Abstract
Using cardiovascular disease (CVD) risk instead of, or in addition to, blood pressure (BP) to guide antihypertensive treatment is an active area of research. The purpose of this review is to provide an overview of studies that may inform this treatment paradigm. We review data from randomized trials on relative and absolute CVD risk reduction that may occur when antihypertensive treatment is guided by CVD risk. We review population-level data on using CVD risk in conjunction with BP for guiding antihypertensive treatment, the broad distribution in CVD risk for people with similar BP levels, and the use of CVD risk for guiding antihypertensive treatment among sub-groups including older adults, young adults and those with diabetes or chronic kidney disease. Also, we review potential challenges in implementing antihypertensive treatment recommendations that incorporate CVD risk. In closing, we provide recommendations for using CVD risk in combination with BP to guide antihypertensive treatment.
Keywords: hypertension, risk prediction, treatment
Observational studies have demonstrated graded associations between both systolic (SBP) and diastolic (DBP) blood pressure and level of risk for cardiovascular disease (CVD) (1,2). The risk for CVD increases continuously between a SBP of at least 115 mm Hg and ≥180 mm Hg, and a DBP of at least 75 mm Hg and ≥110 mm Hg. Despite these graded associations, hypertension is often diagnosed among people with SBP or DBP above certain thresholds, typically an average SBP/DBP ≥140/90 mm Hg (3). Individuals with hypertension are usually advised to make lifestyle changes, and most require antihypertensive medication for control of their blood pressure (BP). Adults with SBP and DBP below the levels used to define hypertension are often not prescribed antihypertensive medication, even though many of them may have high CVD risk. Several studies have considered whether subgroups of people with SBP and DBP below the levels used to diagnose hypertension are likely to benefit from the initiation of antihypertensive medication.
Using absolute CVD risk, instead of or in addition to blood pressure (BP) levels, to guide antihypertensive treatment decisions was first proposed more than 20 years ago and has been adopted in several countries (4–8). In 1997, the sixth report of the Joint National Committee (JNC) on prevention, detection, evaluation and treatment of high blood pressure (JNC6) recommended using CVD risk and BP levels to guide treatment (Table 1) (9). However, in 2003, the JNC7 reverted to a treatment paradigm based entirely on BP levels (3). The goal of this paper is to review the evidence related to incorporating an individual’s CVD risk in the decision to prescribe medication and determine the intensity of antihypertensive medication. We provide an overview of evidence from randomized trials of antihypertensive medication on the prevention of outcomes for patients by CVD risk. Additionally, we review the data related to using a risk-based approach versus using BP alone in making antihypertensive treatment decisions, the variation in CVD risk for people with similar BP levels, the use of CVD risk to guide antihypertensive treatment decisions for population subgroups, including older adults, young adults, and those with diabetes or chronic kidney disease (CKD), and challenges that may be encountered in implementing an approach that incorporates CVD risk to guide antihypertensive medication use. In closing, we provide recommendations for how CVD risk can be used in conjunction with BP levels to make treatment decisions.
Table 1.
Use of CVD Risk in Conjunction With BP Level in Guiding Antihypertensive Medication Treatment in the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC6)
| BP, mm Hg | ≥1 Risk Factor, But Not | |||
|---|---|---|---|---|
| SBP | DBP | No Risk Factors, Organ Damage or Clinical CVD |
Diabetes, Organ Damage, or Clinical CVD |
Organ Damage, Clinical CVD, or Diabetes |
| 130–139 | 85–89 | Lifestyle modification | Lifestyle modification | Drug therapy*,† |
|
| ||||
| 140–159 | 90–99 | Lifestyle modification (up to 12 months) |
Lifestyle modification‡ (up to 6 months) |
Drug therapy† |
|
| ||||
| ≥160 | ≥100 | Drug therapy† | Drug therapy† | Drug therapy† |
Organ damage includes left ventricular hypertrophy, angina, prior myocardial infarction, prior coronary revascularization, heart failure, stroke, transient ischemic attack, nephropathy, peripheral arterial disease, retinopathy. Risk factors include age ≥60 years, male sex or being post-menopausal for women, smoking, dyslipidemia, diabetes mellitus, family history of CVD. Adapted from: National Institutes of Health, National Heart, Lung, and Blood Institute, National High Blood Pressure Education Program (90).
For patients with heart failure, renal insufficiency, or diabetes.
Lifestyle modification is adjunctive for all patients recommended drug therapy
Patients with multiple risk factors should be considered for drug therapy as initial therapy in conjunction with lifestyle modification. CVD = cardiovascular disease; BP = blood pressure; DBP = diastolic blood pressure; SBP = systolic blood pressure.
Evidence From Randomized Trials
Pooled individual-level data from randomized trials comparing pharmacological BP lowering therapy versus placebo, and more versus less intensive antihypertensive treatment regimens reveal potential benefits of focusing antihypertensive medication treatment decisions on predicted CVD risk (10). The relative and absolute differences in CVD events associated with antihypertensive medications were evaluated across a range of absolute CVD risk from 11 trials that enrolled over 50,000 participants. The risk ratio for CVD associated with BP reduction with antihypertensive medication did not vary across level of predicted CVD risk (Figure 1, left). The quite similar relative risk reduction at each CVD risk level corresponded with progressively larger absolute reductions in CVD risk among participants with increasingly higher 5-year predicted CVD risk (Figure 1, right). Also, the authors estimated that treating 1,000 people with a 5-year risk <11% (observed 5-year event rate of 6.5%) would prevent 14 events over 5 years compared with 20, 24, and 38 events prevented if their 5-year risk of CVD was 11% to 15%, 15% to 21%, or >21% (observed 5-year event rates of 13.2%, 20.6%, and 24.8%), respectively. The number needed to treat for 5 years to prevent 1 CVD event was 71, 51, 41, and 26 for participants with a 5-year CVD risk <11%, 11% to 15%, 15% to 21%, or >21%, respectively. The patterns of larger absolute risk reductions with treatment for those with progressively higher predicted CVD risk were also observed for stroke, coronary heart disease (CHD), heart failure, and CVD death.
Figure 1. Relative and Absolute Reduction in CVD Events with BP Reduction by 5-Year Risk of CVD.
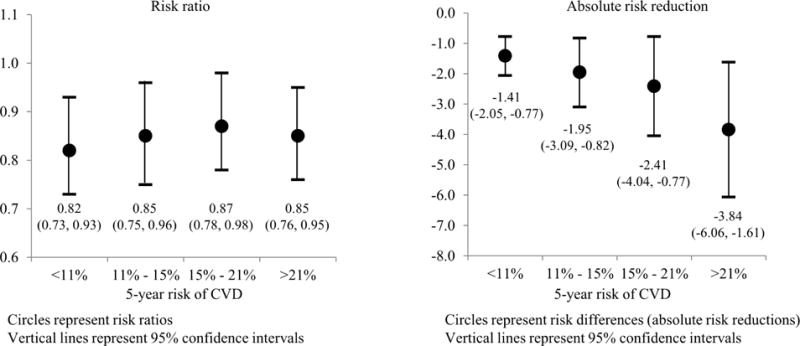
This figure shows data from a pooled analysis of 11 randomized controlled trials of antihypertensive treatment versus placebo (N = 67,475 participants). Participants were grouped into strata on the basis of 5-year predicted risk for cardiovascular disease events. Left: The relative risk for having a cardiovascular disease event during follow-up is similar within strata of predicted cardiovascular disease risk, ranging from 0.82 to 0.87. Right: The absolute risk reduction associated with treatment versus placebo is progressively larger among participants with higher predicted cardiovascular disease risk. The larger absolute risk reduction among those with higher predicted risk means that antihypertensive treatment will reduce more events when treating high-versus low-risk people. Reprinted, with permission, from Blood Pressure Lowering Treatment Trialists’ Collaboration (89). BP = blood pressure; CVD = cardiovascular disease.
Cost-Effectiveness
A simulation analysis using the Third National Health and Nutrition Examination Survey (NHANES III) has demonstrated that using CVD risk in conjunction with BP may prevent more CVD events than using BP levels alone for guiding antihypertensive treatment (11). This study evaluated U.S. adults 30 to 85 years of age who were initially without a history of myocardial infarction, stroke, or heart failure. The approach using CVD risk in conjunction with BP assumed antihypertensive treatment would be allocated to U.S. adults with SBP ≥150 mm Hg or those projected to have an absolute CVD risk reduction >1.7% over 5 years with treatment, whereas the approach using BP alone assumed U.S. adults without diabetes would receive antihypertensive medication if they had SBP ≥140 mm Hg or DBP ≥90 mm Hg (SBP ≥130 mm Hg or DBP ≥85 mm Hg for U.S. adults with diabetes mellitus). Using BP alone to guide treatment resulted in more U.S. adults being prescribed antihypertensive medication compared with the approach that used CVD risk in conjunction with BP (79 million vs. 63 million). However, over 5 years, using CVD risk and BP to guide treatment was projected to prevent 4.2 million CVD events compared with 3.3 million events for the approach using BP alone. Additionally, the model suggested that the CVD risk and BP-based approach would save more quality-adjusted life-years (22.2 million) when compared with the approach using BP alone (19.3 million). The potential benefits of CVD prevention using a CVD risk-informed approach versus a BP-based approach have also been noted in other U.S.-based analyses and for other countries (12,13).
Estimating CVD Risk
Models for predicting CHD and CVD events have been in use for almost 50 years (14,15). The best-known risk prediction models were developed in the Framingham Heart Study (16–18). A model for predicting CHD by Framingham Heart Study investigators was published in 1967 (15). Equations to predict CHD have been refined and updated since that initial publication, and an equation published by the Framingham investigators was incorporated in the U.S. National Cholesterol Education Program Adult Treatment Panel guideline for the management of cholesterol (17–19). This equation was further expanded in 2008, when a model on the basis of Framingham Heart Study experience was developed to predict CHD, stroke, CVD death, heart failure, and claudication (16). In 2013, the American College of Cardiology (ACC) and American Heart Association (AHA) published the Pooled Cohort risk equations (20). These equations are used to estimate 10-year risk for CVD (i.e., CHD and stroke) and were adopted by the 2013 ACC/AHA guideline for the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults (21). This guideline incorporates a treatment approach that is on the basis of CVD risk in conjunction with LDL cholesterol levels. Specifically, adults with LDL cholesterol ≥190 mg/dl are recommended statin therapy. Also, statins are recommended for adults with a history of CVD, diabetes, and those with LDL cholesterol between 70 and 189 mg/dl if their 10-year risk for an atherosclerotic CVD event on the basis of the Pooled Cohort risk equations is ≥7.5%. A number of other risk prediction models have been developed across a variety of study groups (18,22–25). Although the Pooled Cohort risk equations have exhibited good test properties in several studies, the choice of equation to estimate CVD risk in a specific setting relates to validation data showing good discrimination and calibration in the patients of interest (26–29).
Using an equation to estimate 10-year predicted CVD risk has been shown to be substantially more accurate compared with clinician judgment or counting the number of risk factors for a patient (30–32). Also, giving healthcare providers a patient’s predicted CVD risk can change their treatment approach (33). A study using vignettes found that treatment increased among high-risk patients and decreased among low-risk patients when primary care providers were given the 10-year calculated CVD risk for patients. Still, risk prediction is underutilized in clinical practice (34). A survey of 952 physicians recruited from the American Academy of Family Physicians and the American College of Physicians found high awareness (92%) of global CHD risk calculators (34). However, only 41% of physicians reported using CHD risk assessment in their practice. Of those who reported calculating global CHD risk, 48% reported using it to guide antihypertensive medication treatment.
Heterogeneity in CVD Risk
In addition to BP, older age, and history of CVD, diabetes, LDL and HDL cholesterol, and smoking have substantial influences on CVD risk (20). Figure 2 shows the predicted CVD risk associated with SBP of 110 mm Hg and 130 mm Hg for hypothetical adults with low, medium, and high CVD risk estimated using the Pooled Cohort risk equations. The predicted risk for CVD among people with the same SBP can differ by more than 30-fold on the basis of an individual’s age and the presence of other CVD risk factors. The increase in predicted CVD risk with higher BP is small among low-risk individuals, but a more substantial absolute increase in CVD risk is present for those with other CVD risk factors. On the basis of analysis of the NHANES data from 2009 to 2012, 78 million U.S. adults have SBP between 120 and 139 mm Hg or DBP between 80 mm and 89 mm Hg, and 31 million U.S. adults have SBP ≥140 mm Hg or DBP ≥90 mm Hg (35). In each of these populations, there is a wide range of 10-year predicted CVD risk (Figure 3). With the broad distribution of predicted CVD risk in these populations, the absolute risk reduction resulting from taking BP-lowering medication may also vary. Using BP alone to guide treatment decisions would result in treating a sizable group that is likely to obtain only a small short-term reduction in absolute CVD risk.
Figure 2. Ten-Year Predicted CVD Risk for Hypothetical Low-, Intermediate-, and High-Risk Adults at SBP Levels of 110 mm Hg and 130 mm Hg.
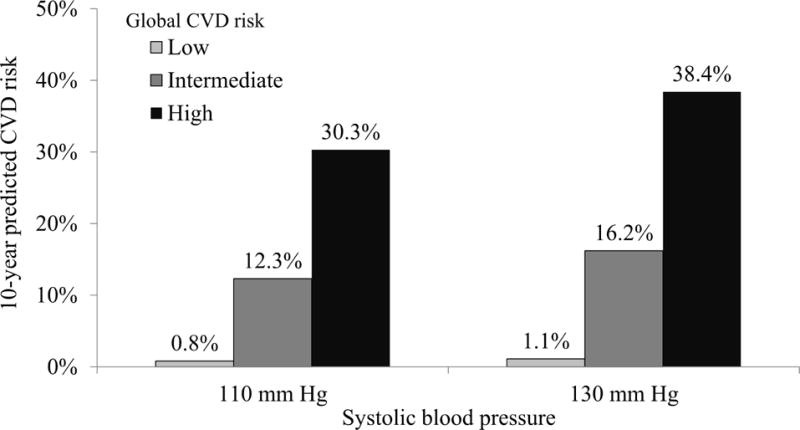
The 10-year predicted cardiovascular disease risk is shown for 6 hypothetical people: 3 people with SBP of 110 mm Hg and 3 with SBP of 130 mm Hg. For each blood pressure level, each of the 3 people has a different cardiovascular disease risk factor profile. This figure shows that people’s 10-year predicted cardiovascular disease risk can vary more than 30-fold, despite having the same blood pressure level. Low risk: 50-year-old, white woman, nonsmoker, without diabetes, not taking antihypertensive medication, total cholesterol of 180 mg/dl and HDL cholesterol of 54 mg/dl. Intermediate risk: 60-year-old, black man, nonsmoker, with diabetes, not taking antihypertensive medication, total cholesterol of 240 mg/dl and HDL cholesterol of 50 mg/dl. High risk: 70-year-old, white man, smoker, with diabetes, not taking antihypertensive medication, total cholesterol of 240 mg/dl and HDL cholesterol of 40 mg/dl. CVD = cardiovascular disease; HDL = high-density lipoprotein; SBP = systolic blood pressure.
Figure 3. Distribution of 10-Year Predicted CVD Risk Among U.S. Adults by Level of SBP and DBP.
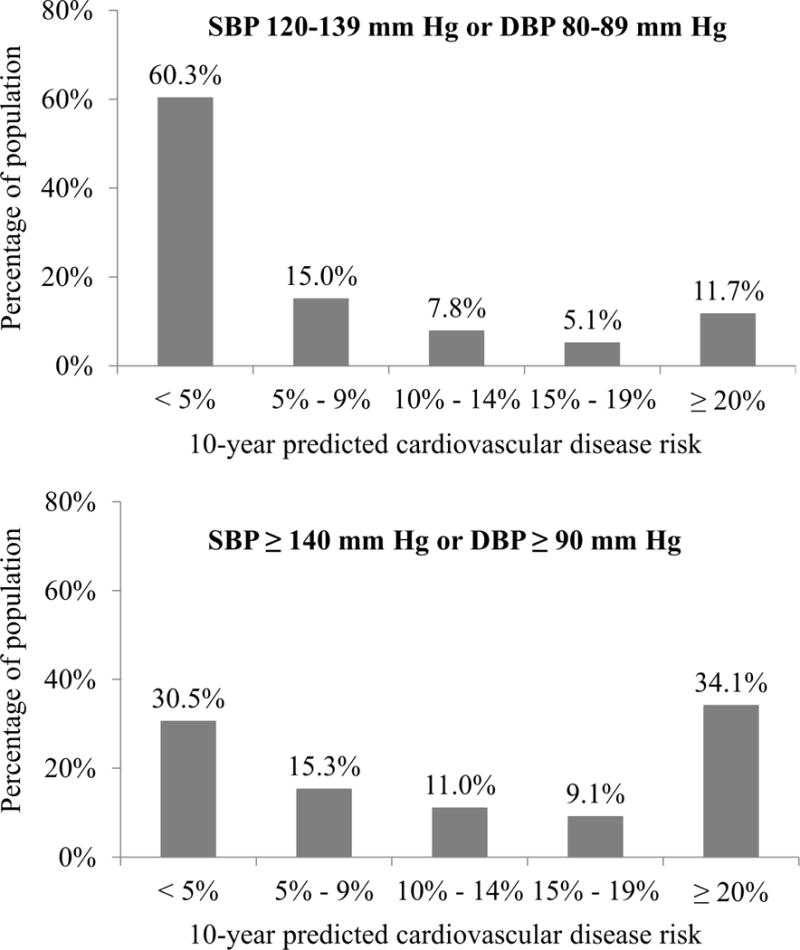
The distribution of 10-year predicted CVD risk among U.S. adults with SBP between 120 and 139 mm Hg and/or DBP between 80 and 89 mm Hg (Top), and for those with SBP ≥ 90 mm Hg (Bottom). These data were calculated using the 2009–2012 National Health and Nutrition Examination Survey. For each blood pressure group, there is a broad range of 10-year predicted CVD risk. DBP = diastolic blood pressure. Other abbreviations as in Figure 1.
Who Has CVD Events?
The vast majority of incident CVD events occur among adults with a 10-year predicted CVD risk ≥7.5%. Over the first 8 years of follow-up in the U.S. population-based REGARDS (REasons for Geographic and Racial Differences in Stroke) study, over 85% of CVD events occurred among participants with a 10-year predicted CVD risk ≥7.5% (27). Although those with SBP ≥140 mm Hg or DBP ≥90 mm Hg have a higher risk for CVD compared with their counterparts with SBP <140 mm Hg and DBP <90 mm Hg, most incident events in contemporary studies do not occur in those in the higher BP group (36). In a pooled analysis of participants not taking antihypertensive medication from 3 observational cohort studies, only 26% of CVD events occurred among participants with SBP ≥140 mm Hg, compared with 65% of events that occurred among participants with a 10-year CVD risk ≥7.5% (36). If BP level alone was used to guide treatment, most participants who went on to have CVD events in this study would not have been recommended antihypertensive medication.
Short-Term BP Variability Versus Variability in CVD Risk
BP levels also present the challenge of measurement error and visit-to-visit variability. In an analysis of ALLHAT (Antihypertensive Lipid-Lowering to Prevent Heart Attack Trial), BP varied substantially within individuals across their follow-up visits (37). Staff members in ALLHAT were trained to measure BP, which was measured twice at each visit following a standardized technique. Visit-to-visit BP variability is likely to be even greater in clinical practice, where BP can vary depending on the choice of sphygmomanometer and the protocol used to measure BP (38). Although treatment decisions should be made on the basis of BP averaged across multiple visits, high visit-to-visit BP variability can lead to misclassification. Additionally, visit-to-visit BP variability can mask the true reduction in BP that occurs following treatment initiation, leading some observers to propose not even conducting follow-up BP assessments following treatment initiation or titration (39).
Although BP varies substantially across visits, studies have shown that predicted CVD risk has less intra-individual changes over short periods of time. Using data from 808 participants enrolled in NHANES III, Ye et al. (40) evaluated changes in BP and 10-year predicted CVD risk by the Pooled Cohort risk equations occurring between 2 clinic visits conducted a median of 18 days apart (range: 3 to 48 days). For individual participants, the change in SBP across these 2 visits ranged from −23 mm Hg to 32 mm Hg (1st and 99th percentiles, respectively). Changes in 10-year predicted CVD risk between visits were smaller. Overall, 34% of participants had an 8 mm Hg or larger difference in SBP between visits, whereas only 8% had a ≥3% difference in 10-year predicted CVD risk. The stability of CVD risk over short periods of time may confer utility for clinical decision-making.
Special Populations
Older and Young Adults
Age is unquestionably the most important determinant of CVD risk, presumably because it represents the cumulative exposure to risk factors (41,42). As CVD risk increases with age, almost all adults ≥65 years of age have a high CVD risk (42). Concern has been raised about antihypertensive treatment for older adults, especially those who are frail (43). In SPRINT (Systolic Blood Pressure Intervention Trial), treating SBP to a goal of 120 mm Hg (intensive goal) was associated with a lower risk of CVD and all-cause mortality when compared with participants treated to a goal SBP of 140 mm Hg (usual goal) (44). The CVD and mortality benefits were present among participants ≥75 years of age and in subgroups with and without indications of frailty (45). It is noteworthy that SPRINT enrolled adults with high CVD risk, defined by a history of CVD, estimated glomerular filtration rate of 20 to 59 ml/min/1.73 m2, age ≥75 years, or 10-year predicted global CVD risk (including heart failure and claudication) ≥15% on the basis of the Framingham CVD risk score (46).
Several guidelines have incorporated global CVD risk into their treatment decision-making recommendations using a 5-year or 10-year time horizon (7,8,21,47). These 5- and 10-year intervals are used because they identify people who are likely to benefit from treatment in the short term. Only a small proportion of adults <50 years of age have a high 10-year predicted CVD risk (48). Using 10-year predicted risk to guide antihypertensive treatment results in a disproportionate selection of older adults, even though a substantial proportion of young adults have a high 30-year and lifetime risk for CVD (49,50). For example, a 45-year-old woman with total cholesterol of 260 mg/dl, HDL cholesterol of 35 mg/dl, and untreated SBP of 160 mm Hg would be predicted to have a 10-year CVD risk <10%, but a 30-year CVD risk of almost 30%. Also, preventing 1 CVD event in a younger adult may result in substantially more life-years gained compared with preventing an event in an older adult. Patients may value the short-term benefit of treatment more than future benefits that accrue over a long time horizon. To account for this valuation, economists often apply a discount that weighs near-term risk reduction benefits more than those that occur in the future. In 1 example, Jackson et al. (51) applied a 3% annual discount such that events that were prevented in the first year of follow-up were weighted at 100%, whereas those prevented in the next year were valued at 97%, and in the year after at only 94% (51,52). Applying a 3% annual discount to allow for this value judgement, and recognizing the higher case-fatality rate associated with CVD events at older age, the difference in average life expectancy gained with antihypertensive treatment between young and old adults is smaller than one would assume. Also, although older adults are more likely to suffer disability from a CVD event, it is possible that younger adults may lose more quality of life than older adults following an event. However, this has yet to be quantified. Another concern with not treating high BP among young adults is the risk for the development of left ventricular hypertrophy or albuminuria. It can be noted that it is uncommon for young adults with high BP, but no other risk factors, to develop organ damage (53).
CKD and/or Diabetes
Antihypertensive medication is a cornerstone therapy among patients with diabetes and CKD (54–56). As most people with CKD or diabetes have high CVD risk, and more patients with CKD die from CVD than develop end-stage renal disease, a risk-based treatment approach may allocate antihypertensive treatment to a higher proportion of those with CKD or diabetes than treatment on the basis of BP alone (57–60). Colantonio et al. (61) used data from the REGARDS study to show that only 8% of adults with CKD not taking statins have a 10-year predicted CVD risk <7.5%. The incidence of CVD was very low in those with CKD and a 10-year predicted CVD risk <7.5% (3 events/1,000 person-years) (61). Using predicted CVD risk would recommend almost all adults with CKD and diabetes receive antihypertensive treatment. The data from the REGARDS study suggests that treatment for the small subgroup of adults with CKD and low 10-year predicted CVD risk can be delayed. However, there may be some young adults with a substantial reduction in estimated glomerular filtration rate or heavy proteinuria for whom treatment may still be warranted, despite having low CVD risk.
Evidence from HOPE-3
The HOPE (Heart Outcomes Prevention Evaluation)–3 trial randomized 12,705 adults at intermediate CVD risk to 12.5 mg hydrochlorothiazide/16 mg candestartan or placebo (62). In a pre-specified subgroup analysis, antihypertensive treatment was associated with a statistically significant 27% risk reduction of the primary outcome (death from cardiovascular causes, nonfatal myocardial infarction, and nonfatal stroke) among participants with baseline SBP >143.5 mm Hg, but no risk reduction was present among those with SBP ≤131.5 mm Hg or 131.6 to 143.5 mm Hg. Although HOPE-3 did not address treatment of high-risk populations, it does support treating all people with SBP ≥140 mm Hg. Additionally, the HOPE-3 data suggest that moderate BP lowering with fixed-dose agents among low- or intermediate-risk patients with SBP <140 mm Hg may not prevent CVD events.
Studies that Have Provided Patients with Global CVD Risk Estimates
Studies have evaluated the impact of providing patients with their global CVD risk on accuracy of perceived risk, intent to start antihypertensive treatment, medication adherence, and reductions in predicted risk (63). In most of these studies, global CVD risk was presented more than 1 time and was done in conjunction with another intervention (e.g., education or counseling). In a systematic review, presentation of global CVD risk combined with education increased the intent to start therapy by 15% to 20% (64–67). Additionally, in focus groups (N = 29 participants, 12 of whom had hypertension), participants reported knowing their global CHD risk provided motivation to reduce this risk (68). However, participants were concerned that risk prediction may be “too simple” and “imprecise.”
Unanswered Questions
The extent to which predicted CVD risk aligns with the observed risk (i.e., calibration of the risk prediction equations) has been extensively studied over the past several years. Some studies have found that risk prediction equations accurately estimate risk, whereas others suggest a discrepancy between predicted and observed risk in the context of contemporary clinical practice with frequent use of risk-lowering medications (26–29). CVD incidence in the United States has declined over the past 30 years in association with greater use of preventive medications, and many risk prediction equations were developed in cohorts that were initiated in the 1980s and 1990s, when preventive medications were used less often (69,70). In countries where the risk of CVD is declining, poor calibration of risk prediction equations could result in treating populations with a lower risk than intended. The application of risk prediction models in populations other than those in which they were developed, including in contemporary clinical practice, may require recalibration (71,72).
Most CVD risk prediction equations are focused on CHD and stroke as outcomes. There are risk prediction models, including the Framingham global CVD risk score for general practice, that include a broader range of CVD outcomes, including heart failure and claudication (16). High BP is associated with an increased risk for left ventricular hypertrophy, CKD, end-stage renal disease, atrial fibrillation, dementia, peripheral artery disease, and retinopathy (73–79). CVD risk scores do not include these as outcomes. Additional research could be done to investigate the development of risk prediction equations that focus on hypertension-related CVD as outcomes, and testing whether these models would be more cost-effective and address patient preferences better than existing risk prediction models.
The discussion of the evidence related to incorporating CVD risk in clinical decisions for antihypertensive medication initiation and treatment intensification should not distract from the need for primordial prevention. It has been previously shown that almost all adults will eventually develop a 10-year predicted atherosclerotic CVD risk ≥7.5%, despite maintaining an optimal risk factor profile (42). Many people have a high CVD risk early in life because they develop modifiable risk factors. Preventing these risk factors will delay the development of high CVD risk into older age. Physical activity, weight loss, reducing alcohol intake, and dietary changes (reduced sodium intake, increasing potassium intake, and following the DASH diet) have been demonstrated in randomized trials to lower BP (80). These factors, as previously studied, are relevant to all people, regardless of CVD risk or BP level.
On the basis of data from observational studies, concern has been raised about potential increased CVD and mortality risk among people with low BP, particularly among those with heart disease or diabetes (81–83). This effect may reflect very low BP not being sufficient for organ perfusion, a low pressure due to preexisting organ damage, or confounding. Meta-analyses of randomized controlled trials have suggested that lowering BP to <120 mm Hg does not increase CVD or mortality risk (84–86). However, there may be little benefit associated with lowering SBP much below 120 mm Hg (85). Considering both observational and randomized controlled trial data, it would be hard to justify prescribing or intensifying antihypertensive medication for people with SBP <120 mm Hg or DBP <60 mm Hg, regardless of their global CVD risk.
Recommendations
We think it is important to consider both CVD risk and BP levels in making treatment decisions. Meta-analyses of randomized trials have demonstrated clear CVD risk reduction benefits with treatment of SBP ≥140 mm Hg or DBP ≥90 mm Hg, but these trials have generally excluded low-risk individuals (87). Additionally, there are data supporting antihypertensive treatment for individuals with lower BP levels, especially among adults with CVD or a high global CVD risk (84). Within this context, for example, one approach might be to treat all adults with SBP ≥140 mm Hg and DBP ≥90 mm Hg, and those with SBP of 130 to 139 mm Hg or DBP of 80 to 89 mm Hg and a 10-year CVD risk ≥10% using the Pooled Cohort risk equations. The 10% CVD risk threshold is consistent with event rates from previous meta-analyses (10,88). Cost-effectiveness studies and consideration of patient preference will be useful for guiding the level of CVD risk at which populations should be recommended antihypertensive medication treatment initiation.
Conclusions
The current review summarizes the evidence related to incorporating patients’ absolute CVD risk, in addition to their BP levels, into the decision for antihypertensive medication use. Using predicted CVD risk in conjunction with BP levels to guide antihypertensive treatment initiation will focus risk reduction on patients who are most likely to receive a benefit from treatment, while avoiding therapy for those who are likely to receive little risk reduction benefit. This approach would acknowledge that people with the same BP level can have markedly different 10-year CVD risks, and that the vast majority of CVD events occur in high-risk populations. In conclusion, using global CVD risk and BP levels to guide antihypertensive treatment may provide a more personalized approach for reducing risk.
Central Illustration.
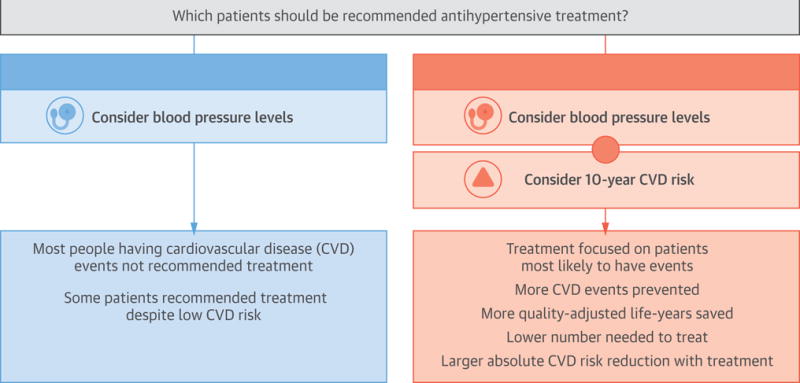
Cardiovascular Risk and Antihypertensive Treatment
CONDENSED ABSTRACT.
Data from randomized controlled trials indicate that patients with higher cardiovascular disease risk will receive a greater absolute risk reduction benefit from antihypertensive medication. Also, simulation studies suggest that using cardiovascular disease risk in conjunction with blood pressure levels, compared with basing treatment solely on blood pressure levels, would result in treating fewer people with antihypertensive medication, while preventing more cardiovascular disease events and saving more quality-adjusted life years. On the basis of these data, we recommend that predicted cardiovascular disease risk be part of antihypertensive treatment decision making.
ABBREVIATIONS AND ACRONYMS
- AHA
American Heart Association
- BP
blood pressure
- CHD
coronary heart disease
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- HDL
high-density lipoprotein
- JNC
Joint National Committee
- LDL
low-density lipoprotein
- SBP
systolic blood pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Whelton is Chair and Dr. Muntner is a member of the American College of Cardiology/American Heart Association Guideline for the Management of Hypertension writing committee. However, this work does not reflect the opinion of the American College of Cardiology, American Heart Association or the writing committee. Dr. Muntner receives research support from Amgen, Inc. unrelated to this paper.
References
- 1.Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 2.Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383:1899–911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, et al. National High Blood Pressure Education Program Coordinating Committee The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–71. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 4.Alderman MH. Blood pressure management: individualized treatment based on absolute risk and the potential for benefit. Ann Intern Med. 1993;119:329–35. doi: 10.7326/0003-4819-119-4-199308150-00013. [DOI] [PubMed] [Google Scholar]
- 5.Jackson R, Barham P, Bills J, et al. Management of raised blood pressure in New Zealand: a discussion document. BMJ. 1993;307:107–10. doi: 10.1136/bmj.307.6896.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts) Eur Heart J. 2007;28:2375–414. doi: 10.1093/eurheartj/ehm316. [DOI] [PubMed] [Google Scholar]
- 7.Gabb GM, Mangoni AA, Anderson CS, et al. Guideline for the diagnosis and management of hypertension in adults - 2016. Med J Aust. 2016;205:85–9. doi: 10.5694/mja16.00526. [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Health and Clinical Excellence. NICE clinical guideline 127. Hypertension: clinical management of primary hypertension in adults. London, UK: NICE; 2011. [Google Scholar]
- 9.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure [Published correction appears in Arch Intern Med 1998;158:573] Arch Intern Med. 1997;157:2413–46. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 10.Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591–8. doi: 10.1016/S0140-6736(14)61212-5. [DOI] [PubMed] [Google Scholar]
- 11.Sussman J, Vijan S, Hayward R. Using benefit-based tailored treatment to improve the use of antihypertensive medications. Circulation. 2013;128:2309–17. doi: 10.1161/CIRCULATIONAHA.113.002290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basu S, Yudkin JS, Sussman JB, Millett C, Hayward RA. Alternative strategies to achieve cardiovascular mortality goals in China and India: a microsimulation of target-versus risk-based blood pressure treatment. Circulation. 2016;133:840–8. doi: 10.1161/CIRCULATIONAHA.115.019985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eddy DM, Adler J, Patterson B, Lucas D, Smith KA, Morris M. Individualized guidelines: the potential for increasing quality and reducing costs. Ann Intern Med. 2011;154:627–34. doi: 10.7326/0003-4819-154-9-201105030-00008. [DOI] [PubMed] [Google Scholar]
- 14.Coronary Risk Handbook: estimating the risk of coronary heart disease in daily practice. New York, NY: American Heart Association; 1973. [Google Scholar]
- 15.Truett J, Cornfield J, Kannel W. A multivariate analysis of the risk of coronary heart disease in Framingham. J Chronic Dis. 1967;20:511–24. doi: 10.1016/0021-9681(67)90082-3. [DOI] [PubMed] [Google Scholar]
- 16.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 17.Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38:46–51. doi: 10.1016/0002-9149(76)90061-8. [DOI] [PubMed] [Google Scholar]
- 18.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 19.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 20.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–9. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 23.Conroy RM, Pyörälä K, Fitzgerald AP, et al. SCORE project group Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118:2243–51. doi: 10.1161/CIRCULATIONAHA.108.814251. 4p following 2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Münster (PROCAM) study. Circulation. 2002;105:310–5. doi: 10.1161/hc0302.102575. [DOI] [PubMed] [Google Scholar]
- 26.Fox ER, Samdarshi TE, Musani SK, et al. Development and validation of risk prediction models for cardiovascular events in black adults: the Jackson Heart Study Cohort. JAMA Cardiol. 2016;1:15–25. doi: 10.1001/jamacardio.2015.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA. 2014;311:1406–15. doi: 10.1001/jama.2014.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeFilippis AP, Young R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162:266–75. doi: 10.7326/M14-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rana JS, Tabada GH, Solomon MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67:2118–30. doi: 10.1016/j.jacc.2016.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pignone M, Phillips CJ, Elasy TA, Fernandez A. Physicians’ ability to predict the risk of coronary heart disease. BMC Health Serv Res. 2003;3:13. doi: 10.1186/1472-6963-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grover SA, Lowensteyn I, Esrey KL, Steinert Y, Joseph L, Abrahamowicz M. Do doctors accurately assess coronary risk in their patients? Preliminary results of the coronary health assessment study. BMJ. 1995;310:975–8. doi: 10.1136/bmj.310.6985.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedmann PD, Brett AS, Mayo-Smith MF. Differences in generalists’ and cardiologists’ perceptions of cardiovascular risk and the outcomes of preventive therapy in cardiovascular disease. Ann Intern Med. 1996;124:414–21. doi: 10.7326/0003-4819-124-4-199602150-00005. [DOI] [PubMed] [Google Scholar]
- 33.Sekaran NK, Sussman JB, Xu A, Hayward RA. Providing clinicians with a patient’s 10-year cardiovascular risk improves their statin prescribing: a true experiment using clinical vignettes. BMC Cardiovasc Disord. 2013;13:90. doi: 10.1186/1471-2261-13-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shillinglaw B, Viera AJ, Edwards T, Simpson R, Sheridan SL. Use of global coronary heart disease risk assessment in practice: a cross-sectional survey of a sample of U.S. physicians BMC Health Serv Res. 2012;12:20. doi: 10.1186/1472-6963-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Center for Health Statistics. National Health and Nutrition Examination Survey 2011–2012 Overview. CDC. Available at: https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Overview.aspx?BeginYear=2011. Accessed March 16, 2017.
- 36.Karmali KN, Ning H, Goff DC, Lloyd-Jones DM. Identifying individuals at risk for cardiovascular events across the spectrum of blood pressure levels. J Am Heart Assoc. 2015;4:e002126. doi: 10.1161/JAHA.115.002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muntner P, Whittle J, Lynch AI, et al. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: a cohort study. Ann Intern Med. 2015;163:329–38. doi: 10.7326/M14-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–61. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 39.Bell KJL, Hayen A, Macaskill P, Craig JC, Neal BC, Irwig L. Mixed models showed no need for initial response monitoring after starting antihypertensive therapy. J Clin Epidemiol. 2009;62:650–9. doi: 10.1016/j.jclinepi.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 40.Ye S, Wang YC, Shimbo D, Newman JD, Levitan EB, Muntner P. Effect of change in systolic blood pressure between clinic visits on estimated 10-year cardiovascular disease risk. J Am Soc Hypertens. 2014;8:159–65. doi: 10.1016/j.jash.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mozaffarian D, Benjamin EJ, Go AS, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 42.Karmali KN, Goff DC, Jr, Ning H, Lloyd-Jones DM. A systematic examination of the 2013 ACC/AHA pooled cohort risk assessment tool for atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2014;64:959–68. doi: 10.1016/j.jacc.2014.06.1186. [DOI] [PubMed] [Google Scholar]
- 43.Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med. 2012;172:1162–8. doi: 10.1001/archinternmed.2012.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williamson JD, Supiano MA, Applegate WB, et al. SPRINT Research Group Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673–82. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ambrosius WT, Sink KM, Foy CG, et al. SPRINT Study Research Group The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT) Clin Trials. 2014;11:532–46. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 48.Bress AP, Tanner RM, Hess R, et al. Prevalence of eligibility criteria for the Systolic Blood Pressure Intervention Trial in US adults among excluded groups: age <50 years, diabetes mellitus, or a history of stroke. J Am Heart Assoc. 2016;5:e003547. doi: 10.1161/JAHA.116.003547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pencina MJ, D’Agostino RB, Sr, Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119:3078–84. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA. 2012;308:1795–801. doi: 10.1001/jama.2012.14312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jackson R, Lawes CM, Bennett DA, Milne RJ, Rodgers A. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual’s absolute cardiovascular risk. Lancet. 2005;365:434–41. doi: 10.1016/S0140-6736(05)17833-7. [DOI] [PubMed] [Google Scholar]
- 52.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276:1253–8. [PubMed] [Google Scholar]
- 53.Ramsay LE. The hypertension detection and follow-up program: 17 years on. JAMA. 1997;277:167–70. doi: 10.1001/jama.277.2.167. [DOI] [PubMed] [Google Scholar]
- 54.American Diabetes Association. (8) Cardiovascular disease and risk management. Diabetes Care. 2015;38(Suppl):S49–57. doi: 10.2337/dc15-S011. [DOI] [PubMed] [Google Scholar]
- 55.Taler SJ, Agarwal R, Bakris GL, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for management of blood pressure in CKD. Am J Kidney Dis. 2013;62:201–13. doi: 10.1053/j.ajkd.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36:646–61. doi: 10.1053/ajkd.2000.16225. [DOI] [PubMed] [Google Scholar]
- 57.Hyre AD, Fox CS, Astor BC, Cohen AJ, Muntner P. The impact of reclassifying moderate CKD as a coronary heart disease risk equivalent on the number of US adults recommended lipid-lowering treatment. Am J Kidney Dis. 2007;49:37–45. doi: 10.1053/j.ajkd.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 58.Cederholm J, Eeg-Olofsson K, Eliasson B, et al. Swedish National Diabetes Register Risk prediction of cardiovascular disease in type 2 diabetes: a risk equation from the Swedish National Diabetes Register. Diabetes Care. 2008;31:2038–43. doi: 10.2337/dc08-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ford ES. Trends in the risk for coronary heart disease among adults with diagnosed diabetes in the U.S: findings from the National Health and Nutrition Examination Survey, 1999–2008. Diabetes Care. 2011;34:1337–43. doi: 10.2337/dc10-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson S, James M, Wiebe N, et al. Alberta Kidney Disease Network Cause of death in patients with reduced kidney function. J Am Soc Nephrol. 2015;26:2504–11. doi: 10.1681/ASN.2014070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Colantonio LD, Baber U, Banach M, et al. Contrasting cholesterol management guidelines for adults with CKD. J Am Soc Nephrol. 2015;26:1173–80. doi: 10.1681/ASN.2014040400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lonn EM, Bosch J, López-Jaramillo P, et al. HOPE-3 Investigators. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374:2009–20. doi: 10.1056/NEJMoa1600175. [DOI] [PubMed] [Google Scholar]
- 63.Sheridan SL, Viera AJ, Krantz MJ, et al. Cardiovascular Health Intervention Research and Translation Network Work Group on Global Coronary Heart Disease Risk The effect of giving global coronary risk information to adults: a systematic review. Arch Intern Med. 2010;170:230–9. doi: 10.1001/archinternmed.2009.516. [DOI] [PubMed] [Google Scholar]
- 64.Sheridan SL, Shadle J, Simpson RJ, Jr, Pignone MP. The impact of a decision aid about heart disease prevention on patients’ discussions with their doctor and their plans for prevention: a pilot randomized trial. BMC Health Serv Res. 2006;6:121. doi: 10.1186/1472-6963-6-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paterson JM, Llewellyn-Thomas HA, Naylor CD. Using disease risk estimates to guide risk factor interventions: field test of a patient workbook for self-assessing coronary risk. Health Expect. 2002;5:3–15. doi: 10.1046/j.1369-6513.2002.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Edelman D, Oddone EZ, Liebowitz RS, et al. A multidimensional integrative medicine intervention to improve cardiovascular risk. J Gen Intern Med. 2006;21:728–34. doi: 10.1111/j.1525-1497.2006.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Montgomery AA, Fahey T, Peters TJ. A factorial randomised controlled trial of decision analysis and an information video plus leaflet for newly diagnosed hypertensive patients. Br J Gen Pract. 2003;53:446–53. [PMC free article] [PubMed] [Google Scholar]
- 68.Sheridan SL, Behrend L, Vu MB, Meier A, Griffith JM, Pignone MP. Individuals’ responses to global CHD risk: a focus group study. Patient Educ Couns. 2009;76:233–9. doi: 10.1016/j.pec.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carson AP, Tanner RM, Yun H, et al. Declines in coronary heart disease incidence and mortality among middle-aged adults with and without diabetes. Ann Epidemiol. 2014;24:581–7. doi: 10.1016/j.annepidem.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosamond WD, Chambless LE, Heiss G, et al. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987–2008. Circulation. 2012;125:1848–57. doi: 10.1161/CIRCULATIONAHA.111.047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.D’Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P, CHD Risk Prediction Group Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 72.Brindle PM, McConnachie A, Upton MN, Hart CL, Davey Smith G, Watt GC. The accuracy of the Framingham risk-score in different socioeconomic groups: a prospective study. Br J Gen Pract. 2005;55:838–45. [PMC free article] [PubMed] [Google Scholar]
- 73.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–50. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 74.Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–61. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 75.Sharrett AR, Hubbard LD, Cooper LS, et al. Retinal arteriolar diameters and elevated blood pressure: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1999;150:263–70. doi: 10.1093/oxfordjournals.aje.a009997. [DOI] [PubMed] [Google Scholar]
- 76.Skoog I, Lernfelt B, Landahl S, et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–5. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- 77.Makin A, Lip GY, Silverman S, Beevers DG. Peripheral vascular disease and hypertension: a forgotten association? J Hum Hypertens. 2001;15:447–54. doi: 10.1038/sj.jhh.1001209. [DOI] [PubMed] [Google Scholar]
- 78.Devereux RB, Pickering TG, Alderman MH, Chien S, Borer JS, Laragh JH. Left ventricular hypertrophy in hypertension. Prevalence and relationship to pathophysiologic variables. Hypertension. 1987;9:II53–60. doi: 10.1161/01.hyp.9.2_pt_2.ii53. [DOI] [PubMed] [Google Scholar]
- 79.Bell EK, Gao L, Judd S, et al. Blood pressure indexes and end-stage renal disease risk in adults with chronic kidney disease. Am J Hypertens. 2012;25:789–96. doi: 10.1038/ajh.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whelton PK, He J, Appel LJ, et al. National High Blood Pressure Education Program Coordinating Committee Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288:1882–8. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- 81.Vamos EP, Harris M, Millett C, et al. Association of systolic and diastolic blood pressure and all cause mortality in people with newly diagnosed type 2 diabetes: retrospective cohort study. BMJ. 2012;345:e5567. doi: 10.1136/bmj.e5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vidal-Petiot E, Ford I, Greenlaw N, et al. CLARIFY Investigators Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet. 2016;388:2142–52. doi: 10.1016/S0140-6736(16)31326-5. [DOI] [PubMed] [Google Scholar]
- 83.Banach M, Aronow WS. Blood pressure j-curve: current concepts. Curr Hypertens Rep. 2012;14:556–66. doi: 10.1007/s11906-012-0314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435–43. doi: 10.1016/S0140-6736(15)00805-3. [DOI] [PubMed] [Google Scholar]
- 85.Bangalore S, Toklu B, Gianos E, et al. Optimal systolic blood pressure target after SPRINT: insights from a network meta-analysis of randomized trials. Am J Med. 2017 Jan 19; doi: 10.1016/j.amjmed.2017.01.004. [E-pub ahead of print], http://dx.doi.org/10.1016/j.amjmed.2017.01.004. [DOI] [PubMed]
- 86.Verdecchia P, Angeli F, Gentile G, Reboldi G. More versus less intensive blood pressure-lowering strategy: cumulative evidence and trial sequential analysis. Hypertension. 2016;68:642–53. doi: 10.1161/HYPERTENSIONAHA.116.07608. [DOI] [PubMed] [Google Scholar]
- 87.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sundström J, Arima H, Jackson R, et al. Blood Pressure Lowering Treatment Trialists’ Collaboration Effects of blood pressure reduction in mild hypertension: a systematic review and meta-analysis. Ann Intern Med. 2015;162:184–91. doi: 10.7326/M14-0773. [DOI] [PubMed] [Google Scholar]
- 89.Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591–8. doi: 10.1016/S0140-6736(14)61212-5. [DOI] [PubMed] [Google Scholar]
- 90.National Institutes of Health, National Heart, Lung, and Blood Institute, National High Blood Pressure Education Program. (NIH publication no. 98-4080).The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. 1997 Available at: https://www.nhlbi.nih.gov/health-pro/guidelines/archive/hypertension-jnc6. Accessed March 9, 2017. [PubMed]


