Abstract
Early intervention in the management of acute kidney injury (AKI) has been shown to improve outcomes. To facilitate early review we have introduced real time reporting for AKI. An algorithm using the laboratory computer system was implemented to report AKI for inpatients. Over 6 months there were 1,906 AKI reports in 1,518 patients: 56.3% AKI1, 26.9% AKI2 and 16.8% AKI3. 51.0% were male. Median age was 78 (interquartile range [IQR] 17) years. 62.6% were from general medical wards, 16.9% from surgical wards, 6.9% from orthopaedic wards and 5.3% from specialty wards. 8.3% were from peripheral hospitals. 31% of patients with AKI reports were clinically coded for AKI. 9% (n = 139) showed progression of AKI (mortality 42%). Patients with AKI had a significantly higher length of stay and mortality than those that did not. 4% of patients with AKI received acute renal replacement therapy (RRT). An e-alert system is feasible, allowing early identification of inpatients with AKI.
KEYWORDS : Acute kidney injury (AKI), e-alert, electronic alert, mortality, outcomes
Introduction
Acute kidney injury (AKI) is a common complication of hospital admissions, with an incidence of 5.4–20%.1–5 Patients with AKI can present to any hospital department and the aetiology of the condition is often iatrogenic. Reported risk factors include age, comorbidity, sepsis, recent surgery, oliguria, drugs and severity of illness.6,7 The presence of acute kidney injury is associated with poorer outcomes, including increased mortality and increased length of stay (LOS).1–5 The AKI Network (AKIN) developed a grading system for AKI based on the earlier RIFLE (risk of renal dysfunction, injury to the kidney, failure of kidney function, loss of kidney function, and end-stage kidney disease) criteria, and this has been incorporated into the current Kidney Disease: Improving Global Outcomes (KDIGO) guidelines.8,9
The National Confidential Enquiry into Patient Outcome and Death (NCEPOD) report highlighted variable outcomes for patients with late identification of AKI and implicated delayed referral for specialist renal review.10 It also suggested that there was a failure by clinicians to recognise and manage the condition appropriately, with omissions of basic medical care such as regular biochemical checks and fluid management. The report went on to state that the prevalence and outcome of this condition would not improve unless attention was paid to the basics. An increased risk of mortality has also been reported for patients with AKI who present to a hospital that does not have a specialist renal team.11
A recent consensus conference held at the Royal College of Physicians of Edinburgh recommended the use of e-alerts to aid early identification of AKI.12 Early intervention in the management of AKI has been shown to improve outcomes,13 so an automated alert system (e-alert) that allows real-time reporting may facilitate early review and lead to improved patient outcomes. We report our experience of an e-alert system over a 6-month period.
Materials and methods
Study population
The Royal Cornwall Hospital is a 657-bed district general hospital that covers a permanent population of 450,000, with doubling of this number over the summer. A single biochemistry laboratory covers the main hospital site, one peripheral hospital, seven community hospitals and 85 general practice surgeries.
Development of electronic reporting system
An algorithm was implemented using the Winpath laboratory computer system (Clinisys, Chertsey, UK) to detect and report AKI. Creatinine was checked for all admitted patients and the result was initially assessed by delta checking (comparing the level against the most recent previous result) to ascertain whether the level had changed by at least 26 μmol/l or had increased by a minimum of 50%; any flagged results were sent automatically to the clinical approval queue, as were creatinine levels >300 μmol/l, other than for patients already known to the dialysis unit. The duty biochemist then checked the creatinine level against a previous result. A stable creatinine level from the last 3 months was used for comparison, if available, but the results were checked back to 1 year if necessary. All four duty biochemists used the same criteria. If no result within the -preceding year was available our algorithm would not identify the abnormality as an episode of AKI.
Once AKI was verified using the AKIN criteria,9 the duty biochemist added an appropriate test code (AKI1, AKI2 or AKI3), which automatically generated a comment on the report stating ‘Significant increase in serum creatinine, probable AKI, stage [1, 2 or 3]’ and referred the user to the trust's local guidelines. For patients with an AKI2 code, the results were given by phone to the requesting location; for patients with an AKI3 code, the results were given by phone to the requesting location and the renal team. This policy was introduced alongside education for junior doctors and ward staff.
The creatinine assay used throughout the study period was a compensated kinetic Jaffe method run on one of two types of analyser supplied by Roche Diagnostics (West Sussex, UK). Most assays are run with p analysers, which have an interassay coefficient of variance (CV) of 2.97% at 147 μmol/l. Integra 800 analysers, which have a CV of 3.6% at 130 μmol/l, are used for urgent work. The normal reference ranges for creatinine levels in adults at our hospital are 44–80 μmol/l for females and 62–106 μmol/l for males, in line with the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC).
Data collection
Data for AKI were collected prospectively, alongside age, sex, location and consultant code. This was then cross-referenced with the patient administration system (PAS) to find the LOS, mortality and coding data.
To look for false positives (that is, overestimation of AKI), a random 5% of the reports of AKI were checked manually by two of the authors through a review of their clinical records; no false positives were found. To look for false negatives (that is, underreporting of AKI), all creatinine results that had not been flagged as AKI from a 24-hour period were manually checked by two of the authors. Of 290 results, eight were deemed to be AKI, giving a false-negative rate of 2.8%. Two of these were missed by the laboratory staff and six were not picked up by the algorithm because of small incremental increases in the creatinine level; all eight cases were classified as AKI1. Of note, 22 (7.5%) cases did not have a previous creatinine level but were not considered to have AKI. Eleven (3.8%) cases had creatinine results at the same level more than 1 year previously; seven (2.4%) of these had subsequent results and were confirmed as not being AKI and four (1.3%) had no previous or subsequent results but were within the normal range and so were felt unlikely to be AKI. The study was registered as an audit and service review within Royal Cornwall Hospital Trust.
Statistical analysis
Categorical variables were described using frequency (%) and analysed using Pearson's chi-squared test. All continuous variables were found to be not normally distributed and were described using the median and interquartile range (IQR). These were analysed using the Mann Whitney ‘U’ and Kruskal-Wallis tests, as appropriate. All statistical significance was set at p<0.05. All analyses, which were based on patient episodes rather than the total number of reports of AKI, were run using SPSS software (version 19; IBM, Portsmouth, UK). If a patient had more than one episode of AKI, the most severe stage was used for the analysis.
Results
Between 1 December 2011 and 31 May 2012, there were 1,906 reports of AKI (1,712 for emergency admissions and 194 for elective admissions) for 1,518 patient episodes. Over the same time period, there were 21,903 admissions without AKI (16,164 emergency and 5,739 elective), excluding attendances to the day-case department, early pregnancy unit and emergency department. This equated to an incidence of AKI of 6.5%.
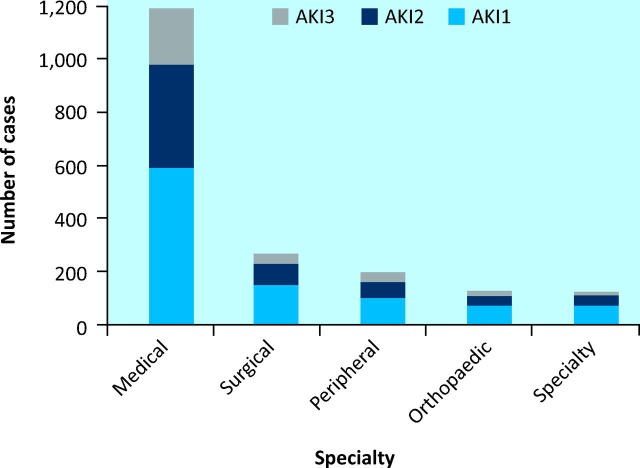
The median age of the patients with AKI was 78 years (IQR 17; range 17–101 years), and 51% (n = 772) were men. Of the reports of AKI, 56.3% (n = 1,073) were classified as AKI1, 26.9% (n = 513) as AKI2 and 16.8% (n = 320) as AKI3 (this included a number of multiple episodes for some patients). Of these cases, 62.6% were medical, 16.9% surgical, 6.9% orthopaedic, 5.3% from speciality wards and 8.3% from peripheral hospitals (Fig 1).
Fig 1.
Episodes of acute kidney injury (AKI) per specialty, by stage of AKI. AKI = acute kidney injury.
Overall, 194 (3.26%) of the elective admissions had AKI compared with 1,324 (7.6%) of the emergency admissions (p<0.05). Three hundred and eight-seven patients had more than one episode of AKI. Of these 187, 99 had multiple admissions with AKI; 139 (9.2%) showed progression of AKI during their single admission and 149 had multiple tests showing AKI at the same level during their single admission. This equates to 6.5% of all patients (n = 1,518) having multiple admissions with AKI, 9.2% showing progression and 9.8% having multiple tests at the same stage. Only 471 (31%) patients identified via the laboratory algorithm were coded for AKI on their discharge summary sheet. Thirty patients received renal replacement therapy (RRT) on the renal unit compared with 33 in the intensive care unit. In total, 4% of patients with AKI received RRT.
Association of acute kidney injury and mortality
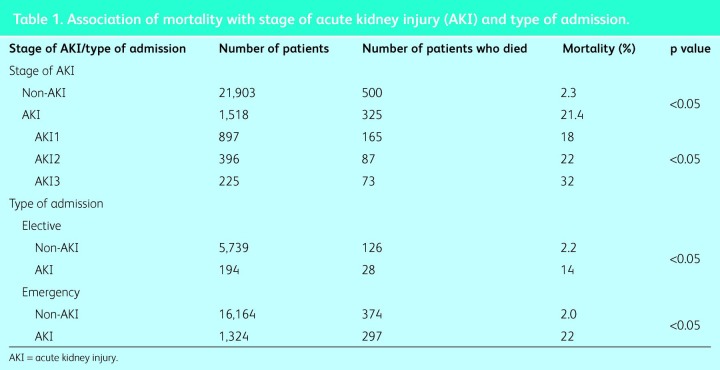
The overall mortality for all admissions was 3.5%, with a significant difference between the mortality of patients with AKI (21.4%) and unadjusted hospital-wide mortality (2.3%) for patients without AKI (Table 1). Associations for mortality between stages of AKI and between elective (not including day-case admissions) and emergency patients are shown in Table 1.
Table 1.
Association of mortality with stage of acute kidney injury (AKI) and type of admission.
Length of stay
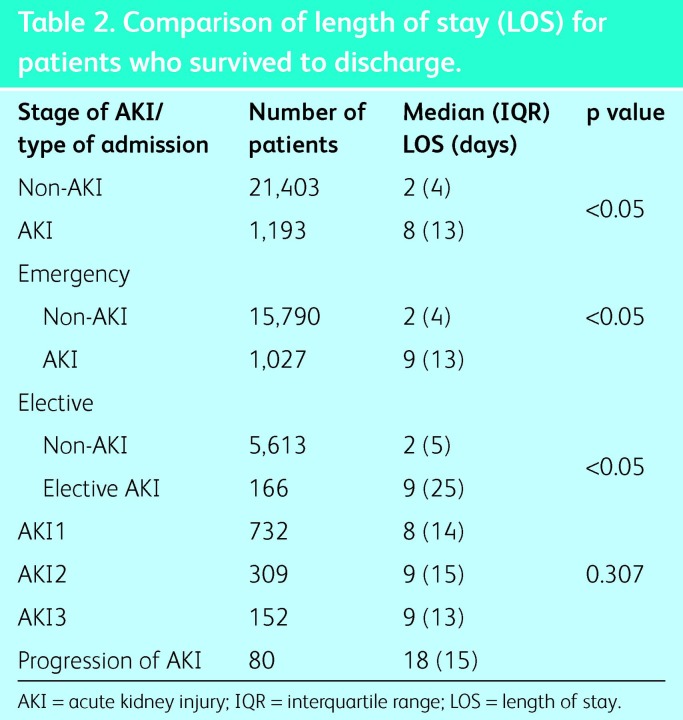
The median length of stay (LOS) for all patients was 2 days (IQR 5 days). Those without AKI also had a median LOS of 2 days (IQR 4 days) compared with 8 days (IQR 13 days) for those with AKI. When the LOS of patients who survived to discharge was compared, initial analysis showed an association between LOS and route of admission (elective/emergency), so the two groups were analysed separately (Table 2). The LOS was significantly higher for patients who had AKI than for those who did not (p<0.005), although there was no difference in LOS between the stages of AKI.
Table 2. .
Comparison of length of stay (LOS) for patients who survived to discharge.
Inpatient progression of acute kidney injury
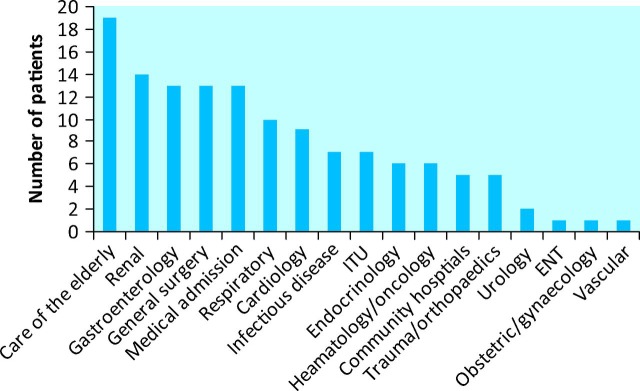
Progression was defined as a patient moving from a lower grade of AKI to a higher grade while they were an inpatient. Of all the patients with AKI, 139 (9.2%) showed evidence of progression. These patients had a median age of 77 (IQR 15) years and 57% were male (n = 79). Fig 2 shows patient location.
Fig 2.
Location of patients with progressive acute kidney injury. ENT = ear, nose and thoat; ITU = intensive treatment unit.
Patients with progression of AKI had a longer LOS than those with AKI3: median 18 (IQR 15) days versus 9 (IQR 12) days (censuring for inpatient death, p < 0.05). Fifty-nine (42.4%) of the 139 patients with progression died compared with 64/204 (31.4%) patients in the AKI3 group (p < 0.05). Sixty-nine (49.7%) of the patients with evidence of progression deteriorated from AKI1 to AKI2.
Discussion
Overall, there were 1,906 reports of AKI for 1,518 patient episodes between 1 December 2011 and 31 May 2012. The incidence of AKI was 6.9%, which is similar to that in other studies.1,2,15 Most reports of AKI involved AKI1, followed by AKI2 then AKI3. About five episodes of AKI1, 2–3 of AKI2 and 1–2 of AKI3 were identified each day. Most of these patients were on the medical wards. Four percent of the patients with AKI received RRT, and 9.2% showed progression of stage of AKI.
We have demonstrated higher mortality and longer LOS for patients with AKI than those without and an association between AKI stage and mortality. Mortality from AKI was similar to that in other studies.1,2,5,16 The LOS did not differ with stage of AKI; the reason for this is unclear, but it could be that the increased mortality in patients with higher stages of AKI resulted in the earlier death of patients during their admission. The highest mortality (42%) was in the group of patients whose AKI -progressed, and this was higher than in the patients who had had AKI3 and no progression.
Two other reports and one abstract on hospital-wide e-alert systems for AKI have been published. Thomas et al reported on 463 patients, for whom a real-time alert message was generated by the computer system for any patient with an increase in creatinine of ≥75%.15 The false-negative rate of 66% was high, as many of the changes seen were <75%. However, mortality in this study was similar to that in our study. Selby et al reported an e-alert system identifying 2,619 episodes of AKI over a 9-month period.14 Mortality and LOS were also similar to those in our study. If no baseline creatinine result was available, an estimated baseline level was generated using the laboratory's computer system. The most appropriate baseline to use is not clear, and altering the baseline alters the number of cases of AKI found.4,17 Devonald et al have published, in abstract form, their experience of an e-alert system that was fully automated and used a theoretical baseline (modification of diet in renal disease [MDRD] using glomerular filtration rate [GFR] of 75 ml/min) if no previous creatinine value was available.18 They reported 13,314 episodes of AKI (8% of total admissions) over a 12-month period, but with a much higher incidence of AKI1 (73.3%) and correspondingly lower numbers of AKI2 and AKI3 than in our study. In-hospital mortality was 8%, 19% and 25% for AKI1, AKI2 and AKI3, respectively. It is difficult to comment on the discrepancy between the results, as demographic data and false-negative/-positive results are not available in the abstract for comparison.
Our system was unable to identify AKI if no previous result was available in the prior 12 months; however, our low false-negative rate suggests good identification of cases. The major contributor to our false-negative rate was patients whose creatinine level had increased slowly over the preceding few weeks. This could be overcome by disregarding creatinine levels in the 2 weeks prior to admission. Our current computer system is unable to do this; however, a future upgrade to the system will enable a baseline creatinine level to be calculated when no previous creatinine result is available, as is the case in other laboratories.1,18
To make best use of resources, initial management of AKI (which is usually straightforward) could be managed by generalists, with educational support as necessary. Specialist renal input could be reserved for patients with severe AKI and those requiring complex renal care.
The high mortality and increased LOS of patients who had evidence of progression is of interest. This could reflect a group of patients who developed AKI during the terminal phase of their illness or those who were not responding to initial treatment, and so evidence of progression could identify a group of patients who may benefit from earlier intervention. This group included patients who progressed from AKI1 to AKI2 and who would not automatically have been referred to the renal team by the biochemistry laboratory. The lack of coding for AKI on the discharge summary sheet suggests that there was under-recognition of this condition, as highlighted in the NCEPOD report.10 Ongoing education, notification of AKI and individual review by the renal team may improve this. Direct coding of AKI from the biochemistry database would also allow more complete coding, but it would not address our colleagues’ lack of understanding of the importance of AKI. Further education is required, and this remains an ongoing challenge.
Limitations
Some episodes of AKI may not be picked up by our system: for example, when there is no previous creatinine result in the last 12 months, when there is lack of recognition by the duty biochemist and when small increments in creatinine from baseline are missed by the delta check.
The data that we have gathered have not been adjusted for confounding factors such as age and comorbidities. The renal team's interventions for the patients were not identified, so we cannot state whether early renal intervention equated to an early change in management.
We also acknowledge that, although all admissions were independent, there were multiple admissions of the same patient in 99 instances in the AKI group. It is unclear what effect this has had on the analysis.
Conclusions
The NCEPOD report suggested that early specialist review would improve mortality and morbidity of patients with AKI. The active e-alert system that we have introduced is a feasible way to identify patients with AKI, which would allow early review by specialist care, and this may lead to an improvement in outcomes.
Acknowledgements
We would like to thank Joanne Palmer in the research and -development department for her help with the statistical analysis; Alan Bromley from the clinical chemistry department, and Phillippa Eddision from the coding department for help with data collection.
References
- 1.Selby NM, Crowley L, Fluck RJ, et al. Use of electronic results reporting to diagnose and monitor AKI in hospitalised patients. Clin J Am Soc Nephrol. 2012;7:533–40. doi: 10.2215/CJN.08970911. [DOI] [PubMed] [Google Scholar]
- 2.Aitken E, Carruthers C, Gall L, et al. Acute kidney injury: outcomes and quality of care. Q J Med. 2013;106:323–32. doi: 10.1093/qjmed/hcs237. [DOI] [PubMed] [Google Scholar]
- 3.Lafrance JP, Miller DR. Acute kidney injury associates with increased long term mortality. J Am Soc Nephrol. 2010;21:345–52. doi: 10.1681/ASN.2009060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lafrance JP, Miller DR. Defining acute kidney injury in database studies: the effect of varying the baseline kidney function assessment period and considering CKD status. Am J Kidney Dis. 2010;56:651–60. doi: 10.1053/j.ajkd.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Uchino S, Bellomo R, Goldsmith D, et al. An assessment of RIFLE -criteria for acute renal failure in hospitalised patients. Crit Care Med. 2006;34:1913–7. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- 6.Stevens PE, Tamimi NA, Al-Hasani MK, et al. Non-specialist -management of acute renal failure. Q J Med. 2001;94:533–40. doi: 10.1093/qjmed/94.10.533. [DOI] [PubMed] [Google Scholar]
- 7.Wallace KR, Andain K, Stratton J, et al. The epidemiology and -mortality of acute kidney injury within Cornwall: a one year -prospective study. Br J Renal Med. 2010;15:19–21. [Google Scholar]
- 8.Kidney Disease: Improving Global Outcomes. KDIGO clinical practice guideline for AKI. Kidney Int Suppl. 2012;2(suppl 1) doi: 10.1016/j.kint.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcome in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Acute kidney injury: adding insult to injury. London: NCEPOD; 2009. National Confidential Enquiry into Patient Outcomes and Death. www.ncepod.org.uk/2009aki.htm. [Accessed 2 December 2013] [Google Scholar]
- 11.Abraham KA, Thompson EB, Bodger K, Pearson M. Inequalities in outcomes of acute kidney injury in England. Q J Med. 2012;105:729–40. doi: 10.1093/qjmed/hcs037. [DOI] [PubMed] [Google Scholar]
- 12.Feehally J, Gilmore I, Barasi S, et al. RCPE UK consensus -conference statement: management of acute kidney injury: the role of fluids, e-alerts and biomarkers. J R Coll Physicians Edinb. 2012;43:37–8. doi: 10.4997/JRCPE.2013.109. [DOI] [PubMed] [Google Scholar]
- 13.Balasubramanian G, Ziyad A, Moiz A, et al. Early nephrologist involvement in hospital-acquired acute kidney injury: a pilot. Am J Kidney Dis. 2011;57:228–34. doi: 10.1053/j.ajkd.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 14.Selby NM, Devonald MAJ. What is the role of e-alerts in acute kidney injury? J R Coll Physicians Edinb. 2012;42(Suppl 19):21–6. doi: 10.4997/JRCPE.2012.105. www.rcpe.ac.uk/sites/default/files/files/rcpe-aki-supplement-2012.pdf. [Accessed 30 January 2014] [DOI] [PubMed] [Google Scholar]
- 15.Thomas M, Sitch A, Dowswell G. The initial development and assessment of an automatic alert warning of acute kidney injury. Nephrol Dial Transplant. 2011;26:2161–8. doi: 10.1093/ndt/gfq710. [DOI] [PubMed] [Google Scholar]
- 16.Fang Y, Ding X, Zhong Y, et al. Acute kidney injury in a Chinese -hospitalised population. Blood Purif. 2010;30:120–6. doi: 10.1159/000319972. [DOI] [PubMed] [Google Scholar]
- 17.Siew ED, Ikizler TA, Matheny ME, et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol. 2012;7:712–9. doi: 10.2215/CJN.10821011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devonald M. The Nottingham Experience. J R Coll Physicians Edinb. 2012;42(Suppl 19):29. www.rcpe.ac.uk/sites/default/files/files/rcpe-aki-supplement-2012.pdf. [Accessed 30 January 2014] [Google Scholar]