Key points
Most doctors struggle with arterial blood gas (ABG) interpretation
ABG interpretation is easy
Break it down into steps
The first priority for the respiratory system is pH
If partial pressure of carbon dioxide (pCO2) goes down, partial pressure of oxygen (pO2) should go up
Mistakes in arterial blood gas (ABG) interpretation are common in clinical practice. The following is a simplified explanation of ABGs, including a practical method for interpreting results. It is simple, perhaps simplistic, but it will hopefully arm the reader with the tools (and confidence) to make better sense of ABG results in future. This is not for the dedicated physiologist. Overly complex explanations can be a barrier to a working understanding of the basics.
On acute medical take, ABGs are used to determine the nature as well as the severity of a problem. The results often have a direct bearing on management. Yet most doctors struggle with interpretation of this common test.
Arterial blood gas measurements
Unlike other ‘blood tests’, which are either ‘high or low’, ABGs present the doctor with six numbers that need to be interpreted as ‘one result’. Given that this can be difficult, there is a need for a simple algorithm for systematically handling each of the numbers in turn, as discussed below. However, I begin with a few basic points to understand.
Respiratory and metabolic systems – in balance
ABGs tell us about activity in two systems; the respiratory system and the ‘metabolic’ system. If one system is disturbed, the other tries to restore balance. Both systems are primarily concerned with keeping blood pH in the normal range. Even for the respiratory system, pH (rather than oxygen) is the priority.
The respiratory system – oxygenation vs pH
In health, we are driven to take our next breath by the arterial partial pressure of carbon dioxide (PaCO2), which is intimately linked to pH. Although there is an additional receptor (the hypoxic centre) in the brain stem that monitors PaO2, it spends most of it time ‘asleep’ and is rather unconcerned about minor fluctuations in the level of oxygenation. This is because individuals generally live at a level of oxygenation well above that which is required to sustain life. This ‘margin of oxygen safety’ enables the respiratory system to focus on pH and to adjust ventilation (to ‘blow off’ or retain CO2) without the fall in oxygenation that underventilation would bring causing any difficulties. Therefore, if, for example, a metabolic alkalosis were to develop, ventilation would fall (at the expense of a small reduction in oxygenation) to retain CO2 and, thus, return pH to the normal range. Only when hypoxia is more severe (approximately PaO2 <8 kPa) does the hypoxic centre ‘wake up’ and take note. Only then, will it drive ventilation to prevent harmful levels of hypoxia.
Respiratory and metabolic systems – the speed of response
The respiratory system can respond quickly to a metabolic derangement, with changes occurring to the blood gases within seconds to minutes. However, the metabolic system (largely regulated by the kidneys excreting or retaining acid or bicarbonate) is much slower and changes can take hours to days.
A step-by-step method for interpreting arterial blood gases
First – look at the pH
Decide whether this is an ‘acidosis’ or ‘alkalosis’ (if it is within the normal range, note whether it is sitting towards the ‘acidotic’ or ‘alkalotic’ end of that range). Fix that fact in your mind because it will not change, no matter what the other numbers are!
Second – look at the PaCO2
Ask the question: is the PaCO2 contributing to, or attempting to compensate for, the problem. If, for example, the problem is an acidosis and the PaCO2 is low, then clearly the respiratory system is attempting to compensate. Thus, one can conclude that the problem is metabolic (similarly with other combinations). Therefore, after looking at only two numbers (pH and PaCO2), most of the interpretation is done.
The other numbers (actual bicarbonate [aHCO3], base excess [BE], PaO2 and so on) might do nothing more than confirm this conclusion. However, they can sometimes add information about time course or provide information on additional derangements, but they will not contradict the conclusion that has already been reached.
Third – look at the ‘base picture’
Actual bicarbonate (aHCO3) vs standard bicarbonate (sHCO3) – what's the difference? What is perhaps surprising is that, after many years of looking at ABGs, those intelligent, enquiring minds have seemingly never once pondered that question.
aHCO3 is the actual measurement of bicarbonate in that actual blood sample (hence the name). The problem with this measurement is that it is markedly affected by PaCO2. If the PaCO2 is high, the aHCO3 is dragged higher and vice versa. What one would like to know is what the HCO3 would have been had the PaCO2 been normal. It is this value that would provide a direct handle on what the metabolic system is doing. One can calculate the value if aHCO3 and PaCO2 are known, although most blood gas machines do this automatically, known as the sHCO3.
What is the base excess?
Base excess (BE) measures all bases, not just bicarbonate. However, because bicarbonate is the greater part of the base buffer, for most practical interpretations, BE provides essentially the same information as bicarbonate. The major advantage of BE is that its normal range is really easy to remember. One could probably have guessed that the expected value of BE was zero (the clue is in the word: ‘excess’). Therefore, a tight range around zero (−3 to +3) is normal. In simple terms, a high BE excess is the same as a high HCO3.
What does the base picture tell us?
If the pH and PaCO2 led to the conclusion that the problem was primarily metabolic, then sHCO3 (or BE) will do little more than confirm that; sHCO3 being high in an alkalosis, low in an acidosis. If one has established that problem is respiratory, then the BE can tell us something of the duration of the problem.
If, for example, in a respiratory acidosis, the sHCO3 has shown no sign of responding (still within the normal range), the probable explanation is that there has not yet been time to respond (ie the problem is an acute respiratory acidosis). In a respiratory ‘acidosis’ (perhaps with the pH in the lower half of the normal range), a high sHCO3 would indicate a longer time course (ie the problem is a chronic -respiratory acidosis). A respiratory acidosis with a low sHCO3 would indicate a combined respiratory and metabolic -acidosis.
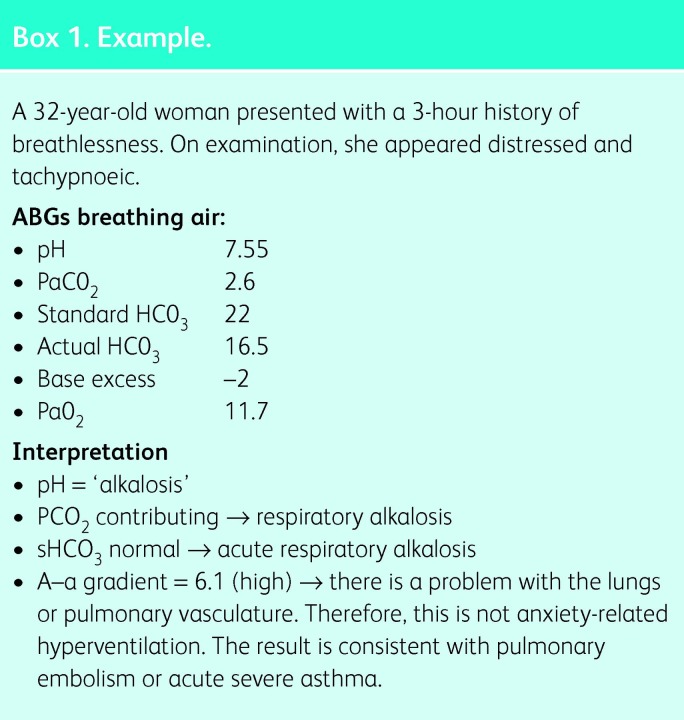
Box 1. Example.
Remember that one cannot live for long with pH outside of the normal range. If the pH is outside the normal range, one should never fall into the trap of assuming the problem is ‘probably all chronic’ (no matter how high the bicarbonate). An abnormal pH means there has to be an acute component to the problem.
Finally, look at the oxygen
It is sometimes thought that type 2 respiratory failure is simply a more severe version of type 1. However, this is not the case. Type 1 and type 2 respiratory failures are due to entirely different mechanisms.
Type 2 respiratory failure is extremely an issue of ventilation, that is, the business of pumping air in and out of the lungs. When underventilation occurs, for what ever reason (eg muscular weakness or opiate overdose), the PaCO2 will increase (the definition of underventilation) and PaO2 must decrease (even if the lungs are perfectly healthy). Type 2 respiratory failure results from underventilation, which can occur even in the context of healthy lungs.
Lung (or pulmonary vascular) disease disturbs the delicate ventilation–perfusion (V/Q) matching system. In such circumstances, oxygen delivered to the lungs by ventilation is handled inefficiently and PaO2 falls. However, provided that overall ventilation is normal, PaCO2 is maintained. When PaO2 is low yet PaCO2 normal, type 1 respiratory failure is present, and such a result implies lung (or pulmonary -vascular) disease.
Type 1 and type 2 respiratory failure can occur simultaneously. Indeed, the combination is common in severe chronic obstructive pulmonary disease, for example. Given that the two conditions result from entirely different mechanisms, with implications for treatment, one should be able to distinguish between them. When the only derangement is PaO2, clearly the failure is type 1. However, when the PaCO2 is high, one has to work out whether the low PaO2 can be accounted for by underventilation alone or whether there is an additional type 1 problem (ie whether there is anything wrong with the lungs).
To do this, one needs to measure the alveolar–arterial gradient, that is, the difference between the alveolar partial pressure of oxygen (PAO2) and the PaO2. The PaO2 is measured in the ABG, the PAO2 has to be calculated using the alveolar gas equation:
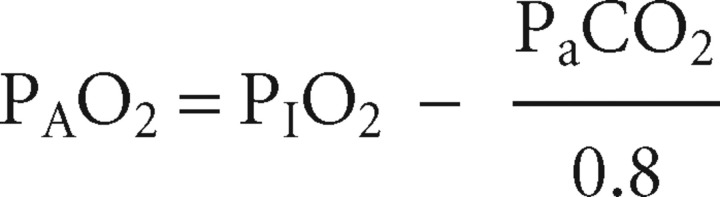 |
where PIO2 is the partial pressure of oxygen in the inspired air (approximately 21 kPa when breathing room air, but 24 kPa when using a 24% Venturi mask and so on) and 0.8 is the ‘respiratory quotient’ (ie the ratio between the CO2 produced and the O2 utilised).
The alveolar–arterial gradient (PAO2–PaO2) can then be calculated. In healthy young adults, the difference should be less than 2 kPa. If the patient is older, breathing higher concentrations of O2 or over ventilating, then the gap can widen, although in healthy patients this would not usually be expected to be greater than 4 kPa. If the alveolar–arterial gradient is higher than it should be, then a type 1 respiratory failure is present. This implies a problem with V/Q matching (ie a problem with either the lungs or the pulmonary vasculature).
Remember!
ABG interpretation is not difficult. Break down the task into steps and do them in order.
For a more detailed review of arterial blood gas interpretation, see Ref 1. Box 1 provides an example of a patient presenting with breathlessness, where ABGs form an important diagnostic test.
Reference
- 1.Gibson GJ. London: Hodder Arnold; 2008. Clinical tests of respiratory function (3rd edn) [Google Scholar]