein tyrosine phosphatase 1B and insulin resistance: role of endoplasmic reticulum stress/reactive oxygen species/nuclear factor kappa B axis. PLoS One. 2013;8:e77228. doi: 10.1371/journal.pone.0077228. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Snyder JG, Prewitt R, Campsen J, Britt LD. PDTC and Mg132, inhibitors of NF-kappaB, block endotoxin induced vasodilation of isolated rat skeletal muscle arterioles. Shock. 2002;17:304–307. doi: 10.1097/00024382-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 41.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–478. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 42.Kranke P, Bennett M, Roeckl-Wiedmann I, Debus S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2004:CD004123. doi: 10.1002/14651858.CD004123.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Bryan N, Ahswin H, Smart N, Bayon Y, Wohlert S, Hunt JA. Reactive oxygen species (ROS)–a family of fate deciding molecules pivotal in constructive inflammation and wound healing. Eur Cell Mater. 2012;24:249–265. doi: 10.22203/ecm.v024a18. [DOI] [PubMed] [Google Scholar]
- 44.Banerjee J, Chan YC, Sen CK. MicroRNAs in skin and wound healing. Physiol Genomics. 2011;43:543–556. doi: 10.1152/physiolgenomics.00157.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balaji S, King A, Crombleholme TM, Keswani SG. The Role of Endothelial Progenitor Cells in Postnatal Vasculogenesis: Implications for Therapeutic Neovascularization and Wound Healing. Adv Wound Care (New Rochelle) 2013;2:283–295. doi: 10.1089/wound.2012.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peppa M, Stavroulakis P, Raptis SA. Advanced glycoxidation products and impaired diabetic wound healing. Wound Repair Regen. 2009;17:461–472. doi: 10.1111/j.1524-475X.2009.00518.x. [DOI] [PubMed] [Google Scholar]
- 47.Peplow PV, Baxter GD. Gene expression and release of growth factors during delayed wound healing: a review of studies in diabetic animals and possible combined laser phototherapy and growth factor treatment to enhance healing. Photomed Laser Surg. 2012;30:617–636. doi: 10.1089/pho.2012.3312. [DOI] [PubMed] [Google Scholar]
- 48.Sugano M, Tsuchida K, Makino N. A protein tyrosine phosphatase inhibitor accelerates angiogenesis in a rat model of hindlimb ischemia. J Cardiovasc Pharmacol. 2004;44:460–465. doi: 10.1097/01.fjc.0000143275.45289.0a. [DOI] [PubMed] [Google Scholar]
- 49.Feldhammer M, Uetani N, Miranda-Saavedra D, Tremblay ML. PTP1B: a simple enzyme for a complex world. Crit Rev Biochem Mol Biol. 2013;48:430–445. doi: 10.3109/10409238.2013.819830. [DOI] [PubMed] [Google Scholar]
- 50.Bourdeau A, Dubé N, Tremblay ML. Cytoplasmic protein tyrosine phosphatases, regulation and function: the roles of PTP1B and TC-PTP. Curr Opin Cell Biol. 2005;17:203–209. doi: 10.1016/j.ceb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Lanahan AA, Lech D, Dubrac A, Zhang J, Zhuang ZW, Eichmann A, Simons M. PTP1b is a physiologic regulator of vascular endothelial growth factor signaling in endothelial cells. Circulation. 2014;130:902–909. doi: 10.1161/CIRCULATIONAHA.114.009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsumoto T, Mugishima H. Signal transduction via vascular endothelial growth factor (VEGF) receptors and their roles in atherogenesis. J Atheroscler Thromb. 2006;13:130–135. doi: 10.5551/jat.13.130. [DOI] [PubMed] [Google Scholar]
- 53.Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res. 2006;12:5018–5022. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.