Key points
Respiratory symptoms are often only apparent when respiratory muscle weakness is severe; assess and monitor lung function
Uncontrolled oxygen therapy can lead to potentially fatal carbon dioxide retention
Patients with severe bulbar impairment might confuse the sensation of choking on lying flat with true orthopnoea and perform volitional lung function tests poorly
In patients without severe bulbar impairment, non-invasive ventilation (NIV) improves symptoms, quality of life and survival, and offers advantages over invasive ventilation, including in the acute setting
It is important to consider not only ventilatory support, but also volume recruitment and assisted cough techniques
In neuromuscular disease (NMD), respiratory muscle weakness (RMW) is common and death often results from respiratory failure. RMW initially causes sleep-related hypoventilation, with sleep disruption. With progression, daytime respiratory failure ensues. Bulbar muscle weakness causes difficulty with speech and swallowing, often complicated by recurrent aspiration. Effective cough and airway clearance requires good inspiratory, expiratory and bulbar muscle function. Severe bulbar impairment is associated with poor tolerance of, and response to, non-invasive ventilation (NIV) in acute and elective settings. In addition to assessing the pattern and severity of muscle involvement, it is important to consider whether the underlying disorder is reversible (eg Guillain–BarrÈ syndrome [GBS]), stable or only slowly progressive (eg post-polio syndrome or myotonic dystrophy) or rapidly progressive (eg amyotrophic lateral sclerosis [ALS]).
Symptoms and signs
Breathlessness often presents late, particularly if mobility is limited, and should be assessed on exertion, performing activities of daily living, talking and in relation to posture. Diaphragmatic weakness causes orthopnoea and breathlessness when immersed in water and intercostal muscle weakness causes breathless when upright; therefore, patients with generalised RMW might be most comfortable semi-reclined. Of importance, patients with bulbar impairment can confuse the sensation of choking on lying flat with orthopnoea.
During sleep, particularly rapid eye movement (REM) sleep, RMW can lead to hypoventilation, apnoeas and hypopnoeas with associated sleep disruption. Symptoms include restless and unrefreshing sleep, vivid dreams, daytime sleepiness, lethargy, poor concentration and mood disturbance. However, other problems can cause sleep disruption, including pain, choking on secretions, and anxiety and depression. Hypercapnia does not usually develop until RMW is severe, and is more pronounced overnight. Symptoms include headache that is worse on wakening, poor appetite, confusion and drowsiness.
Bulbar muscle weakness can cause problems with speech, swallowing, recurrent aspiration, frequent lower respiratory tract infections and weak cough. When the inspiratory and expiratory muscles are also weak, airway clearance is further compromised.
Clinical signs of RMW include rapid shallow breathing, use of accessory muscles, reduced chest expansion, reduced breath sounds, abdominal paradox (inward movement of the abdomen on inspiration; specific to diaphragmatic weakness), and weak cough and sniff. Signs of intercostal muscle weakness with preserved diaphragmatic function (eg spinal muscular atrophy) include bell-shaped chest deformity and chest paradox. Scoliosis, possibly with evidence of surgical correction, might be present, particularly in childhood-onset NMD. Signs of respiratory failure include central cyanosis, coarse tremor, dilated veins, bounding pulse, papilloedema, confusion and drowsiness.
Assessing respiratory function
The severity of RMW is often underestimated clinically and, in rapidly progressive NMD, survival following the onset of symptoms might be only a few weeks or months. In ALS, regular assessment of respiratory symptoms and function is associated with increased use of elective NIV,1 reduced tracheostomy ventilation (TV) and, in patients with good bulbar function, improved survival.2 It is important to assess bulbar function, because this determines the approach to assessment of RMW, and cough effectiveness. The interval between assessments depends on the rate of disease progression (eg GBS: at least daily, ALS: approximately three monthly).
Bulbar function can be assessed by using the Norris Bulbar Score or the bulbar sub-score of the revised Amyotrophic Lateral Sclerosis Functional Rating Scale. Moderate air leak resulting from facial or bulbar muscle weakness can be overcome by the use of an adapted anaesthetic facemask. In patients without severe bulbar impairment, respiratory function should be monitored using spirometry and, if available, sniff nasal inspiratory pressure (SNIP), and maximum inspiratory (MIP) and expiratory (MEP) pressures. Pulse oximetry and, if abnormal, capillary or arterial blood gases should be performed. Spirometry is widely available and reproducible. Vital capacity (VC) predicts survival and, in combination with symptoms, is commonly used to select patients for ventilatory support. Supine VC is difficult to perform if mobility is limited, but compared with sitting VC, it is a better index of diaphragmatic function and a >20% fall indicates diaphragmatic weakness. Compared with VC, SNIP is more sensitive to early RMW and is a better predictor of hypercapnia and death.3 However, SNIP and MIP are less reproducible and possibly less reliable in inexpert hands. Cough effectiveness can be assessed by peak cough flow (PCF).
Patients with severe bulbar impairment perform volitional tests of respiratory function poorly; greater reliance should be placed on oxygen saturation, capillary or arterial blood gases, nocturnal oximetry and transcutaneous pCO2. If sleep-related symptoms are present, limited or full sleep studies are useful.
Assisted ventilation
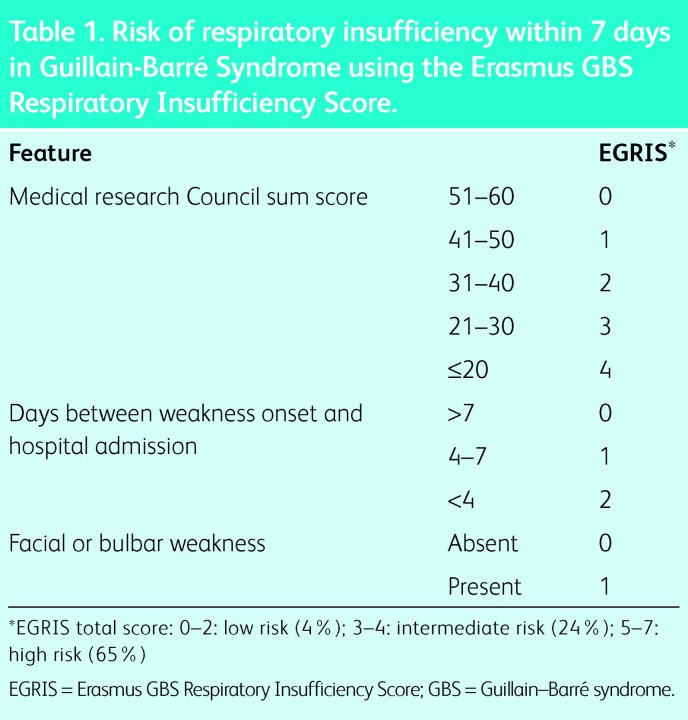
In GBS, RMW can progress rapidly and bulbar function is often impaired. Consequently, invasive ventilation is usually more appropriate than NIV. Clinical features on admission inform the risk of early respiratory failure (Table 1);4 however, this should be combined with frequent monitoring of symptoms and VC.
Table 1. .
Risk of respiratory insufficiency within 7 days in Guillain-BarrÈ Syndrome using the Erasmus GBS Respiratory Insufficiency Score.
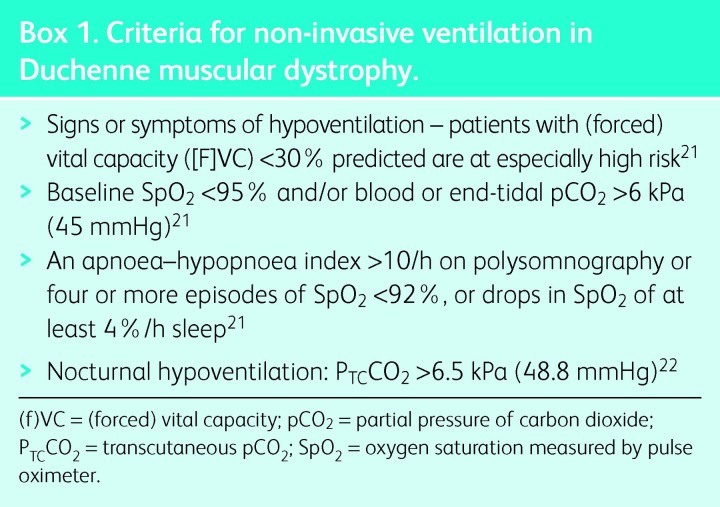
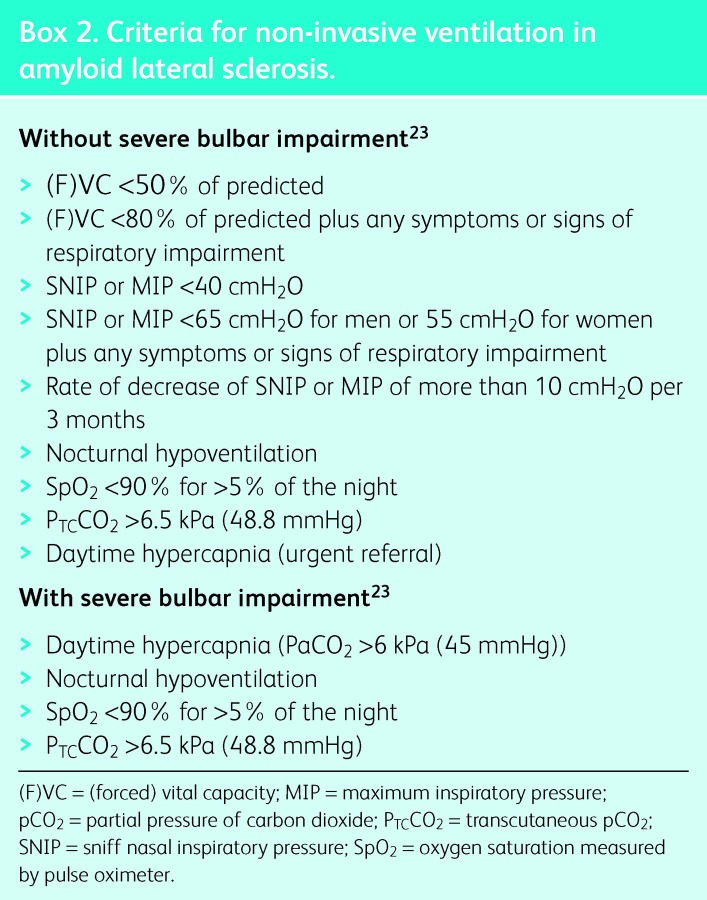
In stable and progressive NMD, NIV improves survival compared with historical controls5–9 and those patients who decline or are intolerant of treatment.10,11 Generally, survival on NIV reflects the rate of disease progression. NIV improves quality of life (QoL) and symptoms, particularly -orthopnoea and symptoms related to sleep disturbance and hypercapnia. The most common conditions encountered in adult life are ALS and Duchenne muscular dystrophy (DMD); indications for elective NIV are shown in Boxes 1 and 2.
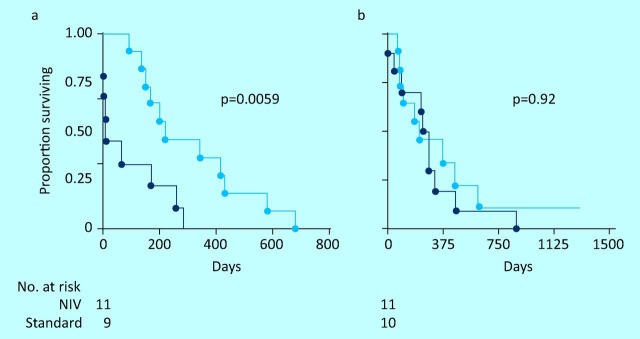
Fig 1.
Survival from randomisation in a randomised controlled trial of non-invasive ventilation () compared with standard care () in patients with amyotrophic lateral sclerosis and (a) normal or only moderately impaired bulbar function and (b) severe bulbar impairment. NIV = non-invasive ventilation.
Box 1. Criteria for non-invasive ventilation in Duchenne muscular dystrophy.
Box 2. Criteria for non-invasive ventilation in amyloid lateral sclerosis.
In ALS, a randomised controlled trial (RCT) showed that NIV improved survival (7 months), symptoms and QoL in patients without severe bulbar impairment. Patients with severe bulbar impairment showed no survival benefit, but sleep-related symptoms improved (Fig 1);12 however, such patients may gain a modest survival advantage if NIV is well tolerated,11 or if the patient is hypercapnic, but not normocapnic, at the time of initiation.2 This might reflect the balance of risks and benefits of administering NIV to patients with poor airway protection. By contrast, in patients with good bulbar function, earlier initiation of NIV in patients with orthopnoea but normal daytime PaCO2 improves outcomes.13 Initiation of NIV based on physiological impairment with minimal or no symptoms has been advocated, but remains controversial. Compared with NIV, TV offers potentially longer survival, particularly if bulbar function is poor, but the burden experienced by carers is greater14 and home care is less feasible. Overall, patients receiving TV report poorer QoL, although this is strongly influenced by the higher proportion of such patients in institutional care.
In DMD, two RCTs comparing early intervention to standard NIV showed conflicting results. In the first trial, early NIV was associated with higher mortality. During respiratory tract infections, patients undergoing NIV were less likely to receive invasive ventilation and most deaths occurred in patients managed at home.15 The more recent RCT included patients with other NMD and chest wall restriction and required confirmation of nocturnal hypoventilation (transcutaneous pCO2 >6.5kPa). In the early intervention arm, NIV improved gas exchange and those who declined early NIV were more likely to require emergency ventilation.16
Once NIV has been initiated, the strongest prognostic indices are compliance13,17 and bulbar function. Other predictors of longer survival include good nutritional status, younger age and good upper limb function.13 Dementia is associated with poor adherence to NIV and gastrostomy feeding and shorter survival.
Volume recruitment and airway clearance
Volume recruitment techniques assist the patient to take a deep breath in, helping to reverse and prevent atelectasis. Examples include frog (glossopharyngeal) breathing and breath-stacking (an ambu-bag and one-way valve is helpful). Alternatively, a ventilator or Mechanical Insufflator-Exsufflator (MI-E) on insufflation mode can be used.
Cough assist techniques should be performed routinely if PCF ≤160 l/min and during respiratory tract infections or following procedures requiring sedation if PCF ≤270 l/min. An effective cough requires a deep breath in, glottic closure and expiratory muscle contraction to generate positive pressure, followed by glottic opening resulting in an explosive cough. In patients with NMD, after a deep breath in, abdominal or thoracoabdominal trusts can be used to assist cough effort. More effective airway clearance can be achieved using a MI-E. In patients with a tracheostomy, MI-E is more effective than deep suctioning and avoids trauma. In patients with poor bulbar function, MI-E is less effective, although a Guedel airway can be used to prevent airway collapse.
Acute decompensation
Episodes of acute decompensation, with acute on chronic type 2 respiratory failure, can be triggered by upper or lower respiratory tract infections, mucus impaction, posture, sedatives or uncontrolled oxygen therapy.18,19 Unfortunately, it is not widely appreciated that uncontrolled oxygen therapy can cause potentially life-threatening carbon dioxide retention in patients with RMW; injudicious use can be the primary precipitant of, or contribute to, acute respiratory failure. Oxygen, if required, should be prescribed and administered by Venturi mask, with target oxygen saturations of 88–92%.20
Most patients have severe RMW and will require long-term ventilation, particularly if the underlying condition is ALS or DMD19 rather than a more slowly progressive condition, such as myotonic dystrophy. In patients without severe bulbar impairment, NIV combined with use of a MI-E reduces the length of stay in the intensive care unit and the incidence of ventilator-associated pneumonia, and probably improves survival. Compared with intubation, sedation is avoided and communication is better preserved, facilitating discussions with the patient about both acute and long-term management. By contrast, in patients with severe bulbar impairment, NIV is associated with poor outcome and, if long-term tracheostomy ventilation is acceptable to the patient, they should be intubated. Otherwise, alternative palliation might be more appropriate. Occasionally, patients with NMD present before diagnosis in respiratory failure. This is more common with respiratory onset disease, and recognition of RMW and diagnosis of the underlying NMD can be delayed.
References
- 1.O’Neill CL, Williams TL, Peel ET, et al. Non-invasive ventilation in motor neuron disease: an update of current UK practice. J Neurol Neurosurg Psychiatry. 2012;83:371–6. doi: 10.1136/jnnp-2011-300480. [DOI] [PubMed] [Google Scholar]
- 2.Farrero E, Prats E, Povedano M, et al. Survival in amyotrophic lateral sclerosis with home mechanical ventilation: the impact of systematic respiratory assessment and bulbar involvement. Chest. 2005;127:2132–8. doi: 10.1378/chest.127.6.2132. [DOI] [PubMed] [Google Scholar]
- 3.Morgan RK, McNally S, Alexander M, et al. Use of sniff nasal-inspiratory force to predict survival in amyotrophic lateral sclerosis. Am J Respir Crit Care Med. 2005;171:269–74. doi: 10.1164/rccm.200403-314OC. [DOI] [PubMed] [Google Scholar]
- 4.Walgaard C, Lingsma HF, Ruts L, et al. Prediction of respiratory insufficiency in Guillain-Barre syndrome. Ann Neurol. 2010;67:781–7. doi: 10.1002/ana.21976. [DOI] [PubMed] [Google Scholar]
- 5.Simonds AK, Elliott MW. Outcome of domiciliary nasal intermittent positive pressure ventilation in restrictive and obstructive disorders. Thorax. 1995;50:604–9. doi: 10.1136/thx.50.6.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nugent AM, Smith IE, Snhneerson JM. Domiciliary-assisted ventilation in patients with myotonic dystrophy. Chest. 2002;121:459–64. doi: 10.1378/chest.121.2.459. [DOI] [PubMed] [Google Scholar]
- 7.Mellies U, Stehling F, Dohna-Schwake C, et al. Respiratory failure in Pompe disease: treatment with noninvasive ventilation. Neurology. 2005;64:1465–7. doi: 10.1212/01.WNL.0000158682.85052.C0. [DOI] [PubMed] [Google Scholar]
- 8.Eagle M, Baudouin SV, Chandler C, et al. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscular Disord. 2002;12:926–9. doi: 10.1016/S0960-8966(02)00140-2. [DOI] [PubMed] [Google Scholar]
- 9.Simonds AK, Muntoni F, Heather S, Fielding S. Impact of nasal ventilation on survival in hypercapnic Duchenne muscular dystrophy. Thorax. 1998;53:949–52. doi: 10.1136/thx.53.11.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vianello A, Bevilacqua M, Salvador V, et al. Long-term nasal intermittent positive pressure ventilation in advanced Duchenne's muscular dystrophy. Chest. 1994;105:445–8. doi: 10.1378/chest.105.2.445. [DOI] [PubMed] [Google Scholar]
- 11.Aboussouan LS, Khan SU, Meeker DP, et al. Effect of noninvasive positive-pressure ventilation on survival in amyotrophic lateral sclerosis. Ann Intern Med. 1997;127:450–3. doi: 10.7326/0003-4819-127-6-199709150-00006. [DOI] [PubMed] [Google Scholar]
- 12.Bourke SC, Tomlinson M, Williams TL, et al. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol. 2006;5:140–7. doi: 10.1016/S1474-4422(05)70326-4. [DOI] [PubMed] [Google Scholar]
- 13.Bourke SC, Bullock RE, Williams TL, et al. Noninvasive ventilation in ALS: indications and effect on quality of life. Neurology. 2003;61:171–7. doi: 10.1212/01.WNL.0000076182.13137.38. [DOI] [PubMed] [Google Scholar]
- 14.Marchese S, Lo Coco D, Lo Coco A. Outcome and attitudes toward home tracheostomy ventilation of consecutive patients: a 10-year experience. Resp Med. 2008;102:430–6. doi: 10.1016/j.rmed.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Raphael JC, Chevret S, Chastang C, Bouvet F. Randomised trial of preventive nasal ventilation in Duchenne muscular dystrophy. French Multicentre Cooperative Group on Home Mechanical Ventilation Assistance in Duchenne de Boulogne Muscular Dystrophy. Lancet. 1994;343:1600–4. doi: 10.1016/S0140-6736(94)93058-9. [DOI] [PubMed] [Google Scholar]
- 16.Ward S, Chatwin M, Heather S, Simonds AK. Randomised controlled trial of non-invasive ventilation (NIV) for nocturnal hypoventilation in neuromuscular and chest wall disease patients with daytime -normocapnia. Thorax. 2005;60:1019–24. doi: 10.1136/thx.2004.037424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo Coco D, Marchese S, Pesco MC, et al. Noninvasive positive-pressure ventilation in ALS: predictors of tolerance and survival. Neurology. 2006;67:761–5. doi: 10.1212/01.wnl.0000227785.73714.64. [DOI] [PubMed] [Google Scholar]
- 18.Gay PC, Edmonds LC. Severe hypercapnia after low-flow oxygen therapy in patients with neuromuscular disease and diaphragmatic dysfunction. Mayo Clin Proc. 1995;70:327–30. doi: 10.4065/70.4.327. [DOI] [PubMed] [Google Scholar]
- 19.Servera E, Sancho J, Zafra M, et al. Alternatives to endotracheal intubation for patients with neuromuscular diseases. Am J Phys Med Rehabil. 2005;84:851–7. doi: 10.1097/01.phm.0000184097.17189.93. [DOI] [PubMed] [Google Scholar]
- 20.O’Driscoll BR, Howard LS, Davison AG. BTS guideline for emergency oxygen use in adult patients. Thorax. 2008;63:vi1–68. doi: 10.1136/thx.2008.102947. [DOI] [PubMed] [Google Scholar]
- 21.Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol. 2010;9:177–89. doi: 10.1016/S1474-4422(09)70272-8. [DOI] [PubMed] [Google Scholar]
- 22.Ward S, Chatwin M, Heather S, Simonds AK. Randomised controlled trial of non-invasive ventilation (NIV) for nocturnal hypoventilation in neuromuscular and chest wall disease patients with daytime -normocapnia. Thorax. 2005;60:1019–24. doi: 10.1136/thx.2004.037424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Institute for Health and Care Excellence The use of non-invasive ventilation in the management of motor neurone disease. London: NICE; 2010. [PubMed] [Google Scholar]