Key points
Patients in whom PD is suspected should be referred quickly, and untreated, to a specialist (a neurologist or a geriatrician)
Levodopa remains the most effective therapy for motor symptoms and should still be considered as first-line therapy
Dopamine agonists may be used as monotherapy in younger patients in whom the risk of levodopa-induced motor complications is higher, but the risk of impulse control disorders should be discussed with them
Monoamine oxidase type B (MAO-B) inhibitors may be used as monotherapy in patients with early disease and mild symptoms
As patients develop motor complications, dopamine agonists, catechol-O-methyl transferase inhibitors (COMT) and MAO-B inhibitors all reduce ‘off’ time and increase ‘on’ time when added to levodopa, with therapy tailored to the individual patient
Introduction
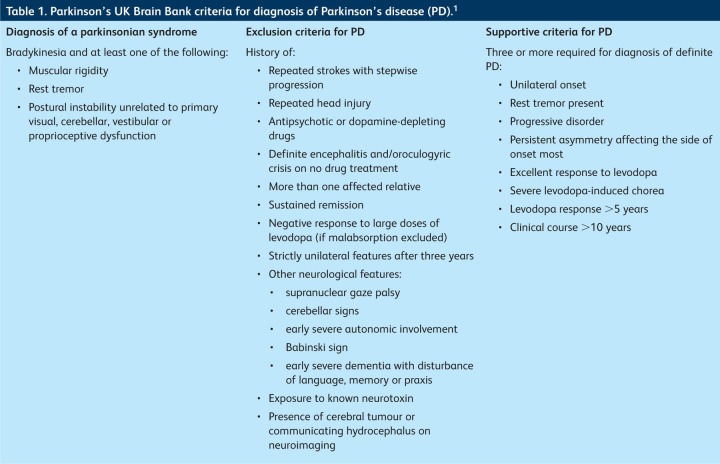
Parkinson's disease (PD) is the second most common neurodegenerative disorder after Alzheimer's disease, affecting around 100,000 people in the UK. Onset is typically with unilateral symptoms in the seventh decade, although the incidence increases with advancing age. A district general hospital serving 250,000 patients would expect to be referred 30–50 new patients per year. The diagnosis is clinical and depends on demonstration of bradykinesia, with at least one of muscular rigidity, rest tremor (4–6 Hz) or postural instability and the absence of a history or symptoms suggestive of an alternative pathology (Table 1).1 Bradykinesia is defined as progressive slowing and decrement in the amplitude of voluntary movement, which is best demonstrated by repetitive tapping of the index finger on the thumb. About 30% of patients with pathologically proven PD have no tremor during life. The National Institute for Health and Clinical Excellence (NICE) recommends that a clinician who suspects a patient has PD should refer them quickly to a specialist (a neurologist or geriatrician) and that the patient should be seen within 6 weeks of referral.2 It is important to refer the patient untreated, as treatment may mask the diagnostic features.
Table 1.
Parkinson's UK Brain Bank criteria for diagnosis of Parkinson's disease (PD).1
Parkinson's disease is incurable and progressive, although the rate of progression varies considerably. The condition has four overlapping stages: diagnosis, maintenance, complex and palliative.3 Non-motor symptoms such as sleep disturbance, depression, anxiety and hypo/anosmia often predate the onset of the motor disorder and become increasingly prevalent and detrimental to quality of life as PD progresses. The onset of psychiatric features, including hallucinations and dementia (up to 80% of patients after 10 years), predicts the need for placement in a care home and reduces life expectancy compared to that in patients with PD without dementia.4 Management of PD should therefore involve a coordinated multidisciplinary approach. This article focuses predominantly on pharmacological therapy of the motor symptoms of PD but touches on the pharmacological management of non-motor symptoms and the main non-pharmacological interventions.
Pharmacological management of motor symptoms
When to start treatment
When to start treatment in PD is controversial. The PD LIFE audit showed that patients started on dopaminergic drugs retained better quality of life for 18 months than those who remained untreated.5 However, in the absence of a proven disease-modifying treatment, the view that patients should remain untreated until they develop disability is still prevalent.
Choice of initial therapy
Currently, no peer-reviewed data comparing the relative efficacy of the commonly used PD medications in early or advanced disease exist. However, the preliminary results of PD MED, a large randomised, multicentre trial comparing therapies in early and late PD in the UK, have been in the public domain since late 2011 and are likely to be published in 2013.
Levodopa
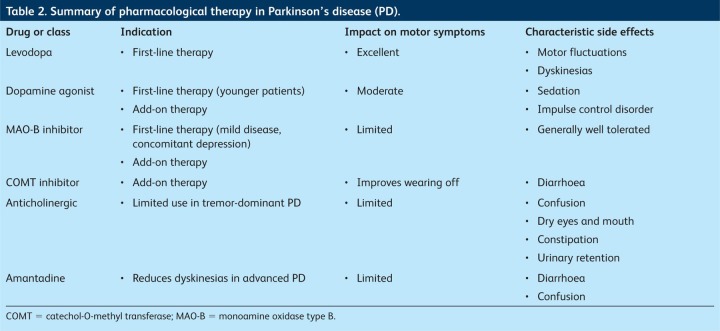
Levodopa in combination with the dopa-decarboxylase inhibitor benserazide (co-beneldopa) or carbidopa (co-careldopa) remains the most efficacious treatment for the motor symptoms of PD.6 As PD progresses, most patients will develop motor complications, including ‘wearing off’ of the treatment response and levodopa-induced dyskinesias (LIDs). Risk factors for LIDs include younger age at onset, longer duration of treatment and higher dose of levodopa.6–8 Fear of these complications has promoted ‘levodopa phobia’, which has delayed the introduction of levodopa. To those clinicians and patients who worry about the ‘risks’ associated with levodopa therapy, I recommend a useful review by Vlaar et al, which dispels much of the mythology.9 As long as the diagnosis has not changed, levodopa should never ‘stop’ working, although the consistency of response declines over time. Moreover, the risk and severity of LIDs can be minimised by keeping the dose of levodopa low. Levodopa should therefore be considered as a potential first-line therapy in all age groups, although caution may be required in younger patients, particularly those younger than 50 years.7
Dopamine agonists
Dopamine agonists (DAs) are another commonly used first-line choice of drug. The adverse effects of nausea, somnolence, dizziness, hallucinosis and impulse control disorder (ICD) tend to be more common than with levodopa, which may be particularly troublesome in older patients.
In younger patients, DAs may be the preferred first-line therapy, as good evidence shows that motor complications are less common than with levodopa.8 However, recent data suggest that ICD (which may include hypersexuality, compulsive gambling, shopping and eating) may affect as many as 17% of patients,10 leading to a re-evaluation of the risks and benefits of DAs. Prescribers should ensure that both the patient and their carers are counselled and given written information about the risk of ICD, as patients may lose insight into their altered behaviour and resist attempts to reduce the DA.11
Pramipexole and ropinirole, both non-ergot oral DAs, can be administered three times daily but are also available as once-daily, extended-release formulations, which may have advantages with respect to drug concordance.12 There is no consistent evidence of superior efficacy or better tolerability compared with standard formulations. With generic preparations now available, repeated switching between brands should be avoided. Rotigotine is a non-ergot DA available as a transdermal skin patch, which may have practical advantages over tablets for some patients.
Monoamine oxidase type B inhibitors
Monoamine oxidase type B (MAO-B) inhibitors such as selegiline and rasagiline are another class of drug recommended by NICE as first-line therapy for PD.2 Their effects are mild, so they are probably best reserved for patients with mild disease; they are generally very well tolerated. Interest has been renewed in their value after data from a delayed-start trial showed that patients with mild PD treated from the outset with 1 mg (but not 2 mg) rasagiline had slightly less severe symptoms at 18 months than those patients in whom treatment was delayed for nine months, which suggests that earlier treatment may confer advantages for some patients.13
Other drugs
Anticholinergic drugs such as benzhexol and the glutamate antagonist amantadine are no longer recommended as first-line treatment in PD, as the evidence for their efficacy is limited and their use is hampered by neuropsychiatric side effects. However, anticholinergics can be useful in some patients with predominant tremor (tremor-dominant PD), and amantadine can be useful for those with LIDs.
Agents used in complex advanced disease
About 10% of patients treated with levodopa per year will develop motor complications including wearing off and LIDs.14 Options include fractionating levodopa (increasing the dose frequency), with the attendant lifestyle/concordance implications of more frequent dosing, and/or the addition of a second agent. Dopamine agonists, catechol-O-methyl transferase (COMT) inhibitors and MAO-B inhibitors can be used safely in conjunction with levodopa, reducing motor fluctuations and ‘off’ time and increasing ‘on’ time.15 The MAO-B inhibitors work by inhibiting dopamine breakdown in the brain, thus prolonging its effect. The choice between these agents depends largely on clinician preference and patient characteristics, as there is no clear evidence of an advantage of one class of drug over another.2
Table 2.
Summary of pharmacological therapy in Parkinson's disease (PD).
The COMT inhibitors entacapone and tolcapone reduce the metabolism of levodopa, prolonging the half-life by 30–50% and increasing levodopa concentrations by 25–100% without increasing plasma concentrations.16 They increase ‘on’ time in patients with advanced PD. The advice from NICE is that combination preparations containing levodopa, carbidopa and entacapone (Stalevo) should be offered to patients to reduce the numbers of tablets and therefore aid concordance.2 Adverse effects, which are generally attributable to an enhanced dopaminergic effect, include nausea, confusion and hallucinations. Diarrhoea is relatively common and may require cessation of the drug.17 Tolcapone is rarely associated with fatal hepatic toxicity and thus should be used only if entacapone has failed; intensive hepatic monitoring is required and the licensing requirements in some countries are restrictive.
Other treatments for advanced Parkinson's disease
Levodopa is absorbed exclusively from the jejunum. Gut motility is reduced in patients with PD, and absorption of levodopa may become increasingly unreliable as the disease progresses, contributing to development and maintenance of motor fluctuations and dyskinesias. To circumvent the problems of delayed gastric emptying, continuous subcutaneous infusion of apomorphine, a potent dopamine agonist, may reduce peak dose LIDs. Similarly, continuous infusion of a levodopa and carbidopa gel into the jejunum via a percutaneous jejunostomy tube may have advantages. However, both have practical limitations and are expensive. Surgical intervention with deep brain stimulation remains a powerful tool for those with motor complications refractory to best medical treatment but is generally reserved for a small minority of non-demented patients younger than 70 years who are fit enough to tolerate the procedure.
Non-motor symptoms
Non-motor symptoms of PD, such as fatigue, anxiety, depression, sleep disturbance, bladder dysfunction, constipation, cognitive impairment and dementia, are common. They are often undeclared by patients and overlooked by clinicians. These symptoms contribute to disability and are an important determinant of quality of life.
Evidence for the management of specific non-motor symptoms in PD is limited. An evidence-based review of published Class I evidence concluded that pramipexole (depression), clozapine (psychosis), rivastigmine (dementia) and botulinum toxin (sialorrhoea) were the only interventions with evidence of definite efficacy; nortriptyline and desipramine (depression) and macrogol (constipation) were also likely to be effective.18 Although many non-motor symptoms are thought to reflect involvement of non-dopaminergic systems, encouraging recent preliminary data indicate that continuous dopaminergic drug delivery may be beneficial for aspects of sleep19 and other non-motor symptoms.20
Non-pharmacological management
Parkinson's disease places a significant social, psychological and physical burden on patients and carers. As important as pharmacological interventions are the support networks created around patients. The NICE guidelines2 state that every patient with PD should have access to:
specialist nursing care
physiotherapy
occupational therapy
speech and language therapy
palliative care services.
Summary
Parkinson's disease is a common, progressive, debilitating disease with substantial physical, psychological and social implications. Pharmacological management is complex and should be individualised according to the needs of the patient. In early disease, treatment is generally highly effective, but medication becomes increasingly inadequate in controlling motor fluctuations and dyskinesias as the disease progresses. Non-motor symptoms, especially depression and dementia, require a holistic, multidisciplinary approach to maximise quality of life for patients and their carers.
For the future, the ideal solution remains neuroprotection and restoration. Progress has been hampered by the lack of animal models that reflect the widespread brain pathology presumed to cause both motor and non-motor symptoms of PD in humans. Currently, agents are undergoing clinical trials in early, mildly affected patients, such as the plant-derived substance PYM50028 (Cogane), which promotes expression of endogenous neural growth factors and has shown promise in vitro and in animal models. Gene-therapy trials in progress rely on the viral vectors used to deliver the enzymatic machinery required for dopamine synthesis to the striatum.
As PD progresses, adequate control of motor symptoms depends increasingly on continuous drug delivery, and greater physiological stimulation of dopamine receptors may help to prevent the development of LIDs and motor fluctuations. Efforts thus are afoot to develop better delivery systems for levodopa, and a new sustained-release formulation is in development.
References
- 1.Gibb WRG. Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:745–52. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Collaborating Centre for Chronic Conditions. Parkinson's disease: national clinical guideline for diagnosis and management in primary and secondary care. London: Royal College of Physicians; 2006. [PubMed] [Google Scholar]
- 3.MacMahon DG. Thomas S. Practical approach to quality of life in Parkinson's disease: the nurse's role. J Neurol. 1998;245(Suppl 1):S19–22. doi: 10.1007/PL00007732. [DOI] [PubMed] [Google Scholar]
- 4.Hobson P. Meara J. Ishihara-Paul L. The estimated life expectancy in a community cohort of Parkinson's disease patients with and without dementia, compared with the UK population. J Neurol Neurosurg Psychiatry. 2010;81:1093–8. doi: 10.1136/jnnp.2009.198689. [DOI] [PubMed] [Google Scholar]
- 5.Grosset D. Taurah L. Burn DJ, et al. A multicentre longitudinal observational study of changes in self reported health status in people with Parkinson's disease left untreated at diagnosis. J Neurol Neurosurg Psychiatry. 2007;78:465–9. doi: 10.1136/jnnp.2006.098327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fahn S. Oakes D. Shoulson I, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004;351:2498–508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 7.Ku S. Glass GA. Age of Parkinson's disease onset as a predictor for the development of dyskinesia. Mov Disord. 2010;25:1177–82. doi: 10.1002/mds.23068. [DOI] [PubMed] [Google Scholar]
- 8.Stacy M. Galbreath A. Optimizing long-term therapy for Parkinson disease: levodopa, dopamine agonists, and treatment-associated dyskinesia. Clin Neuropharmacol. 2008;31:51–6. doi: 10.1097/WNF.0b013e318065b088. [DOI] [PubMed] [Google Scholar]
- 9.Vlaar A. Hovestadt A. van Laar T. Bloem BR. The treatment of early Parkinson's disease: levodopa rehabilitated. Pract Neurol. 2011;11:145–52. doi: 10.1136/practneurol-2011-000011. [DOI] [PubMed] [Google Scholar]
- 10.Voon V. Sohr M. Lang AE, et al. Impulse control disorders in Parkinson disease: a multicenter case-control study. Ann Neurol. 2011;69:986–96. doi: 10.1002/ana.22356. [DOI] [PubMed] [Google Scholar]
- 11.Scottish Intercollegiate Guidelines Network. SIGN guideline No. 113. Edinburgh: SIGN; 2010. Diagnosis and pharmacological management of Parkinson's disease. [Google Scholar]
- 12.Grosset KA. Bone I. Grosset DG. Suboptimal medication adherence in Parkinson's disease. Mov Disord. 2005;20:1502–7. doi: 10.1002/mds.20602. [DOI] [PubMed] [Google Scholar]
- 13.Olanow CW. Rascol O. Hauser RA, et al. A double-blind, delayed-start trial of rasagiline in Parkinson's disease. N Engl J Med. 2009;361:1268–78. doi: 10.1056/NEJMoa0809335. [DOI] [PubMed] [Google Scholar]
- 14.Ahlskog JE. Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–58. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- 15.Rascol O. Brooks DJ. Melamed E, et al. Rasagiline as an adjunct to levodopa in patients with Parkinson's disease and motor fluctuations (LARGO, lasting effect in adjunct therapy with rasagiline given once daily, study): a randomised, double-blind, parallel-group trial. Lancet. 2005;365:947–54. doi: 10.1016/S0140-6736(05)71083-7. [DOI] [PubMed] [Google Scholar]
- 16.Bonifati V. Meco G. New, selective catechol-O-methyltransferase inhibitors as therapeutic agents in Parkinson's disease. Pharmacol Ther. 1999;81:1–36. doi: 10.1016/S0163-7258(98)00032-1. [DOI] [PubMed] [Google Scholar]
- 17.Reichmann H. Boas J. Macmahon D, et al. Efficacy of combining levodopa with entacapone on quality of life and activities of daily living in patients experiencing wearing-off type fluctuations. Acta Neurol Scand. 2005;111:21–8. doi: 10.1111/j.1600-0404.2004.00363.x. [DOI] [PubMed] [Google Scholar]
- 18.Seppi K. Weintraub D. Coelho M, et al. The Movement Disorder Society evidence-based medicine review update: treatments for the non-motor symptoms of Parkinson's disease. Mov Disord. 2011;26(Suppl 3):S42–80. doi: 10.1002/mds.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trenkwalder C. Kies B. Rudzinska M, et al. Rotigotine effects on early morning motor function and sleep in Parkinson's disease: a double-blind, randomized, placebo-controlled study (RECOVER) Mov Disord. 2011;26:90–9. doi: 10.1002/mds.23441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honig H. Antonini A. Martinez-Martin P, et al. Intrajejunal levodopa infusion in Parkinson's disease: a pilot multicenter study of effects on nonmotor symptoms and quality of life. Mov Disord. 2009;24:1468–74. doi: 10.1002/mds.22596. [DOI] [PubMed] [Google Scholar]