Abstract
Rheumatoid arthritis (RA) is a chronic disease associated with significant morbidity. The 2009 NICE guidance advises on the management of patients with RA. In this study, we undertook a survey to assess the implementation of the guidance into practice across the Midlands. In total, 19 rheumatology units participated, of which nine have designated early inflammatory arthritis clinics (EIAC). Data for 311 patients with RA attending clinics were collected during a two week period. The median time from symptom onset to first visit was four months. Of the patients, 95.6% were seen within 12 weeks of referral. Of those seen in EIAC, 75.9% had erosions documented on X-rays versus 49.4% of non-EIAC patients. In addition, 57.9% of patients were offered combination disease-modifying antirheumatic drugs (DMARD) therapy in EIAC, versus 30.4% in non-EIAC units. Monthly disease-activity scores were calculated more in patients attending EIAC than non-EIAC units (51.1% versus 25.4%). Based on our results, there is significant regional variation in implementation of the NICE guidance. In addition, patients with RA attending EIACs are more likely to receive a treat-to-target approach.
Key Words: rheumatoid arthritis, early arthritis clinic, NICE guidance, treatment
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease that causes joint destruction, functional impairment and increased mortality. It is associated with elevated mortality rates because of higher rates of myocardial infarction, infection and certain malignancies.1 RA has an estimated prevalence of 0.8% in the UK, with approximately 12,000 people developing it per year.2
Approximately one-third of people stop work because of RA within two years of onset and this prevalence increases thereafter.3 This costs the National Health Service (NHS) an estimated £560 million annually in healthcare costs; the additional cost to the economy of sick leave and work-related disability is £1.8 billion per year.4
Of patients with new-onset RA, 70% develop bony erosions within the first three years,5 with 25% displaying erosions evident on radiographs within three months of disease onset.6 Therefore, a delay in treatment is associated with poorer outcomes, which emphasises the need to diagnose and treat RA within the early ‘window of opportunity’. Evidence shows that combination treatment with two or more disease-modifying antirheumatic drugs (DMARDs), such as methotrexate (MTX), sulfasalazine (SASP), leflunomide (LEF) and hydroxychloroquine (HCQ), results in higher rates of disease remission.7–9 This, coupled with tightly controlled monitoring of disease activity (using a composite measure, such as the 28-joint Disease Activity Score (DAS-28) and regular adjustment of treatment (the Treat-to-Target approach) leads to improved outcomes.10,11 DAS-28 is calculated based on the physician's assessment of: the number of tender and swollen joints identified on examination of the patient, current inflammatory markers and a visual analogue score, which is completed by the patient regarding their overall health. Each time a patient attends the clinic, these measures can be checked to build up a picture of how active the disease is. The disease activity can, in turn, influence the treatment given.
Designated early inflammatory arthritis clinics (EIAC) are one way in which patients with suspected RA can be appropriately investigated, diagnosed, treated and reviewed. EIACs are not available in all rheumatology units, although the number is steadily increasing as treatment for RA becomes evermore specialised. The 2009 National Institute for Health and Clinical Excellence (NICE) guidelines provide best practice advice on the care of adults with RA. Within these guidelines, the key priorities for implementation include prompt referral for specialist treatment, early initiation of DMARDs, appropriate monitoring of disease activity to assess response to treatment and access to a member of the multidisciplinary team (MDT) responsible for coordinating their care.
Objectives
A multicentre rheumatology regional audit programme has been running in the Midlands for 10 years. Using this programme, we assessed to what extent NICE guidelines are being implemented into clinical practice across the Midlands, and ascertained whether there are differences in the service provided between centres with EIACs and those without (non-EIAC).
Methods
Study design
Of the 22 rheumatology units approached, 19 participated in the study, covering a diverse population of approximately 10 million people across the East and West Midlands. Data regarding the services provided at each unit were gathered using a pre-audit questionnaire. The questionnaire gathered information on whether individual units had a designated EIAC and was emailed to the rheumatology specialist trainee at each unit one month before audit data collection. An audit proforma was developed in accordance with the NICE guideline audit tool and initially piloted at several centres. The proformas were anonymously completed for all patients with definite or probable RA that had been diagnosed since February 2009 and who attended outpatient rheumatology clinics over a two-week period during May 2011. Completed proformas were returned by post. The information recorded included: demographic data; time to referral to rheumatology from general practitioner (GP); antibodies and radiographs checked; use of combination DMARD therapy and/or glucocorticoids; whether DAS-28 was utilised at baseline and monthly for the first three months; and whether the patient was treated using a multidisciplinary approach. Given that the NICE guidelines were published in February 2009, patients who had received a diagnosis before this date were not included.
Audit standard
Briefly, the NICE guidelines recommend urgent referral of all patients with suspected inflammatory arthritis for specialist opinion and institution of combination DMARD therapy within three months of symptom onset. Prognostic indicators, including rheumatoid factor (RF), anti-citrullinated peptide antibody (ACPA) and presence of erosion on radiographs of hands and feet must be recorded at baseline. The disease activity, including measurement of C-reactive protein (CRP), should be done on a monthly basis until the desired level of control is achieved and all patients should have access to a named member of the MDT responsible for coordinating their care. To inform their decision making, patients should also have access to written information on their condition, treatment and the service providing their care.
Data management and statistical analysis
Completed questionnaires were scanned optically onto a Microsoft Access database. Descriptive data analysis was performed using Microsoft Excel and StatsDirect statistical software version 2.7.8, and p values were calculated by chi-square analysis using VassarStats.
Results
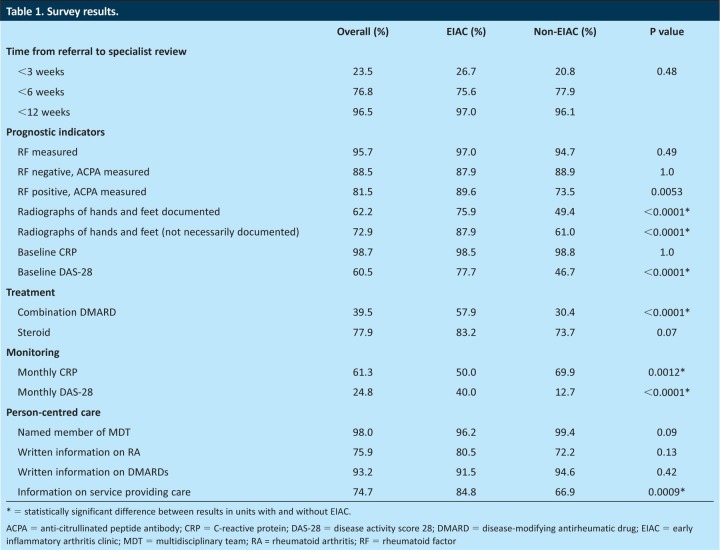
Results are summarised in Table 1. Nine of the units had an EIAC and 10 did not. A total of 579 audit proformas were completed. Of these, 311 were usable responses with probable or definite RA diagnosed after February 2009, comprising 70.5% women and 29.5% men. The remainder had ‘undifferentiated inflammatory arthritis’ or ‘other definite diagnosis’ and, therefore, were excluded from the analysis. In total, 81.4% had definite RA according to the clinician completing the questionnaire. Between two and 34 proformas were submitted per unit, with 136 patients seen in an EIAC and 175 in a non-EIAC setting.
Table 1.
Survey results.
Time to specialist review
The median time from symptom onset to specialist review was four months (interquartile (IQ) range 2–8 months). Time from referral to the first rheumatology clinic appointment was <3 weeks in 23.5%, <6 weeks in 76.8% and <12 weeks in 95.6% of patients. There was no significant difference between units with and without an EIAC (p=0.48).
Prognostic indicators
RF was measured in 95.7% of cases and, of patients who were RF positive, 78.5% also had ACPA measured (89.6% in EIAC, 73.5% in non-EIAC, p=0.0053). Of the patients who were RF negative, 11.5% did not have ACPA measured (12.1% in EIAC, 11.1% in non-EIAC, p=1.0).
Units with EIAC had more patients with X-rays taken of both hands and feet (87.9% versus 61.0% non-EIAC, p<0.0001). In those patients who had X-rays taken of their hands and/or feet, the presence and/or absence of bone erosions was clearly documented in 75.9% of patients in units with an EIAC, and in 49.4% of patients in non-EIAC units (p<0.0001).
Treatment
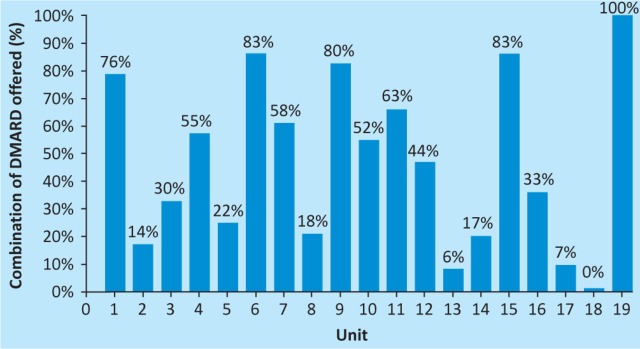
Baseline DAS-28s were completed for 110 patients, of which 68 had DAS-28 >5.1, indicating active disease; 35 patients had a DAS-28 of between 2.6 and 5.1, and seven patients had a DAS-28 of <2.6 at baseline, indicating remission. No data were available as to whether this guided the choice of treatment. Combination DMARD therapy was offered in 39.5% of cases. There was a significant difference between units with an EIAC, where 57.9% of patients were offered combination therapy, compared with 30.4% of patients in non-EIAC units (p<0.0001). There was also wide variation across units, with some not offering any combination therapy and some offering it to all patients (Fig 1). Glucocorticoid therapy was offered in 77.9% of cases, and intramuscular (IM) administration was the most popular.
Fig 1.
Percentage of patients treated with combination disease-modifying antirheumatic drug therapy at each rheumatology unit.
Monitoring
Baseline CRP was checked in 98.7% of patients, with 61.3% having it checked monthly. Half the patients attending EIACs had monthly CRPs compared with 69.9% in non-EIAC units (p=0.0012). Units with EIACs were more likely to measure DAS-28 monthly, with this being done in 40.0% of patients, but only in 12.7% patients attending non-EIAC units (p<0.0001).
Person-centred care
A named member of the MDT was recorded for 98.0% of patients. Written information was documented as being given on RA in 75.9% of cases, on DMARDs in 93.2% of cases and on the service providing care in 74.7% of cases.
Discussion
This survey provides not only valuable insight into variations in practice in the UK on the management of RA, but also a comparison between the service offered by units with and without an EIAC. The first three months after symptom onset are an important therapeutic window for RA12 and disease duration at the time of DMARD initiation has been shown to be the primary predictor of response to DMARD treatment.13 The survey shows that most patients (95.6%) were assessed by a rheumatologist within three months, which differs positively from the results of a recent European study examining delays to specialist treatment, where the percentage of patients seen within this time ranged from 8% to 42%.12 Moreover, the survey results are an improvement upon a previous Midlands audit on RA in 2008, when 84.5% of patients were seen within three months.14 In the patients who presented after the three-month target, the delay was mostly before referral, similar to previous data, which suggests that the delay in presentation to primary care is the main reason why patients with RA are seen late by rheumatologists.15 There was no significant statistical difference between units with and without EIACs, suggesting that, in the current climate of short waiting times in rheumatology, there was no difference in access to timely specialist review in non-EIAC compared with EIAC units.
NICE recommends that all patients with suspected RA have their RF measured, and that ACPA is measured if RF is negative. Most patients had their RF measured, but many also had ACPA measured, even if they were RF positive. This common practice of measuring both antibodies reflects the additional prognostic information conferred, because they are independently poor prognostic indicators, particularly at high titres.16,17 The detection and assessment of erosive disease might be more likely within the EIAC setting, because X-rays of hands and feet were performed more frequently and the presence and/or absence of erosions was better documented. The presence of erosive disease at outset is a poor prognostic marker and can influence how aggressively patients are treated with DMARD therapy.5
MTX is recommended as the first-line DMARD, and this was offered to most patients, while units with EIACs were significantly more likely to utilise combination therapy and glucocorticoids. This might be because patients are monitored and assessed more frequently in the EIAC setting, providing clinicians with greater confidence in prescribing combination DMARD therapy at the outset. Importantly, 60% of patients did not receive treatment as outlined in the NICE guidance, which advises combination DMARD therapy and glucocorticoids for all patients with active RA. However, the guidelines do not define precisely what constitutes active RA. This recommendation was based on randomised controlled trials (RCTs) using combination DMARDs and glucocorticoids.8,9,18 However, entry criteria to these trials differed so that some patients with less active RA received combination therapy. In the trials comparing combination therapy with monotherapy, glucocorticoid use was increased in the combination arm, which might explain the superiority of combination therapy.19 Therefore, clinicians might vary in their threshold to use combination DMARDs. Although NICE recommends combination treatment, European16 and American guidelines,20 which appraised the same trials, suggest monotherapy, which puts in context the finding that less than half of patients were offered combination therapy.
Monthly CRP and DAS-28 are recommended by NICE until an agreed level of disease control is achieved. This is based on two RCTs in which patients with recent-onset RA in the aggressive arm who were monitored monthly had improved outcomes and greater remission rates compared with those that were monitored every three months.10,21 A recent systematic review found that few studies used RCT settings to test the value of treatment to a specific target.11 However, all studies investigating early disease showed significantly better clinical outcomes of the targeted approach. The survey shows that CRP was checked monthly in two-thirds of patients and was done so more frequently in non-EIAC centres. Clinical trials evaluating the use of DMARDs and biological drugs in early RA continue to use erythrocyte sedimentation rate (ESR) in screening suitable patients for entry. This might be one explanation why certain centres are utilising ESR more than CRP. Furthermore, units might be more likely to utilise ESR on a monthly basis rather than CRP because the latter is more cost effective. Monthly DAS-28 was recorded in significantly higher proportions in EIACs than in non-EIACs, which reflects one of the greatest theoretical advantages of EIACs, that is, the capacity for frequent specialist review. It might also explain why EIACs were better at providing information on the service delivering their care, because there was more frequent interaction with the patient. However, it is noteworthy that, even in EIACs, compliance with monthly DAS-28 fell short of 50%.
Conclusion
This regional survey provides important data regarding the accessibility and quality of care for patients with RA. In addition, the survey provides novel comparative data on the care provided between units with and without EIACs. Although there is no difference in how rapidly patients were assessed by a rheumatologist, the results of the survey suggest that EIACs facilitate meeting the NICE recommendations. EIACs provide an opportunity for frequent clinical assessment and the ability to tailor treatment better, as highlighted by increased measurement of radiographic prognostic indicators, monitoring disease activity and treatment with combination DMARD therapy. Therefore, patients with RA attending dedicated EIACs are more likely to receive a treat-to-target approach. The variation in clinician practice regarding DMARD treatment reflects the variety of local, national and international treatment guidelines. Individual centre results have been provided to the relevant units, as well as the overall anonymised results of other centres; these have also been presented and discussed at regional meetings.
Acknowledgements
We would like to thank the Russell's Hall Hospital Audit Department, and the rheumatology clinicians at the following: Dudley Group NHS Foundation Trust; Royal Wolverhampton Hospitals NHS Trust; Sandwell and West Birmingham Hospitals NHS Trust; University Hospitals Birmingham NHS Foundation Trust; Heart of England NHS Foundation Trust; University Hospitals Coventry and Warwickshire NHS Trust; South Warwickshire NHS Foundation Trust; Wye Valley NHS Trust; Shrewsbury and Telford Hospital NHS Trust; University Hospital of North Staffordshire NHS Trust; Mid Staffordshire NHS Foundation Trust; Worcestershire Acute Hospitals NHS Trust; Derby Hospitals NHS Foundation Trust; Sherwood Forest Hospitals NHS Foundation Trust; Nottingham University Hospitals NHS Trust; Kettering General Hospital NHS Foundation Trust; Northampton General Hospital NHS Trust; and United Lincolnshire Hospitals NHS Trust.
References
- 1.Wolfe F. Mitchell DM. Sibley JT, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 2.National Institute of Clinical Excellence (NICE) Clinical Guideline 79. Rheumatoid arthritis: The management of rheumatoid arthritis in adults. London: NICE; 2009. [Google Scholar]
- 3.Barrett EM, Scott DG. Wiles NJ. Symmons DP. The impact of rheumatoid arthritis on employment status in the early years of disease: a UK community-based study. Rheumatology. 2000;39:1403–9. doi: 10.1093/rheumatology/39.12.1403. [DOI] [PubMed] [Google Scholar]
- 4.National Audit Office. Services for people with rheumatoid arthritis. London: National Audit Office; 2009. [Google Scholar]
- 5.van der Heijde DM. van Leeuwen MA. van Riel PL. van de Putte LB. Radiographic progression on radiographs of hands and feet during the first 3 years of rheumatoid arthritis measured according to Sharp's method (van der Heijde modification) J Rheumatol. 1995;22:1792–6. [PubMed] [Google Scholar]
- 6.Nell VP. Machold KP. Eberl G, et al. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology. 2004;43:906–14. doi: 10.1093/rheumatology/keh199. [DOI] [PubMed] [Google Scholar]
- 7.O'Dell JR. Haire CE. Erikson N, et al. Treatment of rheumatoid arthritis with methotrexate alone, sulfasalazine and hydroxychloroquine, or a combination of all three medications. N Engl J Med. 1996;334:1287. doi: 10.1056/NEJM199605163342002. [DOI] [PubMed] [Google Scholar]
- 8.Landewé RB. Boers M. Verhoeven AC, et al. COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheum. 2002;46:347–56. doi: 10.1002/art.10083. [DOI] [PubMed] [Google Scholar]
- 9.Möttönen T. Hannonen P. Leirisalo-Repo M, et al. Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. FIN-RACo trial group. Lancet. 1999;353:1568–73. doi: 10.1016/S0140-6736(98)08513-4. [DOI] [PubMed] [Google Scholar]
- 10.Grigor C. Capell H. Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364:263–6. doi: 10.1016/S0140-6736(04)16676-2. [DOI] [PubMed] [Google Scholar]
- 11.Schoels M. Knevel R. Aletaha D, et al. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search. Ann Rheum Dis. 2010;69:638–43. doi: 10.1136/ard.2009.123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raza K. Stack R. Kumar K, et al. Delays in assessment of patients with rheumatoid arthritis: variations across Europe. Ann Rheum Dis. 2011;70:1822–5. doi: 10.1136/ard.2011.151902. [DOI] [PubMed] [Google Scholar]
- 13.Anderson JJ. Wells G. Verhoeven AC. Felson DT. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum. 2000;43:22–9. doi: 10.1002/1529-0131(200001)43:1<22::AID-ANR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Sandhu RS. Treharne GJ. Justice EA, et al. Accessibility and quality of secondary care rheumatology services for people with inflammatory arthritis: a regional survey. Clin Med. 2007;7:579–84. doi: 10.7861/clinmedicine.7-6-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar K. Daley E. Carruthers DM, et al. Delay in presentation to primary care physicians is the main reason why patients with rheumatoid arthritis are seen late by rheumatologists. Rheumatology. 2007;46:1438–40. doi: 10.1093/rheumatology/kem130. [DOI] [PubMed] [Google Scholar]
- 16.Smolen JS. Landewé R. Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69:964–75. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raza K. Breese M. Nightingale P, et al. Predictive value of antibodies to cyclic citrullinated peptide in patients with very early inflammatory arthritis. J Rheumatol. 2005;32:231–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Hetland ML. Stengaard-Pedersen K. Junker P, et al. Combination treatment with methotrexate, cyclosporine, and intraarticular betamethasone compared with methotrexate and intraarticular betamethasone in early active rheumatoid arthritis. Arthritis Rheum. 2006;54:1401–9. doi: 10.1002/art.21796. [DOI] [PubMed] [Google Scholar]
- 19.Smolen JS. Aletaha D. Keystone E. Superior efficacy of combination therapy for rheumatoid arthritis: fact or fiction? Arthritis Rheum. 2005;52:2975–83. doi: 10.1002/art.21293. [DOI] [PubMed] [Google Scholar]
- 20.Saag KG. Teng GG. Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–84. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 21.Verstappen SM. Jacobs JW. van der Veen MJ, et al. Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial) Ann Rheum Dis. 2007;66:1443–9. doi: 10.1136/ard.2007.071092. [DOI] [PMC free article] [PubMed] [Google Scholar]