Abstract
Diabetes mellitus is an emerging problem in the developing world. In sub-Saharan Africa, for example, the incidence and prevalence of the disease is unknown, diagnosis is often made on the basis of poor information and a loosely defined set of criteria, and access to oral hypoglycaemic agents and insulin is patchy and expensive. The best system of management is currently unclear and this article explores lessons learnt, good practice and the applicability of the structured ‘directly observed treatment, short course’ (DOTS) approach (the current best care system for tuberculosis disease management in resource-poor settings) to the management of chronic diseases such as diabetes.
Key Words: diabetes mellitus, developing word, global health, tubercular diabetic, DOTS for chronic disease management
Introduction
Diabetes is one of the greatest contributors to the global burden of disease. The management of both type 1 and type 2 diabetes mellitus represents a significant challenge in the developing world, which is faced with many pressing health issues, especially infectious diseases. In this context, non-communicable diseases are poorly understood and often under-prioritised by governments. Of all patients with diabetes, 80% live in developing countries. The country with the greatest prevalence is the Micronesian island of Nauru, where 31% of its 14,000 inhabitants have diabetes (the UK has a prevalence of 5%).1 The 2007 STEPS survey showed how the prevalence of diabetes increases with age and was found to be 24.1% in the 35–44 age group, 37.4% among 45–55-year-olds and 45% in the 55–64 age group.2
The most recent International Diabetes Federation (IDF) atlas, published in 2011, pointed to an even greater current and future problem, calculating that, at present, diabetes affects 336 million people worldwide, with a projected rise to 552 million by 2030.3 Each new report highlights the fact that low- and middle-income countries will bear the brunt of the increase and that Africa especially will contribute significantly to this rise in prevelance.4
The reasons for the increasing incidence of diabetes (the majority of which is type 2) are manifold. First, there is an increased recognition and awareness of the problem, but this is accompanied by increasing urbanisation and adoption of westernised lifestyles, leading to dysglycaemia and cardiovascular disease. Of particular importance in the developing world is the influence of tuberculosis and HIV on both pancreatic and endocrine function, as well as the diabetogenic effect of anti-retroviral medications themselves.
In many developing countries, there is little physician experience of, or post-graduate training specific to, diabetes and endocrinology. Consequently, diabetes mellitus tends to be managed by non-specialists with sub-optimal resources and relatively little experience of managing the condition. There are also the practical constraints of limited access to oral hypoglycaemic agents (OHAs), insulin, injection devices and equipment for self-monitoring of blood glucose (SMBG). Type 1 Diabetes mellitus can still be a death sentence for some patients if they, or their family, are unable to purchase insulin. In rural Mozambique, for example, the life expectancy of a child who is diagnosed with type 1 diabetes has been estimated to be as short as 7 months, equal to that in Britain in the pre-insulin era, nearly 100 years ago.5
Africa is facing a rapidly growing chronic non-communicable disease burden, while at the same time experiencing continual high rates of infectious disease. Diabetes has been associated with a three-fold incident risk of tuberculosis, and it is hypothesised that tuberculosis might also increase the risk of developing diabetes. For patients who present with co-morbid tuberculosis and diabetes, the outcomes are worse for both disease states.6
The question, therefore, is how best can we manage diabetes in resource-poor settings? A recent paper from Cohen et al.,7 described the challenges facing a diabetes clinic in Malawi where patients were unable to achieve adequate control of their glycaemia and hypertension, and where micro-vascular complications were very common. For example, in this clinic, the only measure of glycaemia is a fasting blood-glucose measurement on clinic day. Measurement of lipids or glycosolated haemoglobin are not available, neither are urine test sticks for micro-albuminuria.
Are there transferrable areas of best practice that can be applied here, or are there any alternative solutions to meet local challenges? Perhaps physicians in the developed world who have access to point of care testing, insulin pumps, islet cell transplants and increasing numbers of new OHAs are suffering from an embarrassment of riches. In this article, I explore the considerations necessary when seeking to provide a basic, yet essential, diabetes service and present a starting point for deliberation on this difficult issue.
Difficulties in calculating epidemiological data
The impact of an increasingly globalised world on disease burden goes beyond infections to non-communicable diseases (NCDs), which are rising in prevalence in middle- and low-income countries as the population ages and lifestyles and diets change. Diabetes exemplifies this process: in 2000, developing countries were estimated to carry 67% of the global burden of diabetes mellitus, but this proportion is predicted to rise to 78% by 2030.8
One problem, from the clinical point of view, is the identification of the condition. Lack of awareness of the signs or symptoms of diabetes — ‘the flesh melting into the urine’ for younger patients with type 1 diabetes, or the vague insidious nature of hyperosmolar symptoms for older individuals with type 2 diabetes — can mean that the condition is wrongly labelled, or more often ignored. There are also malnutrition-related and atypical forms of type 2 diabetes (e.g. Ketosis-prone diabetes) to be aware of.
There is often a paucity of population-level data from health registries or screening surveys, especially estimates of disease prevalence in rural areas, as well as the difficulties that people with diabetes experience in accessing medical services resulting from geographical distance and/or cost. The IDF estimates a current diabetes prevalence of 2.4% in continental Africa,9 and the diseases is known to be even more prevalent in certain ethnic groups, such as the descendants of Asian migrants.10
The pre-existing communicable disease burden is large, but the chronic disease burden is growing more quickly and the public health and healthcare delivery systems are not at all equipped to address the gathering storm.
Practical problems in diabetes management
In most developing world settings, especially sub-Saharan Africa, the management of non-communicable diseases is sub-optimal. There are few diabetes disease specialists, limited training of dedicated nurses, an absence of standardised management guidelines and protocols, frequent fluctuations in the availability of medications and no register or database of health records. Health education is often under resourced and health-related behaviours can be complex and difficult to change. For example, patients might not be financially able to make the dietary modifications necessary to manage their diabetes adequately. In addition, an individual might be well be resentful because of both the personal and the cultural implications of having diabetes and being singled out as ‘other’.11
When the diagnosis is confirmed, the ‘western’ treatment route for those with type 1 diabetes is to go straight for insulin (as an essential life-saving agent), whereas those with type 2 diabetes are usually treated with an oral hypoglycaemic agent.
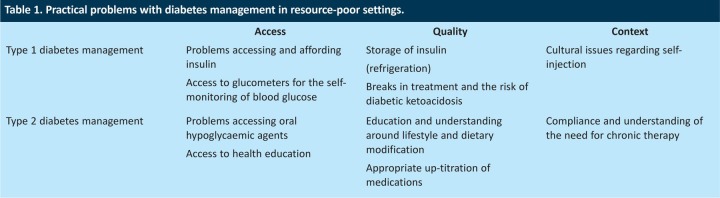
In resource-poor settings, however, a number of practical problems that do not have easy solutions are associated with these paths for diabetes management (Table 1).
Table 1.
Practical problems with diabetes management in resource-poor settings.
Lifestyle
People with diabetes are often advised, not only to control their diet, but also to exercise and lose weight; choices that may seem anathema to many in the developing world. In Botswana, for example, a marker of social success is an elevated body mass index (BMI), which Alexander McCall Smith describes in the Mama Ramotswe series as a ‘traditionally built’ body habitus. Recent anthropological research from Cameroon also showed that obesity can be a deep-rooted status symbol.12
Acute metabolic complications commonly develop in those with diabetes and these are associated with high mortality: 10–30% in diabetic ketoacidosis13 and up to 41% in hyperglycaemic hyperosmolar non-ketotic coma (HONK).14 These mortality rates are often caused by lack of insulin and delayed presentation.
Health education
Healthcare in developing countries primarily focuses on acute disease, relying little on laboratory services, and offers limited patient follow-up. By contrast, chronic disease management requires sustainable laboratory services, training of the healthcare workforce, the availability of appropriate drugs, and patient education in nutrition and self-care. Lack of resources, poor infrastructure and the loss of healthcare workers to developed countries have all slowed progress in these areas.9 The costs of patient education need not be great: this is one of the least expensive diabetes treatments and is a major and effective part of all of the currently described care-delivery packages.
Lack of diabetologists and drugs
In the developing world, sub-standard care is frequent and the complications of diabetes are often not screened for, recognised or treated. It could be argued that generic forms of oral hypoglycaemic agents such as metformin and glibenclamide are relatively cheap and affordable, but interruptions to the supply of essential drugs are all too common. Unstructured and unmonitored clinical care is unfortunately the norm and there is little in the way of reliable information about treatment outcomes, morbidity and mortality.
There is little doubt that the prevalence of micro- and macro-vascular complications has increased in keeping with the rising occurrence of diabetes. This is likely to strain the health budgets of resource-poor countries.15 For example, a recent study in Tanzania showed that treatment of diabetic complications accounted for 31% of total outpatient costs in the main hospital in Dar es Salaam, with a yearly cost of US $138 per person; 19 times more than the average cost of treating someone for a year.16
Suggestions as to how these practical difficulties might be overcome are numerous. No one measure alone can deal with the problem, but it might be possible to consider a number of improvements in concert, as part of a care package akin to those delivered by the multi-disciplinary care teams that we are so used to in the developing world:17
diabetes register to ensure that patients are tracked over time and to allow population level data to be collected and analysed;
standardised assessment checklist to enable optimal screening;18
increased training for specialist diabetes nurses, educators and pharmacists who not only could provide diabetes-related information but who would also be able to undertake most aspects of the diabetes review;
an agreed management protocol to allow escalation of glycaemic treatments when there is failure to achieve tailored targets; and
an improved, reliable supply of essential drugs through closer liaison with the local pharmacy.
TB, HIV and diabetes — the perfect storm?
Several recent articles have highlighted the issue of the ‘tubercular diabetic’.19,20 The large-scale spread of HIV/AIDS in the developing world, especially in sub-Saharan Africa, has precipitated a significant rise in tuberculosis (TB) in immunosuppressed individuals. There is a long-recognised but underappreciated connection between TB and diabetes, in which diabetes makes a substantial contribution to the burden of incident TB around the world and can also worsen TB severity and treatment outcome. The dual management of these diseases is challenging but must be addressed, both in developed and developing countries, because the rising worldwide diabetes burden poses a threat to global TB control.
Diabetes is known to increase the risk of acquisition of active TB and can be a serious co-morbidity factor for existing TB. There is, however, no evidence to suggest how this dual pathology should be managed in low-, middle- or high-income countries.
TB treatments themselves may also be problematic for those with diabetes; for example, rifampicin can have hyperglycaemic effects and pyrazinamide can derange glycaemic control. Where these two conditions co-exist, their integrated management would clearly optimise outcomes. In the past, an association between TB and diabetes was widely accepted. Indeed, half a century ago, expert clinics were established for ‘tuberculous diabetics’ and these appeared to be successful in reducing the otherwise high mortality rate.19
DOTS and how it may work for diabetes mellitus
The structured ‘directly observed treatment, short course’ (DOTS) approach has enabled large-scale administration, monitoring and planning of TB treatment in developing countries, but no comparable systematic service exists for the management of chronic diseases such as diabetes. Could the management of chronic diseases in resource-poor settings take lessons from TB management? It has been suggested that the DOTS approach could be successfully extrapolated for the management of diabetes in an attempt to provide regulated, monitored management that is both affordable and sustainable.20,21
The DOTS model for TB incorporates political commitment, passive diagnosis in patients who present to healthcare facilities, a standardised treatment protocol, a standardised monitoring and evaluation system, and uninterrupted drug supplies. These five facets of the framework for TB control are equally relevant to diabetes control and could be adapted to form a framework for the standardised diagnosis, treatment, monitoring and planning necessary for the long-term management of diabetes patients.22
Diet and education are key components of diabetes management (especially when there is little else to offer). It is possible to envisage a situation in which diabetes educators, who are not healthcare professionals and who have undergone only a short course of training, could work to raise the understanding of diabetes within the community and to advise families on healthier eating. Such individuals already exist in many developing countries and are employed under a public health remit in sex education and in improving understanding of women's health issues. Such workers often have experience of overcoming the cultural prejudices that might hinder optimal healthcare, and the extrapolation of their skill set to meet needs in chronic disease management is entirely plausible.
In the long term, the implementation and sustainability of the DOTS approach in any given setting will depend upon the way in which health services are organised and whether there is ongoing political support. Allain et al.23 have recently shared the lessons they learnt from delivery of a DOTS model for monitoring and evaluating individuals with diabetes in Malawi. They had previously shown how DOTS could be implemented for patients with HIV/AIDS in Malawi and had overcome one of the major hurdles, namely the need for life-long rather than time-limited treatment. If life-long anti-retroviral treatment could be managed and monitored using a modified DOTS framework, then in principle such a framework could also be applied to chronic disease states such as diabetes.
The experience in Malawi has been very interesting and Allain et al23 have become the first to show how the DOTS monitoring system works successfully. The clinicians at Queen Elizabeth Central Hospital in Malawi were able to generate quarterly and cumulative cohort data to monitor and report on their diabetes patients. This allowed not only the collection of strategic information, but also the rapid identification of patients who were not being treated to target. The use of electronic data allows graphical feedback of parameters such as BMI, fasting blood glucose and HbA1c, which enhanced consultations and improved understanding. The system also prompts clinicians to screen for complications and can monitor drug compliance or attrition rates. A further bonus is that when there are common co-morbidities, such as TB and HIV/AIDS, duplication of treatment monitoring is less likely to occur. Plans are currently underway to expand the DOTS system to other district hospitals and to the main central hospital in Lilongwe. The adoption of such a model will then make it possible to provide a national monitoring system that captures comprehensive clinical data on patients with diabetes.
The problem with insulin
Insulin is a difficult medication to replace, store or circumvent. It is relatively expensive, available only to those who can afford it and presents problems in terms of storage and delivery. Some insulins have slightly different storage needs; they need to be kept at temperatures below 25°C and insulin that is not being used needs to be refrigerated. It might be possible to obtain insulin in vials or the like, but the chronic need for consumables such as clean needles and insulin syringes often represents an unsolvable problem.
Perhaps western drug and device manufacturers should become more charitable in their provision for such needs, or perhaps western diabetes centres should even form outreach links or partnerships with centres in the developing world to allow the use of excess or out-of-date stocks of insulin-related consumables. Everything from blood and urine test strips to SMBG diaries would be useful and potentially life saving if provided in out-patient settings where there currently is next to no help available. Optimistically, there are intervention studies and twinning projects that are beginning to show a variety of benefits in varying locations.24 In other effective projects, such as those involving partnered dialysis units, enthusiastic doctors from developing countries have been happy to develop links with centres of excellence in the developed world.25 How much assistance could be provided in such a manner is unknown, but it is worth taking up the initiative.
Summary and conclusions
The prevalence of diabetes is increasing throughout the world. In the developed world, we increasingly have a feel for how the disease should best be managed by making use of clinical algorithms, easy access to medications, close follow up, extensive experience and also greater understanding of the pathophysiology of the condition. This improved understanding has led to new treatments, such as GLP-1 analogues,26 intelligent insulin pumps27 and islet cell transplants.28 The management of diabetes in the developing world also benefits from the work of strong charities such as Diabetes UK and mass-media public health campaigns, which have led to greater awareness among the general public.
The situation in the developing world is starkly different. Diabetes is on the rise but not on the radar. In Africa, for example, epidemiological issues and heath economic factors are problematic. Medical education is limited, meaning that dealing with diabetes on both a personal and population level is difficult. Patients with diabetes do not get the specialist input that we, as developed world diabetologists, would desire. Healthcare systems often do not support the chronic disease management that is so badly needed for diabetes care. It is possible to suggest how preventative measures could be shored up, and we would strongly support this, but experience from the developed world demonstrates how difficult it can be to promote and deliver simple health measures. The socio-cultural impetus that has precipitated the development of metabolic diseases such as hypercholesterolaemia, coronary heart disease and diabetes is hugely overwhelming. Much of this discussion has been touched on by the work of John Yudkin, one of the pioneering researchers who first examined the link between sugar and various degenerative illnesses. In his classic text ‘Pure, white and deadly: the problem of sugar',29 Yudkin showed how refined sugars and eating habits precipitated a surge in diabetes.
In summary, diabetes management in a resource-poor setting faces a number of diverse problems. Some of the barriers to effective treatment are practical, but there are also the political, cultural and social issues that must be attended to. From our fortunate situation, we have the potential to provide aid in various forms — time, experience, education and excess diabetes-related consumables — through the formation of links with embryonic diabetes centres in the developing world. We must work towards a local solution to a globalised problem — one that diabetologists should be at the forefront of responding to.
Acknowledgments
Many thanks go to Dr Sara-Lou Bailey at the London School of Hygiene and Tropical Medicine, to members of the BSUH–Lusaka link project, and to Christopher Bates and Derek Lington.
References
- 1.Horton R. Diabetes — a global threat. Lancet. 2009;373:1735. doi: 10.1016/S0140-6736(09)60954-5. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) 2007 STEPS Survey Nauru. 2007. www.wpro.who.int/countries/nru/en/index.html [Accessed 7 December 2012].
- 3.International Diabetes Federation (IDF) IDF Atlas. 2011. www.idf.org/diabetesatlas [Accessed 7 December 2012].
- 4.Levitt NS. Diabetes in Africa: epidemiology, management and healthcare challenges. Heart. 2008;94:1376–82. doi: 10.1136/hrt.2008.147306. [DOI] [PubMed] [Google Scholar]
- 5.Beran D. Yudkin JS. de Courten M. Access to care for patients with insulin-requiring diabetes in developing countries: case studies of Mozambique and Zambia. Diabetes Care. 2005;28:2136–40. doi: 10.2337/diacare.28.9.2136. [DOI] [PubMed] [Google Scholar]
- 6.Young F. Critchley JA. Johnstone LK. Unwin C. A review of co-morbidity between infectious and chronic disease in sub Saharan Africa: TB and diabetes mellitus, HIV and metabolic syndrome, and the impact of globalization. Global Health. 2009;5:9. doi: 10.1186/1744-8603-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen DB. Allain TJ. Glover S, et al. A survey of the management, control, and complications of diabetes mellitus in patients attending a diabetes clinic in Blantyre, Malawi, an area of high HIV prevalence. Am J Trop Med Hyg. 2010;83:575–81. doi: 10.4269/ajtmh.2010.10-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wild S. Roglic G. Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 9.Longo-Mbenza B. On'kin JB. Okwe AN, et al. Metabolic syndrome, aging, physical inactivity, and incidence of type 2 diabetes in general African population. Diab Vasc Dis Res. 2010;7:28–39. doi: 10.1177/1479164109346362. [DOI] [PubMed] [Google Scholar]
- 10.Lopez AD. Mathers CD. Ezzati M, et al. Global and regional burden of disease and risk factors: systematic analysis of population health data. Lancet. 2006;367:1747–57. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 11.Cameron F. Teenagers with diabetes — management challenges. Aust Fam Physician. 2006;35:386–90. [PubMed] [Google Scholar]
- 12.Cohen E. Pasquet P. Development of a new body image assessment scale in urban Cameroon: an anthropological approach. Ethn Dis. 2011;21:288–93. [PubMed] [Google Scholar]
- 13.Otieno CF. Kayima JK. Omonge EO. Oyoo GO. Diabetic ketoacidosis: risk factors, mechanisms and management strategies in sub-Saharan Africa: a review. East Afr Med J. 2005;82(12 Suppl):S197–203. doi: 10.4314/eamj.v82i12.9382. [DOI] [PubMed] [Google Scholar]
- 14.Kolawole BA. Ajayi AA. Prognostic indices for intra-hospital mortality in Nigerian diabetic NIDDM patients. J Diabetes Complications. 2000;14:84–9. doi: 10.1016/S1056-8727(00)00069-6. [DOI] [PubMed] [Google Scholar]
- 15.Tesafaye S. Gill G. Chronic diabetes complications in Africa. Afr J Diabetes Med. 2011;19:4–8. [Google Scholar]
- 16.World Health Organization. Core health indicators: the latest data from multiple WHO sources. United Republic of Tanzania. Geneva: WHO; 2006. [Google Scholar]
- 17.Windus DW. Ladenson JH. Merrins CK, et al. Impact of a multidisciplinary intervention for diabetes in Eritrea. Clin Chem. 2007;53:1954–9. doi: 10.1373/clinchem.2007.095067. [DOI] [PubMed] [Google Scholar]
- 18.Grant P. The perfect diabetes review. Prim Care Diabetes. 2010;4:69–72. doi: 10.1016/j.pcd.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Grant P. Bailey SL. ‘The tubercular diabetic’: the impact of diabetes mellitus on tuberculosis and its threat to global TB control. Clin Med. 2011;11:344–7. doi: 10.7861/clinmedicine.11-4-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruslami R. Aarnoutse RE. Alisjahbana B, et al. Implications of the global increase of diabetes for tuberculosis control and patient care. Trop Med Int Health. 2010;15:1289–99. doi: 10.1111/j.1365-3156.2010.02625.x. [DOI] [PubMed] [Google Scholar]
- 21.Jeon CY. Harries AD. Baker MA, et al. Bi-directional screening for tuberculosis and diabetes: a systematic review. Trop Med Int Health. 2010;15:1300–14. doi: 10.1111/j.1365-3156.2010.02632.x. [DOI] [PubMed] [Google Scholar]
- 22.Harries AD. Jahn A. Zachariah R. Enarson D. Adapting the DOTS framework for tuberculosis control to the management of non-communicable diseases in sub-Saharan Africa. PLoS Med. 2008;5:124. doi: 10.1371/journal.pmed.0050124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allain TJ. Oosterhout JJ. Douglas GP, et al. Applying lessons learnt from the ‘DOTS’ tuberculosis model to monitoring and evaluating persons with diabetes mellitus in Blantyre, Malawi. Tropical Med Int Health. 2011;16:1077–84. doi: 10.1111/j.1365-3156.2011.02808.x. [DOI] [PubMed] [Google Scholar]
- 24.Ramaiya K. Tanzania and diabetes: a model for developing countries? BMJ. 2005;330:679. doi: 10.1136/bmj.330.7492.679. [DOI] [Google Scholar]
- 25.Gregory MC. Cost-effective dialysis for the developing world. Ethn Dis. 2009;19(Suppl 1 S1):65–7. [PubMed] [Google Scholar]
- 26.Wajcberg E. Amarah A. Liraglutide in the management of type 2 diabetes. Drug Des Devel Ther. 2010;4:279–90. doi: 10.2147/DDDT.S10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant P. Intelligent control of diabetes. Fuzzy logic and insulin pump technology. Med Eng Phys. 2007;29:824–7. doi: 10.1016/j.medengphy.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Srinivasan P. Huang GC. Amiel SA. Heaton ND. Islet cell transplantation. Postgrad Med J. 2007;83:224–9. doi: 10.1136/pgmj.2006.053447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yudkin P. Pure, white and deadly: the problem of sugar. London: Davis-Poynter Ltd; 1972. [Google Scholar]