Abstract
The capacity to respond to temperature fluctuations is critical for microorganisms to survive within mammalian hosts, and temperature modulates virulence traits of diverse pathogens. One key temperature-dependent virulence trait of the fungal pathogen Candida albicans is its ability to transition from yeast to filamentous growth, which is induced by environmental cues at host physiological temperature. A key regulator of temperature-dependent morphogenesis is the molecular chaperone Hsp90, which has complex functional relationships with the transcription factor Hsf1. Although Hsf1 controls global transcriptional remodeling in response to heat shock, its impact on morphogenesis remains unknown. Here, we establish an intriguing paradigm whereby overexpression or depletion of C. albicans HSF1 induces morphogenesis in the absence of external cues. HSF1 depletion compromises Hsp90 function, thereby driving filamentation. HSF1 overexpression does not impact Hsp90 function, but rather induces a dose-dependent expansion of Hsf1 direct targets that drives overexpression of positive regulators of filamentation, including Brg1 and Ume6, thereby bypassing the requirement for elevated temperature during morphogenesis. This work provides new insight into Hsf1-mediated environmentally contingent transcriptional control, implicates Hsf1 in regulation of a key virulence trait, and highlights fascinating biology whereby either overexpression or depletion of a single cellular regulator induces a profound developmental transition.
Author summary
For human pathogens, the capacity to respond to elevated temperature is required for survival, with elevated temperature in the form of fever as a conserved host response to defend against infection. One of the leading fungal pathogens of humans in Candida albicans, which is capable of growing in both a yeast and filamentous state. The ability to transition between these forms is a key virulence trait, and one that is highly temperature-dependent. A pivotal regulator of filamentous growth is the temperature-responsive molecular chaperone Hsp90, which has complex relationships with the transcription factor Hsf1. Although Hsf1 regulates changes in gene expression in response to heat shock, its impact on morphogenesis remains unknown. Here, we uncover an intriguing phenomenon whereby overexpression or depletion of C. albicans HSF1 induces morphogenesis. We observe that HSF1 depletion compromises Hsp90 function, thereby driving filamentation. In contrast, HSF1 overexpression induces a dose-dependent expansion of its transcriptional targets that drives overexpression of positive regulators of filamentous growth. This work illuminates novel mechanisms through which tuning the levels of an environmentally contingent transcription factor drives a key developmental program.
Introduction
Microorganisms that colonize mammals are continually challenged with a myriad of environmental stimuli, such as osmotic imbalances, oxidative stresses, nutrient limitation and temperature fluctuations, with fever as one of the most ubiquitous responses to infection. The capacity to sense and respond to temperature change is critical for pathogens to survive, and the circuitry governing responses to thermal stress is one of the most conserved responses in nature[1]. For fungal pathogens, temperature sensing also has a profound impact on developmental programs that are crucial for virulence. This is exquisitely clear for thermally dimorphic fungi, including species of Histoplasma, Blastomyces, Coccidioides and Paracoccidiodes, which grow as non-pathogenic filamentous molds in the soil and respond to the temperature increases of a mammalian host by switching to a pathogenic yeast form[2].
Although the opportunistic fungal pathogen Candida albicans resides within warm-blooded mammals and occupies a thermally buffered niche, it has retained the heat shock response[3, 4] and temperature influences diverse aspects of its biology including mating, phenotypic switching, and drug resistance[5]. Temperature also has a profound effect on the transition between distinct morphological states, including the yeast form and filamentous pseudohyphal and hyphal forms[6]. The capacity to transition between morphotypes is strongly correlated with virulence[7, 8], as both forms play critical roles in infection. The transition from yeast to filamentous growth is induced by diverse host relevant environmental cues, including exposure to serum, nutrient limitation, and neutral pH, all of which require the physiologically relevant temperature of 37°C[5, 6]. Response to these morphogenetic cues is regulated by complex signaling cascades, which include many transcription factors that positively regulate filamentation, such as Efg1, Cph1, Flo8[5, 6], Ume6[9, 10] and Brg1[11], as well as repressors of morphogenesis, such as Tup1 and Nrg1[5, 6]. Although filamentation in response to different cues can depend upon distinct genetic circuitry and culminate in contrasting cellular and sub-cellular phenotypes, filamentation in response to most cues is accompanied by a common global transcriptional remodeling, which includes the expression of a suite of filament-specific genes that encode adhesin proteins such as Als3 and Als5, secreted aspartyl proteases such as Sap4 and Sap5, cyclins such as Hgc1, and the hyphal wall protein Hwp1[12, 13]. Although genetic screens have revealed many genes that influence filamentation[5–8, 14], the molecular basis of how environmental signals such as temperature are sensed and transduced to activate this developmental program remains largely enigmatic.
A fascinating example of temperature sensing relevant to morphogenesis involves the coordinated and rapid induction of a suite of cellular heat shock proteins (HSPs) that enable adaptation to thermal insults. At the core is Hsp90, an essential and conserved molecular chaperone that stabilizes diverse client proteins integral to many signaling pathways[15]. Hsp90 represses C. albicans morphogenesis via the cyclic AMP-protein kinase A (cAMP-PKA) signaling pathway[16, 17], which positively regulates filamentation in response to diverse inducing cues. The elevated temperature normally required for filamentation creates a state of cellular stress that exceeds the functional capacity of Hsp90 to chaperone client proteins, thereby relieving Hsp90-mediated repression of the filamentation program[17, 18]. Compromise of Hsp90 function genetically or pharmacologically also relieves repression of cAMP-PKA signaling and induces a morphological transition from yeast to filamentous growth in the absence of an inducing cue or elevated temperature[17]. Filamentation in response to Hsp90 inhibition is dependent on most components of the cAMP-PKA pathway[17], interactions with the cell cycle kinase Cdc28[19], and signaling through the cyclin Pcl1, cyclin-dependent kinase Pho85 and transcription factor Hms1[20]. Further, Hsp90 function is regulated by complex interactions with co-chaperones that regulate its ATPase activity and mediate interactions with client proteins, and by post-translational modifications such as phosphorylation and acetylation[15, 21], which can have profound effects on cellular responses to thermal stress and on morphogenesis[16, 22].
Global transcriptional remodeling in response to elevated temperature is orchestrated by the essential heat shock transcription factor, Hsf1. Hsf1 is a conserved temperature sensor, whose expression and activity are elevated in response to acute increases in temperature, in order to enable crucial cellular responses to thermal stress[3, 23]. In C. albicans and related yeast species, Hsf1 is constitutively trimerized and located in the nucleus where it drives the basal expression of HSP90 and other chaperone genes and is poised to induce their expression upon heat shock[3, 4, 24]. Hsp90 physically interacts with Hsf1 and represses its function, such that compromising Hsp90 function leads to activation of Hsf1 and induction of the heat shock response[25]. We recently established that C. albicans Hsf1 binds to an expanded set of targets in response to heat shock, and engages not only with genes involved in protein folding, but also genes important for filamentation, pathogenesis, adhesion and biofilm formation[4]. This is consistent with findings that mutations in HSF1 that block its activation prevent thermal adaptation and attenuate virulence in a mouse model of systemic disease[26], although Hsf1 has yet to be implicated in morphogenesis. C. albicans Hsf1 binds to distinct motifs in nucleosome-depleted promoter regions that resemble conserved Hsf1 binding sites, referred to as Heat Shock Elements (HSEs), which are found in other eukaryotes including Saccharomyces cerevisiae[24], Cryptococcus neoformans[27], Drosophila species[28] and humans[29]. Importantly, there are 4,879 C. albicans genes with an Hsf1 consensus sequence but no detectable Hsf1-binding under basal or heat shock conditions, suggesting that Hsf1 binds to distinct targets in response to different environmental conditions[4]. As an environmentally contingent transcriptional regulator of cellular circuitry, Hsf1 is poised to regulate temperature-dependent developmental programs in C. albicans.
Here, we establish that tuning the expression level of HSF1 has a profound impact on C. albicans morphogenesis, such that depletion or overexpression of HSF1 is sufficient to induce a transition from yeast to filamentous growth in the absence of any external cues. We demonstrate that the filaments induced by HSF1 depletion or overexpression are structurally distinct, are contingent upon different genetic circuitry, and are induced through independent mechanisms. Our findings support the model that HSF1 depletion induces filamentation by compromising Hsp90 function, independent of its role in regulating HSP90 transcription. HSF1 overexpression does not impact Hsp90 function, but rather induces filamentation by driving the overexpression of positive regulators of morphogenesis and repressing the expression of negative regulators of morphogenesis. For example, global ChIP-seq and RNA-seq analyses reveal that HSF1 overexpression expands the set of bona fide targets, thereby influencing the expression of many morphogenetic regulators, including the positive regulators Ume6 and Brg1, and the negative regulator Nrg1. Reducing the ambient temperature abolishes filamentation induced by HSF1 overexpression, but does not affect filamentation induced by HSF1 depletion. This work provides new insight into Hsf1-mediated environmentally contingent transcriptional control, implicates Hsf1 in regulation of a key virulence trait, and highlights fascinating biology whereby either overexpression or depletion of a single cellular regulator induces a profound developmental transition.
Results
HSF1 overexpression and depletion induce filamentation through distinct mechanisms
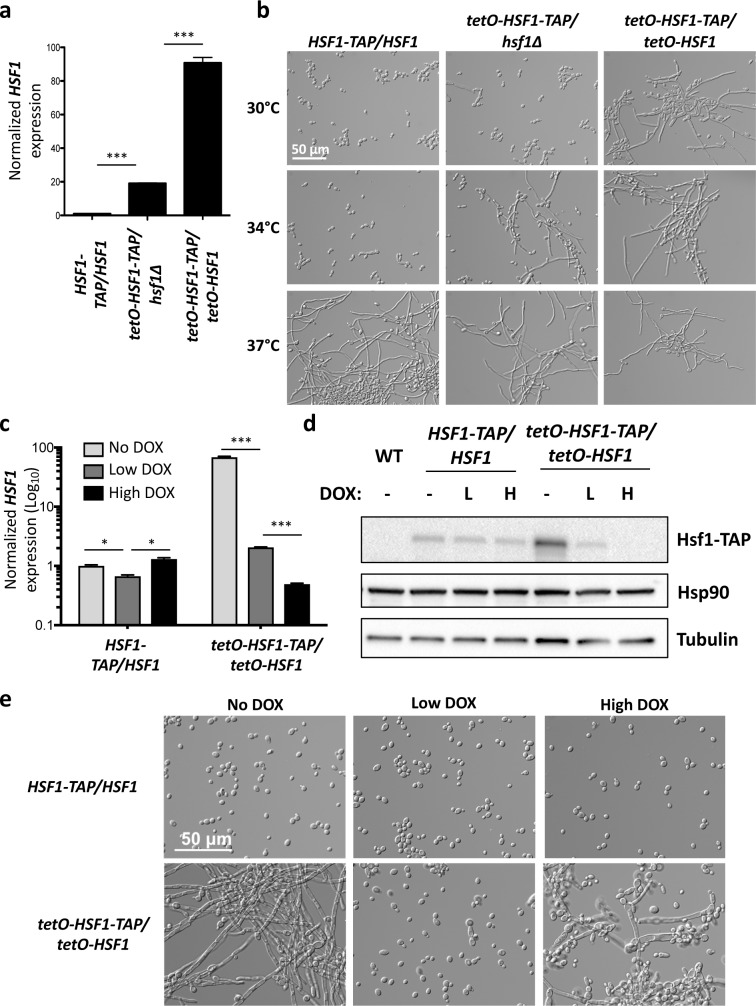
To investigate the role of Hsf1 in C. albicans morphogenesis, we engineered a strain in which one allele of HSF1 is deleted and the remaining allele is regulated by the tetracycline-repressible promoter tetO (tetO-HSF1/hsf1Δ), which is repressed by tetracycline or the analog doxycycline (DOX). As HSF1 is generally expressed at low levels, replacing its native promoter with the strong tetO promoter caused HSF1 overexpression in the absence of DOX (Fig 1A). Although C. albicans morphogenesis is normally contingent upon a temperature of 37°C, we observed that overexpression of HSF1 in the tetO-HSF1/hsf1Δ strain enabled filamentation at 34°C (Fig 1B). In a strain where both alleles of HSF1 were regulated by tetO (tetO-HSF1/tetO-HSF1), the magnitude of HSF1 overexpression in the absence of DOX was much greater (Fig 1A), completely bypassing the requirement for elevated temperature and inducing filamentation even at 30°C in the absence of an inducing cue (Fig 1B). Filamentation is not a general response to overexpressing genes in this system, as overexpression of other genes, including the morphogenetic regulator HSP90, did not promote filamentous growth (S1A Fig). Contrary to findings in S. cerevisiae where HSF1 overexpression causes severe growth defects and toxicity[30, 31], overexpression of HSF1 in C. albicans did not cause a defect in growth or viability (S1B Fig). Thus, HSF1 overexpression is sufficient to drive morphogenesis, and HSF1 levels modulate the temperature-dependence of an important developmental program.
Fig 1. Overexpression or depletion of HSF1 induces filamentation.
a) HSF1 levels can be overexpressed to different levels by placing one or both alleles of HSF1 under the control of the tetracycline-repressible promoter, tetO. Strains were grown in the absence of doxycycline (DOX) to mid-log phase. HSF1 transcript levels were measured by quantitative RT-PCR and normalized to ACT1 and GPD1. Data are means +/- standard error of the means for triplicate samples. *** indicates P value <0.005, unpaired t test. b) Overexpression of HSF1 can bypass the temperature requirement for morphogenesis. Strains were grown in the absence of DOX for 4 hours at the indicated temperature. c) The tetO-HSF1-TAP/tetO-HSF1 strain can be used to monitor the effect of both HSF1 overexpression and depletion. HSF1 is overexpressed in the tetO-HSF1-TAP/tetO-HSF1 strain when grown in the absence of DOX (No DOX). HSF1 expression is lowered when the strain is grown in the presence of 0.1 μg/mL DOX (Low DOX), and is depleted when grown with 20 μg/mL DOX (High DOX). HSF1 transcript levels were measured by quantitative RT-PCR and were normalized to ACT1 and GPD1. Data are means +/- standard error of the means for triplicate samples and are graphed on a log10 scale. *** indicates P value <0.005, * indicates P value <0.05, unpaired t test. d) Hsf1 protein levels are overexpressed when the tetO-HSF1-TAP/tetO-HSF1 strain is grown in the absence of DOX, reduced when grown in 0.1 μg/mL DOX (Low DOX, labelled L), and depleted when grown in 20 μg/mL DOX (High DOX, labelled H). WT indicates the wild type, untagged control. Tubulin levels serve as loading control. e) HSF1 overexpression or depletion induces filamentation in the absence of an inducing cue. Strains were grown in the presence of no DOX, 0.1 μg/mL DOX (Low DOX), or 20 μg/mL DOX (High DOX) at 30°C.
To further explore the relationship between HSF1 levels and filamentation, we grew the tetO-HSF1/tetO-HSF1 strain in varying concentrations of DOX to repress HSF1 expression. As expected, when the tetO-HSF1/tetO-HSF1 strain was grown in the absence of DOX, HSF1 transcript and protein levels were dramatically overexpressed (Fig 1C and 1D), causing robust filamentation at 30°C (Fig 1E). When grown in a very low concentration of DOX (0.01 μg/mL), HSF1 levels were reduced to an intermediate level of overexpression that still promoted filamentous growth (S2 Fig). Filamentation induced by HSF1 overexpression was reversible, as growth in a low concentration of DOX (0.1 μg/mL) reduced HSF1 expression to a similar level as observed in the wild-type strain (Fig 1C, Fig 1D and S2 Fig), and the cells grew in the yeast form (Fig 1E and S2 Fig). Remarkably, when the tetO-HSF1/tetO-HSF1 strain was grown in the presence of a ten-fold higher concentration of DOX (1 μg/mL) some filamentation was observed (S2 Fig), and filamentation was more robust when the strain was grown in high concentrations of DOX (10–20 μg/mL) that lead to a significant reduction in HSF1 levels (Fig 1C, Fig 1D, Fig 1E and S2 Fig). Although Hsf1 is essential in C. albicans[3, 8, 26], depletion of HSF1 with 20 μg/mL DOX in these conditions had only a minor impact on viability (S1B Fig). We observed that overexpression or depletion of HSF1 induced filamentation in multiple strain backgrounds, confirming the robustness of the phenotypes (S3 Fig). Analysis of published RNA sequencing (RNA-seq) data of wild-type C. albicans grown in diverse environmental conditions revealed that HSF1 expression is highly responsive to the environment and varies over 100-fold across experiments (S4A Fig). In particular, HSF1 levels are transcriptionally upregulated upon a 30°C to 42°C heat shock, as well as during biofilm growth (S4B Fig). This upregulation leads to a level of HSF1 that is similar to that observed in the tetO-HSF1/hsf1Δ strain when grown in the absence of DOX (S4B Fig), which we demonstrated promotes filamentation at 34°C (Fig 1B). Together, this defines a fascinating paradigm in which tuning the levels of a single environmentally-responsive transcriptional regulator drives C. albicans morphogenesis.
Hsf1 is exquisitely responsive to temperature, as its expression and activity increase in response to temperature upshifts, thereby enabling thermal adaptation[23]. Given that modulating HSF1 expression altered the requirement of elevated temperature for morphogenesis (Fig 1B), we hypothesized that further reduction in temperature might impair filamentation in response to altered HSF1 levels. To test this, we assayed whether filamentation induced by overexpression or depletion of HSF1 was maintained at temperatures below 30°C. Filamentation in response to HSF1 overexpression was completely blocked at 23°C, but filamentation in response to HSF1 depletion was maintained (Fig 2A). The temperature dependence of filamentation induced by HSF1 overexpression was striking and distinct from the robust filamentation observed at 23°C in response to depletion of HSP90, deletion of the transcriptional repressors TUP1 and NRG1, or overexpression of the positive regulators of filamentation UME6 and BRG1 (S5 Fig). This suggests that Hsf1 activity is strongly influenced by temperature, with profound effects on its capacity to induce morphogenesis.
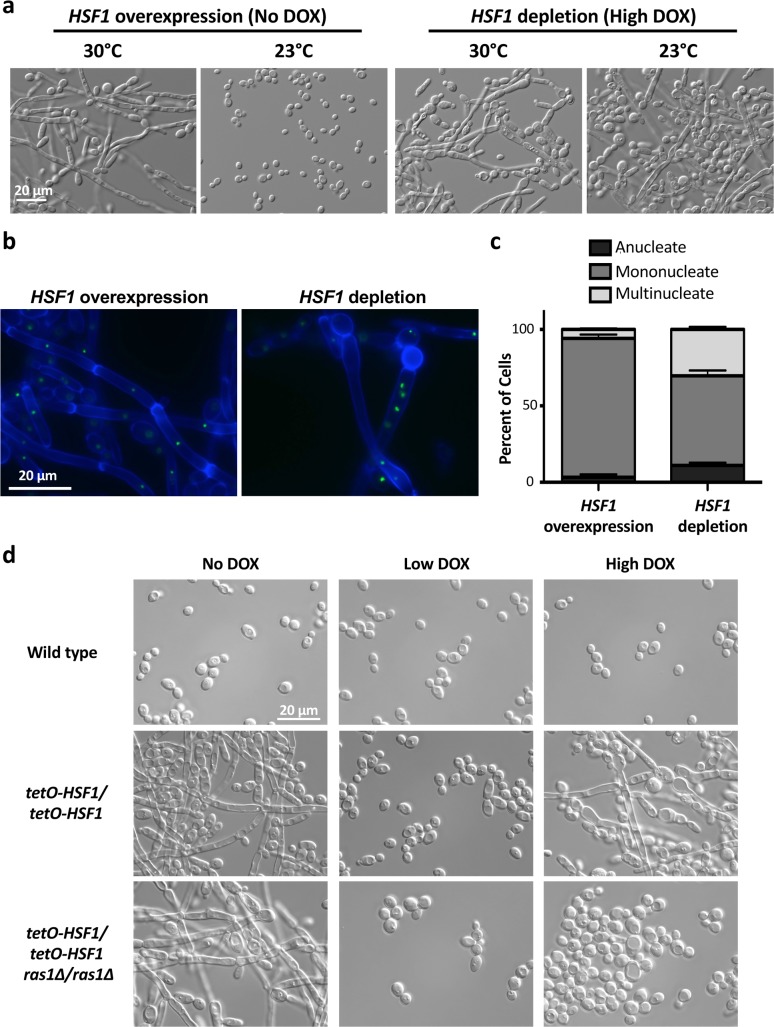
Fig 2. HSF1 overexpression and depletion induce filaments that differ in their morphology, nuclear content and dependence on Ras1.
a) Reduced temperature blocks filamentation in response to HSF1 overexpression but not in response to HSF1 depletion. Strains were grown in the absence or presence of high levels of DOX (20 μg/mL) at the indicated temperature. b) Filaments induced by HSF1 overexpression and depletion have distinct features. Cell walls and septa were visualized using calcofluor white and the nuclei were visualized using a strain with the nucleolar protein, Nop1, GFP tagged. c) Filaments induced by HSF1 depletion have a significant increase in the percentage of cells that are multinucleate compared to filaments induced by HSF1 overexpression. The number of nuclei in at least 300 cells were counted for each condition, for two biological replicates. Means are graphed with the error bars displaying the standard error of means. Unpaired t-test indicates a significant difference in percentage of multinucleate cells, P value is 0.0056. d) Filaments induced by HSF1 depletion, but not overexpression, are dependent on the GTPase Ras1. Strains were grown in rich medium with 80 mg/L uridine added, in the presence of no DOX, 0.1 μg/mL DOX (Low DOX), or 20 μg/mL DOX (High DOX) at 30°C.
We further explored the morphology of filaments induced by HSF1 overexpression and depletion using the GFP-tagged nucleolar protein Nop1 to visualize the nuclei and calcofluor white to label the septa and cell wall[32]. When HSF1 was overexpressed, filaments formed parallel cell walls with distinct, mononucleate compartments defined by regularly spaced septa (Fig 2B). In contrast, HSF1 depletion induced filaments with variable widths, less-defined compartments harboring sporadically spaced septa, and often anucleate or multinucleate compartments (Fig 2B). Quantification of the number of nuclei per cellular compartment confirmed that HSF1 depletion induced filaments with a significant increase in multinucleate cells compared to filaments induced by HSF1 overexpression (Fig 2C). This is consistent with findings that HSF1 depletion in S. cerevisiae leads to large, amorphous cells with 4C DNA content[33, 34]. Thus, we observed structural differences between the filaments induced by HSF1 overexpression and depletion, suggesting that the mechanisms underpinning the cellular responses are distinct.
Next, we assessed whether filamentation in response to HSF1 overexpression and depletion is dependent on the same genetic circuitry. To do so, we engineered tetO-HSF1/tetO-HSF1 strains in a RAS1 homozygous deletion mutant background. Ras1 is a GTPase that regulates cAMP-PKA signaling and is a critical regulator of filamentation in response to many cues[5, 17, 35]. Intriguingly, loss of Ras1 completely blocked filamentation induced by HSF1 depletion but did not affect filamentation induced by HSF1 overexpression (Fig 2D). Ras1 remained necessary for filamentation induced by HSF1 depletion even when the tetO-HSF1/tetO-HSF1 strain was grown in a higher concentration of DOX (50 μg/mL), confirming that the block in filamentation is not a result of insufficient HSF1 depletion (S6 Fig). Taken together, we establish that overexpression of HSF1 induces filaments that differ from those induced by depletion of HSF1 in terms of their temperature dependence, cell biology and genetic requirements.
HSF1 depletion induces filamentation by compromising Hsp90 function
We focused first on elucidating the mechanism through which HSF1 depletion induces filamentation. We confirmed that HSF1 depletion induced filamentation when HSF1 is repressed in the tetO-HSF1/hsf1Δ strain in the presence of DOX and with the independent MAL2p repressible promoter system, which is repressed when cells are grown in the presence of glucose (S7A Fig). The tetO-HSF1/hsf1Δ strain was used for our mechanistic studies as there are profound changes in physiology associated with switching carbon sources, and the concentrations of DOX required for this system are phenotypically neutral. Further, this strain grows in the yeast form in the absence of DOX (Fig 1B and S7A Fig) simplifying the genetic system to study the mechanisms governing filamentation upon HSF1 depletion. To provide an initial portrait of transcriptional changes in response to HSF1 depletion, we performed comparative microarray analysis of the tetO-HSF1/hsf1Δ strain grown in the absence or presence of 20 μg/mL of DOX to deplete HSF1. This identified 1,346 genes whose expression were altered by at least 1.5-fold upon HSF1 depletion (P value < 0.05; S1 Data), with GO term enrichments for oxidation-reduction processes and many processes related to morphogenesis (S7B Fig and S1 Data). Gene Set Enrichment Analysis (GSEA) was employed to identify transcriptional profiles with significant correlation to the microarray profile induced upon HSF1 depletion. This analysis identified a significant correlation (P value < 0.0001) between the profile of genes upregulated upon HSF1 depletion with profiles of genes upregulated in filaments induced by serum at 37°C[12], treatment with cell cycle inhibitors[36], and treatment with geldanamycin[20], an Hsp90 inhibitor that binds with high affinity to the unusual Hsp90 ATP binding pocket[37] (S7C Fig, S7D Fig and S1 Data). This suggests that depletion of HSF1 induces a core transcriptional program that is common to filamentation in response to diverse cues.
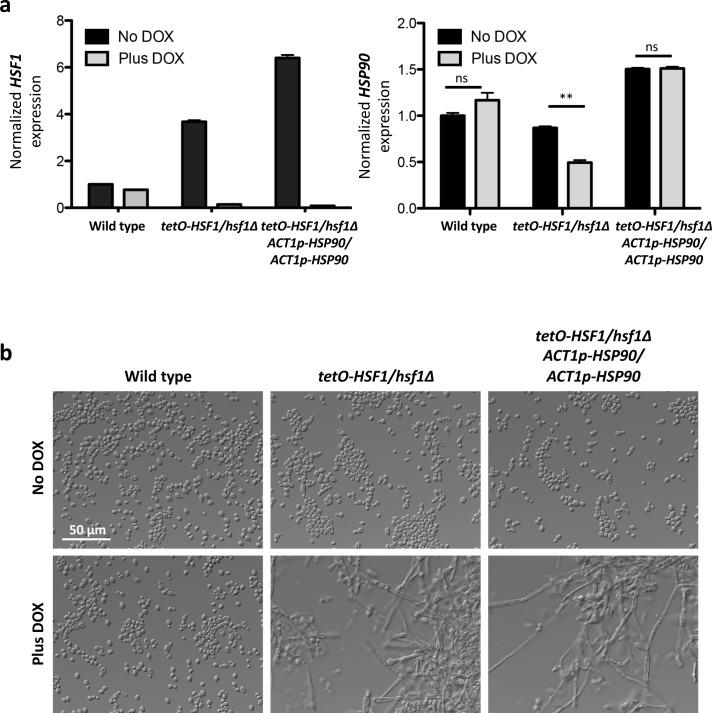
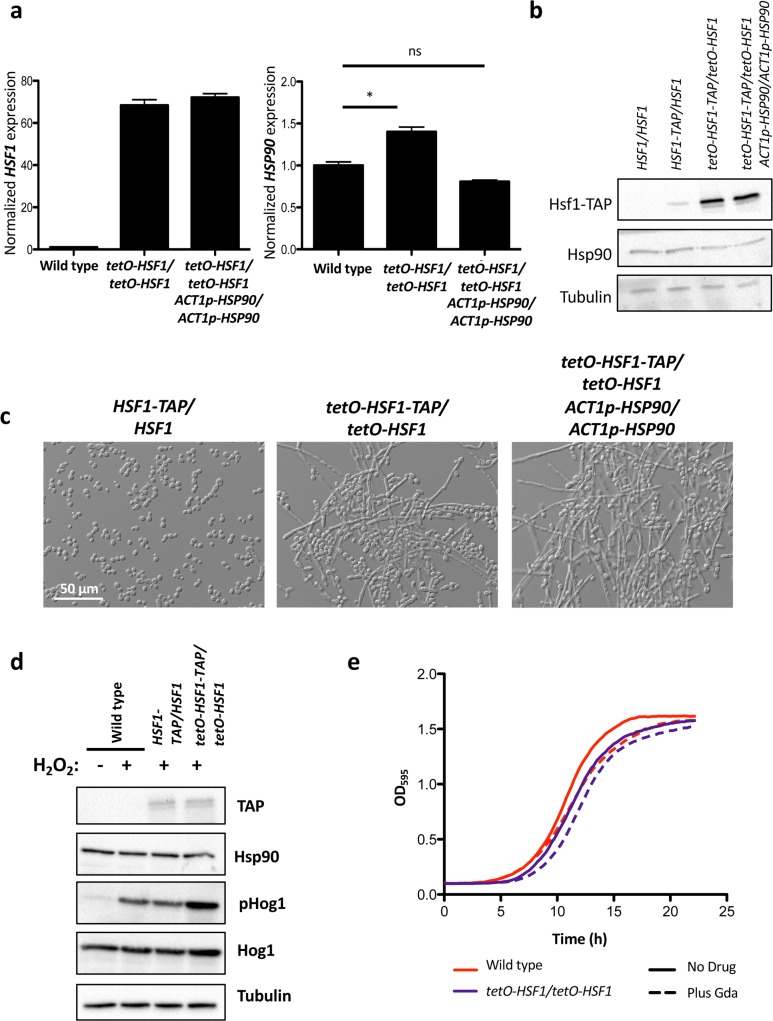
Based on the observations that Hsf1 regulates the expression of HSP90 and co-chaperone genes important for Hsp90 function[3, 4], that Hsp90 regulates temperature-dependent morphogenesis[17, 18], and that filaments induced by compromised Hsp90 function and HSF1 depletion have structural similarities including an increase in multinucleate cells[19], we hypothesized that filamentation in response to HSF1 depletion is a consequence of reduced HSP90 expression or function. To determine if HSF1 induces filamentation via reduction of HSP90 expression, we engineered a strain in which HSF1 could be depleted without affecting HSP90 levels. In the tetO-HSF1/hsf1Δ strain, we ectopically integrated HSP90 under the control of the strong, constitutive promoter ACT1p, which is independent of Hsf1[4]. In these strains, DOX-mediated transcriptional repression of HSF1 occurred without significant alterations in HSP90 transcript or protein levels (Fig 3A and Fig 4A). Genetic depletion of HSF1 induced filamentation despite constitutive HSP90 expression (Fig 3B), indicating that morphogenesis induced by depletion of HSF1 occurs in a manner that is independent of HSP90 expression. This suggests that HSF1 depletion induces morphogenesis via impaired Hsp90 function or that it does so via circuitry that is independent of Hsp90.
Fig 3. HSF1 depletion induces filamentation independently of changes in HSP90 expression.
Strains were grown in the absence or presence of 20 μg/mL DOX in rich medium at 30°C. a) A strain was engineered where HSP90 is under the control of a constitutive promoter, ACT1p, in the tetO-HSF1/hsf1Δ background. In the ACT1p-HSP90 strain, HSF1 levels were depleted (left panel) with DOX without altering HSP90 expression (right panel). HSF1 and HSP90 transcript levels were normalized to ACT1 and GPD1. Data are means +/- standard error of the means for triplicate samples. ** indicates P value <0.01, ns indicates no significant difference, unpaired t test. b) HSF1 depletion induces filamentation independently of changes in HSP90 expression.
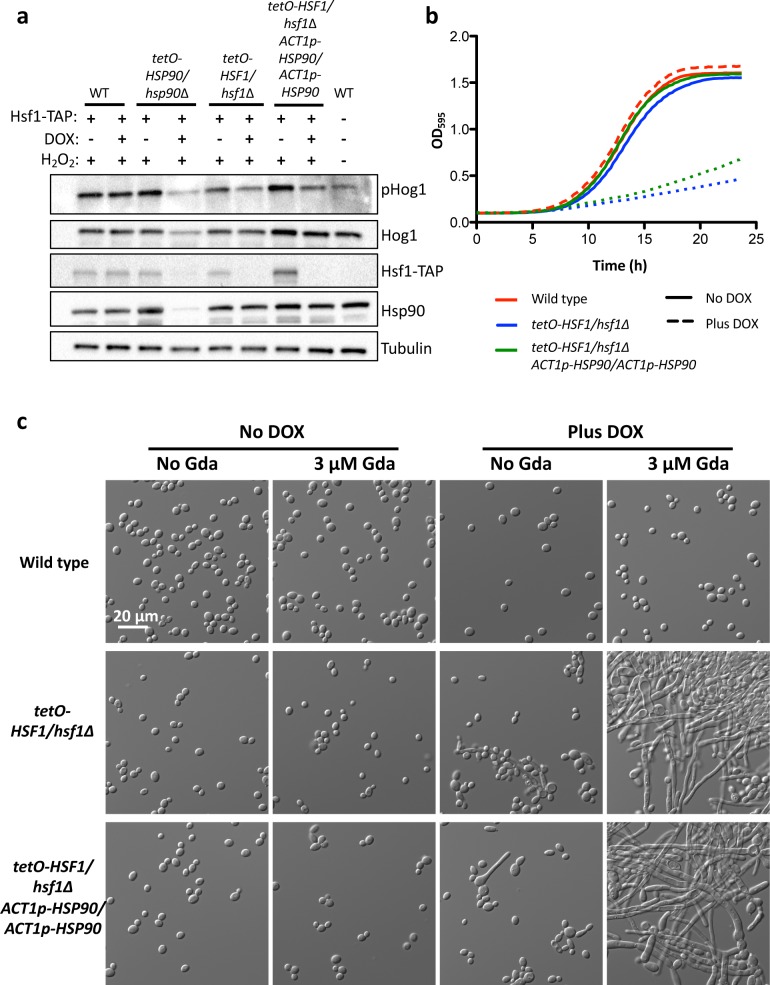
Fig 4. HSF1 depletion induces filamentation by compromising Hsp90 function, independently of changes in HSP90 expression.
a) Western blot analysis was performed to assay if HSF1 depletion compromises Hsp90 function by monitoring the Hsp90 client protein Hog1. Strains were grown in the absence or presence of 20 μg/mL DOX. Cells were treated with 5 mM hydrogen peroxide (H2O2) for 10 minutes to induce oxidative stress before protein extraction. Depletion of Hsf1 reduces the levels of phosphorylated Hog1 (pHog1) but not total Hog1 levels, even in the isogenic strain with constitutive HSP90 expression. Tubulin levels serve as loading control. WT indicates the wild type control. Experiment was performed in biological quadruplicate and a representative image is shown, with the western blots for the additional replicates shown in S8 Fig. b) HSF1 depletion causes a hypersensitivity to the Hsp90 inhibitor geldanamycin, even when HSP90 expression is constitutive and independent of Hsf1. Growth curves were generated by measuring the optical density of cells grown in the absence or presence of 20 μg/mL DOX in the presence of a concentration of geldanamycin that does not inhibit the growth of the wild-type strain (3.13 μM). Optical density at 595 nm was measured every 15 minutes with a TECAN plate reader grown with high orbital shaking at 30°C. Experiment was performed in biological quadruplicate, with one representative graph shown. c) HSF1 depletion causes filamentation at lower concentrations of geldanamycin (Gda) than is necessary to induce filamentation of the wild-type strain. Strains were grown in static conditions in the presence of no DOX or 20 μg/mL DOX, and in the presence of no geldanamycin (Gda) or 3.13 μM geldanamycin (Gda) at 30°C. In static growth conditions, 3.13 μM geldanamycin does not inhibit growth of the strains.
To determine if Hsp90 function was compromised upon HSF1 depletion, we monitored activation of the Hsp90 client protein Hog1, as Hsp90 enables activation of this mitogen activated protein kinase (MAPK)[38]. Depletion of HSF1 caused a small but reproducible reduction in the levels of phosphorylated Hog1, even in the strain with constitutive HSP90 expression (Fig 4A and S8 Fig). HSF1 depletion did not affect total Hog1 levels (Fig 4A), highlighting that HSF1 depletion does not have the same magnitude of impact as direct compromise of Hsp90 function. The reduction of phosphorylated Hog1 levels upon HSF1 depletion are unlikely to be caused by direct transcriptional effect of Hsf1 on the Hog1 pathway, as the expression levels of HOG1 and the upstream kinases PBS2 or SSK2[39] are unaffected by HSF1 depletion (S1 Data). Together, this is consistent with the model that Hsf1 is required for proper Hsp90 function to enable the accumulation of activated Hog1. As a complementary approach to confirm that HSF1 depletion compromises Hsp90 function, we monitored sensitivity to the Hsp90 inhibitor geldanamycin, as strains with compromised Hsp90 function are hypersensitive to this inhibitor[22]. We observed that HSF1 depletion caused hypersensitivity to geldanamycin, even with constitutive HSP90 expression (Fig 4B). Strains with compromised Hsp90 function have also been observed to filament at lower concentrations of geldanamycin than the wild-type strain[40]. At a time point before HSF1 depletion induces filamentation, HSF1 depletion caused filamentation at a significantly lower concentration of geldanamycin (3.13 μM) than is necessary to induce filamentation in the wild-type strain (10 μM) (Fig 4C and Fig 5A). Together, these results are consistent with a model in which HSF1 depletion compromises Hsp90 function, which provides a mechanism through which HSF1 depletion can induce filamentation.
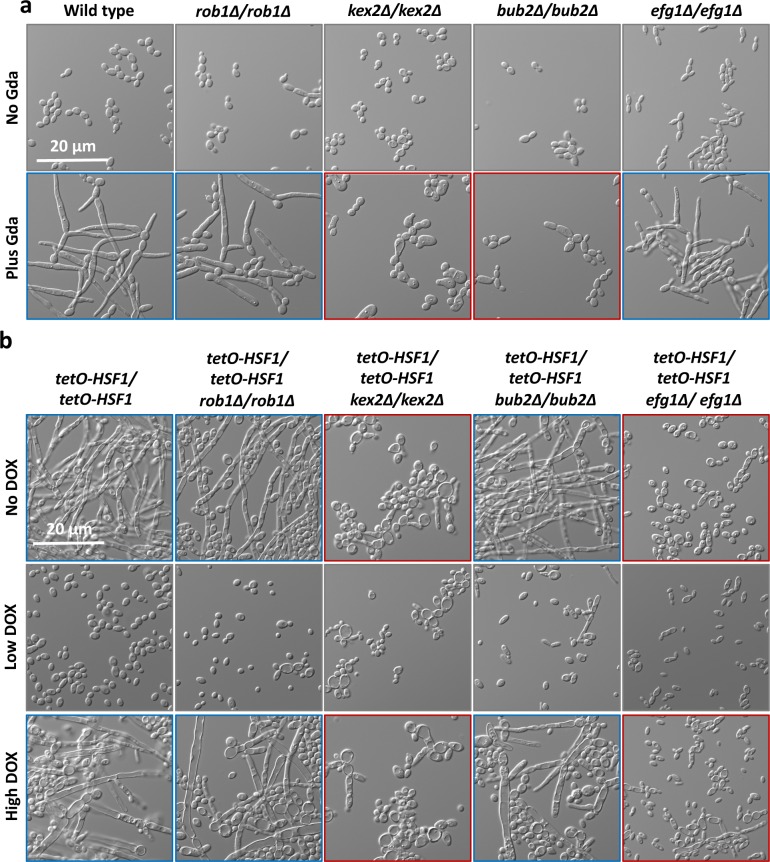
Fig 5. The genetic circuitry through which Hsf1 and Hsp90 regulate filamentation are distinct.
a) Filaments induced by compromised Hsp90 function are not dependent on the transcription factors Rob1 or Efg1, but are dependent on the protease Kex2 and the cell cycle checkpoint protein Bub2. Cultures were grown in the absence or presence of 10 μM of geldanamycin (Gda) for 6.5 hours to inhibit Hsp90 function. b) Unlike filaments induced by compromised Hsp90 function, filaments induced by HSF1 depletion are not dependent on Bub2 but are dependent on Efg1. Cultures were treated with no DOX, 0.1 μg/mL DOX (Low DOX), or 20 μg/mL DOX (High DOX) at 30°C. For both a) and b), blue outlines indicate that the homozygous deletion mutant of the morphogenetic regulator filaments to a comparable level as the non-mutant control and red outlines indicates that the mutant blocks filamentation.
Hsf1 might promote Hsp90 function by driving the transcription of Hsp90 co-chaperone genes that are important for chaperone function. Indeed, Hsf1 binds within the promoter region of six known Hsp90 co-chaperone genes in C. albicans: AHA1, CDC37, CPR6, HCH1, SBA1, and STI1[4]. We hypothesized that HSF1 depletion could lead to reduction in the levels of these critical regulators of Hsp90 function, thereby inducing filamentous growth. To test the impact of reduced co-chaperone levels on filamentation, we generated deletion or depletion strains for each of the Hsf1-regulated Hsp90 co-chaperones and found that loss of any co-chaperone alone was not sufficient to induce filamentation (S9 Fig). This suggests that HSF1 depletion does not compromise Hsp90 function and drive filamentation through effects on a single co-chaperone, but rather, the effects could be mediated through multiple co-chaperones or through an additional regulator of Hsp90 function such as a kinase or deacetylase. Given that Hsf1 regulates the expression of many proteostasis genes including the Hsp70 genes SSA1 and SSA2[4], it is also possible and consistent with findings in S. cerevisiae[41], that HSF1 depletion could cause an accumulation of misfolded proteins, which could alter Hsp90 function indirectly by overwhelming the functional capacity of Hsp90.
To further explore the genetic circuitry governing filamentous growth upon HSF1 depletion, we compared the genes necessary for filamentation in response to HSF1 depletion with those genes necessary for filamentation upon inhibition of Hsp90 with geldanamycin. To do this, we generated tetO-HSF1/tetO-HSF1 strains in homozygous deletion mutant backgrounds of genes important for filamentation, including EFG1[42], BUB2[19], KEX2[43], and ROB1[44]. We determined that the transcription factor Rob1 was dispensable for filamentation induced by compromised Hsp90 function and modulation of Hsf1 levels (Fig 5). Surprisingly, unlike filamentation induced by compromised Hsp90 function[17, 20], we observed that filamentation induced by HSF1 depletion was dependent on Efg1 (Fig 5A and Fig 5B). We also observed that although the cell cycle checkpoint protein Bub2 was necessary for filamentation in response to geldanamycin[19] (Fig 5A), it was dispensable for filamentation induced by depletion of HSF1 (Fig 5B). This suggests that although compromised Hsp90 function and HSF1 depletion induce filamentation using some of the same genetic circuitry, including Ras1[17] (Fig 2D) and the protease Kex2 (Fig 5), depletion of HSF1 also governs filamentous growth through circuitry independent of Hsp90.
HSF1 overexpression drives expression of morphogenetic regulators
To determine if HSF1 overexpression also influences filamentation through effects on Hsp90 expression or function, we generated tetO-HSF1/tetO-HSF1 strains in which HSP90 expression is independent of HSF1 by placing HSP90 under the control of the ACT1 promoter. Despite stable HSP90 transcript (Fig 6A) and protein levels (Fig 6B), the strain filamented robustly in response to HSF1 overexpression (Fig 6C). Further, HSF1 overexpression did not affect Hsp90 function, as HSF1 overexpression did not cause a reduction in phosphorylated Hog1 or total Hog1 levels (Fig 6D), or hypersensitivity to geldanamycin (Fig 6E). Thus, HSF1 overexpression does not influence morphogenesis through effects on Hsp90 expression or function.
Fig 6. HSF1 overexpression induces filamentation independently of changes in HSP90 transcript or protein levels, and function.
a) To monitor the effects of HSF1 overexpression on filamentation independently of changes in HSP90 expression, we engineered strains where HSP90 is under the control of a constitutive promoter, ACT1p, in the tetO-HSF1/tetO-HSF1 strain. HSF1 and HSP90 transcript levels were normalized to ACT1 and GPD1. Data are means +/- standard error of the means for triplicate samples. In the ACT1p-HSP90 strain, HSF1 levels are overexpressed (left panel) but HSP90 levels do not differ substantially from the wild-type levels (right panel). * indicates P value <0.05, ns indicates no significant difference, unpaired t test. b) Western blot analysis shows that overexpression of HSF1 does not impact the levels of Hsp90 protein. Tubulin serves as loading control. c) HSF1 overexpression induces filamentation independently of changes in HSP90 expression. Strains were grown in the absence of DOX at 30°C. d) Western blot analysis was performed to assay if HSF1 overexpression compromises Hsp90 function by monitoring the Hsp90 client protein Hog1. Cells were treated with 5 mM hydrogen peroxide (H2O2) for 10 minutes to induce oxidative stress before protein extraction. Overexpression of HSF1 does not affect the stability of Hog1, nor does it block its activation. Tubulin serves as a loading control. e) HSF1 overexpression does not cause hypersensitivity to the Hsp90 inhibitor geldanamycin (Gda). Growth curves were generated by measuring the optical density of cells grown in the absence and presence of 20 μg/mL DOX in the presence of 3.13 μM geldanamycin. Optical density measurements at 595 nm were taken every 15 minutes with a TECAN plate reader. Experiment was performed in biological quadruplicate, with one representative graph shown.
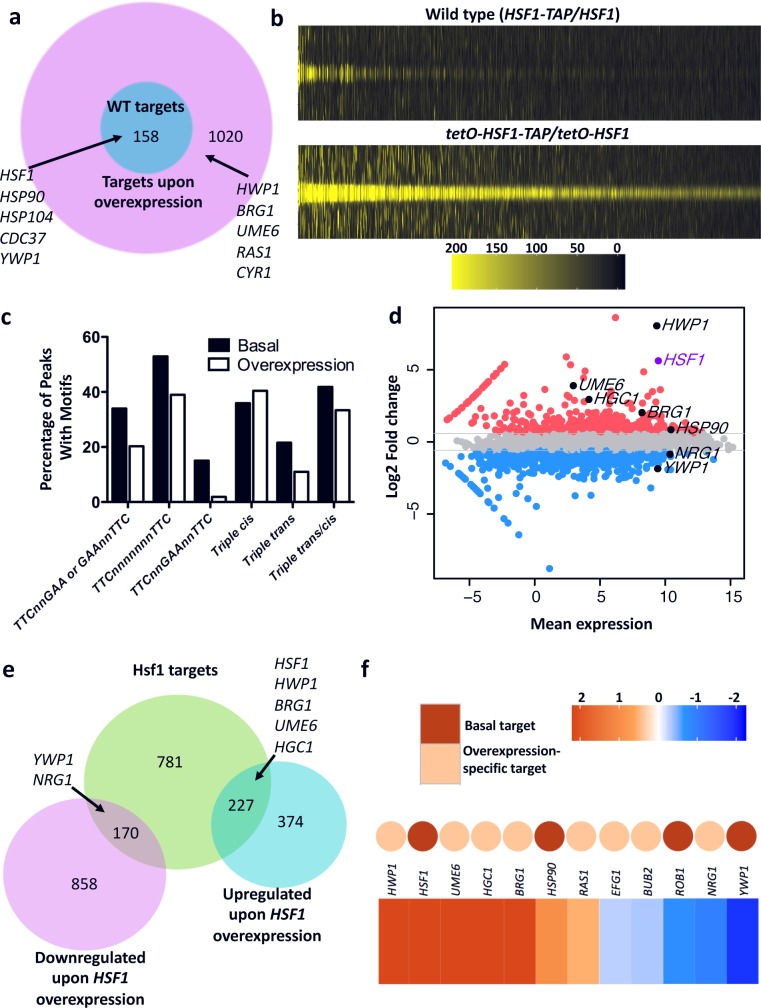
As a transcription factor, Hsf1 is poised to induce filamentation via transcriptional regulation of target genes, but it is not clear whether Hsf1 would bind to and control the same or distinct sets of target genes when expressed at different levels. To determine this and the direct targets of Hsf1, we performed chromatin immunoprecipitation of TAP-tagged Hsf1 coupled with sequencing (ChIP-seq) on strains expressing basal (HSF1-TAP/HSF1) or overexpressed (tetO-HSF1-TAP/tetO-HSF1) levels of Hsf1. Model-based Analysis of ChIP-seq (MACS) and promoter analyses identified 158 promoters to which Hsf1 binds when expressed at a basal level (referred to as basal targets), including many known targets of Hsf1 such as HSF1 itself, HSP104, HSP90, and six Hsp90 co-chaperones (AHA1, CDC37, CPR6, HCH1, SBA1, and STI1)[4] (Fig 7A and S2 Data). Consistent with the known functions of Hsf1-regulated genes[3, 4], GO term analysis of the basal targets identified an enrichment of processes related to protein folding, protein refolding, and response to temperature stimulus (S2 Data). In the HSF1 overexpression strain, Hsf1 bound to the promoters of all the basal targets, as well as an additional 1,020 targets (referred to as overexpression-specific targets) (Fig 7A and S2 Data). Close examination of the Hsf1 ChIP-seq signal under basal conditions revealed that the majority of these overexpression-specific targets had weak but distinct binding signals (Fig 7B), suggesting that these targets are also bound at a low level under basal conditions. De novo motif analysis by Multiple EM for Motif Elicitation (MEME) of the 200 bp sequences spanning the binding sites of the Hsf1 basal and overexpression-specific targets identified that both sets of targets had a significant enrichment for Hsf1 binding motifs following the conserved Heat Shock Element (HSE) pattern of inverted nGAAn repeats (Fig 7C and S3 Data). The presence of these conserved Hsf1 binding motifs in the overexpression-specific targets together with the low level of Hsf1 binding to these targets under basal conditions suggests that they are bona fide Hsf1 targets. GO term analysis of the overexpression-specific targets showed an enrichment in genes involved in symbiosis, growth, response to stimulus, and many processes related to filamentous growth (S2 Data). The overexpression-specific target set included many genes encoding morphogenetic regulators such as Ras1; the transcription factors Efg1, Ume6 and Brg1; and the hypha-specific G1 cyclin-related protein Hgc1 (S2 Data). Thus, overexpressed Hsf1 engages with an expanded target set including many morphogenetic regulators, suggesting that it drives morphogenesis via overexpression of genes that promote filamentation.
Fig 7. Hsf1 binds to an expanded set of taget genes upon overexpression, inducing the expression of positive regulators of filamentation.
a) ChIP-seq was performed to determine the targets of Hsf1 in strains expressing basal (HSF1-TAP/HSF1) and overexpressed (tetO-HSF1-TAP/tetO-HSF1) levels of Hsf1, which identified an expansion of Hsf1 targets upon overexpression. Genes previously identified as Hsf1 targets and genes involved in C. albicans filamenation are indicated. b) Hsf1 signals at the summit of Hsf1 ChIP-seq peaks in wild-type (basal conditions, top) and HSF1 overexpression (bottom) strains. Weak Hsf1 ChIP-seq signal can be seen at the overexpression-specific target sites under basal conditions, suggesting that Hsf1 also binds these sites under basal conditions albeit at a lower level. Legend shows Hsf1 ChIP-seq signal across a 2 kb window spanning Hsf1 binding summits identified by MACS analysis. c) Both basal and HSF1 overexpression-specific targets contain the conserved heat shock element (HSE) binding sites. The percentage of ChIP-seq peaks with the HSE motifs TTCnnGAA, TTCn7TTC, TTCnnGAAnnTTC, or variations of the motifs termed triple cis motifs (GAA n0-3 GAA n0-3 GAA), triple trans motifs (GAA n0-3 TTC n0-3 GAA) or triple trans/cis motifs (TTC n0-3 GAA n0-3 GAA, GAA n0-3 GAA n0-3 TTC, TTC n0-3 TTC n0-3 GAA, GAA n0-3 TTC n0-3 TTC) are shown for basal and overexpression-specific targets. d) MA plot showing difference in gene expression between wild-type and HSF1 overexpression strains. Genes more than 1.5-fold up-regulated in the HSF1 overexpression strain as compared to the wild-type strain are shown in red, whereas genes more than 1.5-fold down-regulated are shown in blue. Genes of interest based on their roles in filamentation are indicated. HSF1 is highlighted in purple. e) A Euler diagram depicting the overlap between differentially regulated genes upon HSF1 overexpression determined by RNA-seq and Hsf1 bound targets determined by ChIP-seq. Hsf1 targets includes those promoters bound in either the wild-type or overexpression strain. Selected genes associated with filamentation are indicated in the diagram. f) A heat map showing gene expression changes upon HSF1 overexpression for filamentation regulators. Colored dots are included to indicate whether the respective gene is bound by Hsf1 in the wild-type strain (in red) or only when Hsf1 is overexpressed (in orange). The colour bar depicts the change in expression as determined by RNA-seq.
To identify the Hsf1 targets that are also transcriptionally modulated upon HSF1 overexpression, we performed genome-wide expression profiling analysis using RNA-seq. Comparison of the transcriptional profiles of the tetO-HSF1-TAP/tetO-HSF1 strain with the wild-type strain grown in the absence of DOX revealed 1,629 genes for which expression was altered by at least 1.5-fold upon HSF1 overexpression (Fig 7D and S4 Data). Among the differentially expressed genes, 397 were in fact direct targets of Hsf1 (Fig 7E). Intriguingly, 53% (83/158) of the basal targets had altered expression upon HSF1 overexpression, including HSP90, HSP104 and all six of the Hsf1-dependent Hsp90 co-chaperone genes (S4 Data), and 31% (314/1,020) of the overexpression-specific targets had significantly altered expression. This included the upregulation of many filament-induced genes, including ALS3, ECE1, IHD2, PHR1 and RBT1[6] which were also upregulated upon HSF1 depletion (S1 Data and S4 Data). It also included the induction of positive regulators of filamentation such as HWP1, HGC1, UME6 and BRG1, and downregulation of the negative regulator of filamentation NRG1 (Fig 7E and 7F). Finally, the HSF1 overexpression-specific targets encompassed genes known to be regulated by other morphogenetic regulators including the heat shock factor (HSF)-type transcription factors Sfl2 and Skn7[45, 46]. These Hsf1-dependent morphogenetic regulators are ideal candidates for targets through which HSF1 overexpression could induce morphogenesis.
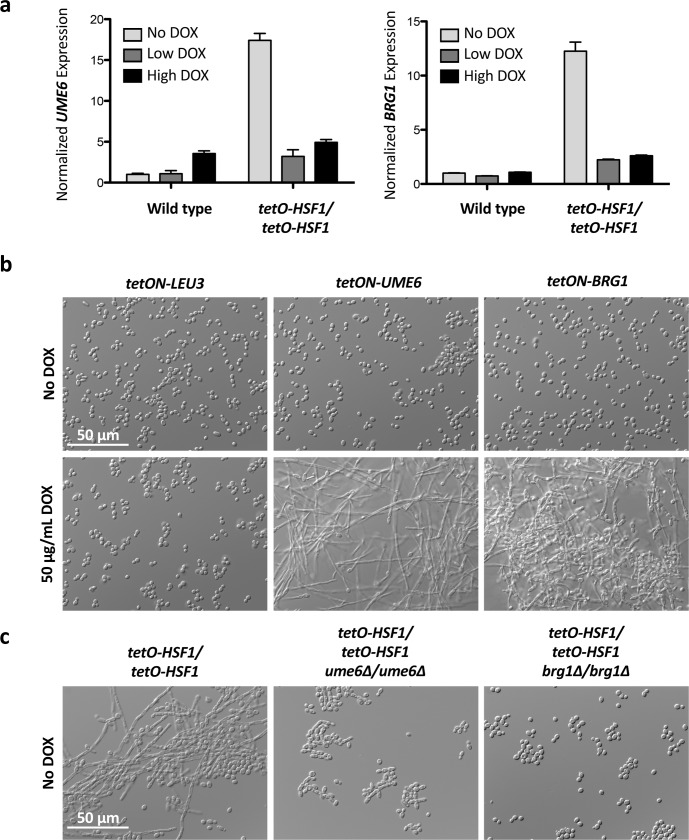
We focused on two well-established positive regulators of filamentation, Ume6 and Brg1, for which overexpression is sufficient to induce morphogenesis[10, 47]. We validated the RNA-seq analysis and confirmed that HSF1 overexpression drives overexpression of UME6 and BRG1 (Fig 8A). The overexpression of UME6 and BRG1 is dependent on HSF1 levels, as the overexpression was lost when HSF1 was depleted with DOX (Fig 8A). Next, we confirmed that overexpression of UME6 or BRG1 was sufficient to induce filamentation using tetracycline-inducible conditional expression strains in which target genes are overexpressed in the presence of DOX[47] (Fig 8B and S10A Fig). Overexpression of the leucine biosynthesis gene LEU3, a gene that does not influence the yeast-to-filament transition, was included as a control. Intriguingly, we observed that the overexpression of UME6 and BRG1 upon HSF1 overexpression was lost when the tetO-HSF1/tetO-HSF1 strain was grown at 23°C (S10B Fig), a temperature at which HSF1 overexpression is not able to induce filamentation (Fig 2A). Finally, to assess the impact of Ume6 and Brg1 on filamentation induced by HSF1 overexpression, tetO-HSF1/tetO-HSF1 strains were generated in homozygous deletion mutants of UME6 or BRG1. Loss of either Ume6 or Brg1 was sufficient to block filamentation in response to HSF1 overexpression (Fig 8C). Although Ume6 and Brg1 are necessary for filamentation in response to many cues[9, 11], they are not strictly required for polarized growth as neither Ume6 or Brg1 are required for filamentation in response to geldanamycin[20, 44], and Brg1 is dispensable for filamentation in RPM1 and Lee’s Medium[44]. Together, this is consistent with a model in which HSF1 overexpression drives filamentation through the upregulation of positive regulators of filamentation, including UME6 and BRG1.
Fig 8. Overexpression of HSF1 induces filamentation through overexpression of positive regulators of filamentation.
a) Overexpression of HSF1 drives the overexpression of UME6 and BRG1. Strains were grown in the presence of no DOX, 0.1 μg/mL DOX (Low DOX), or 20 μg/mL DOX (High DOX) at 30°C. UME6 and BRG1 transcript levels were normalized to ACT1 and GPD1. Data are means +/- standard error of the means for triplicate samples. b) Overexpression of UME6 or BRG1 induces filamentation. Strains with a tetracycline-inducible promoter (tetON) driving the expression of target genes were grown in the presence of 50 μg/mL DOX to induce overexpression at 30°C. Overexpression of LEU3 acts as a negative control. c) Loss of Ume6 or Brg1 blocks filamentation induced by HSF1 overexpression. Strains were grown in the absence of DOX at 30°C.
Discussion
C. albicans morphogenesis is regulated by complex genetic circuitries that enable sensing and responding to environmental inducing signals. Here, we implicate Hsf1 as a novel regulator of filamentation, expanding its function beyond its classical role in regulating the heat shock response and providing a fascinating example of how homeostasis of Hsf1 levels is required to maintain the yeast state (Fig 9). Depletion of HSF1 leads to an impairment of Hsp90 function even when HSP90 levels are held constant and independent of Hsf1 (Fig 3 and Fig 4), which provides a mechanism through which HSF1 depletion could induce filamentous growth. HSF1 depletion also induces filamentation through mechanisms distinct from Hsp90, which require the cAMP-PKA pathway and Efg1 (Fig 5). Overexpression of HSF1 also induces filamentous growth, but does so by means of engaging with an expanded set of targets, thereby up-regulating the expression of positive regulators of filamentation such as Ume6 and Brg1, and down-regulating the expression of negative regulators of filamentation such as Nrg1 (Fig 7 and Fig 8). As a central transcriptional regulator that is exquisitely responsive to environmental cues, Hsf1 serves as a master regulator of C. albicans morphogenesis.
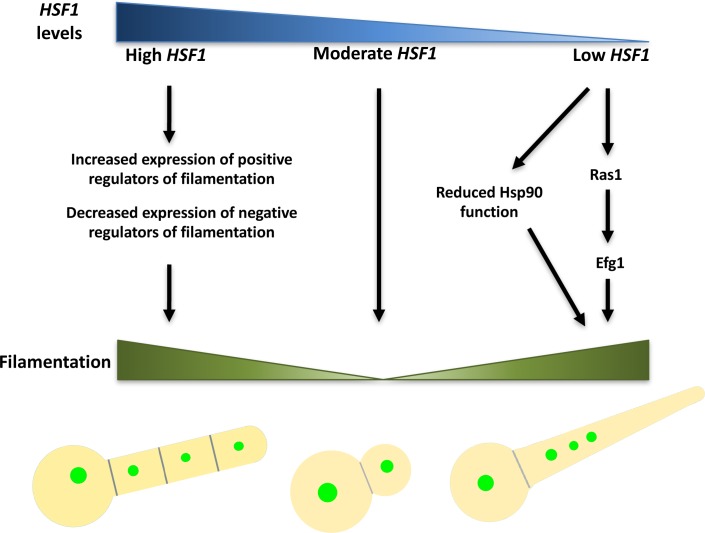
Fig 9. Model for how HSF1 overexpression and depletion induce filamentation.
Under basal conditions, HSF1 levels are moderate and C. albicans exists in the yeast form. A reduction in HSF1 levels leads to filamentous growth both by compromising Hsp90 function and through circuitry that is independent of Hsp90 but dependent on Efg1. An increase in HSF1 levels induces a dose-dependent expansion of Hsf1 direct targets that drives overexpression of positive regulators of morphogenesis, including Brg1 and Ume6, and decreased expression of negative regulators of morphogenesis, such as Nrg1, resulting in filamentous growth. Filaments induced by HSF1 overexpression and depletion are structurally distinct, require different genetic circuitry and are induced through distinct mechanisms.
We establish that in addition to Hsf1’s role in transcriptional regulation of HSP90, Hsf1 also influences Hsp90 function (Fig 4), providing an additional layer of complexity to the known regulatory feedback loop between Hsf1 and Hsp90 in which Hsf1 drives HSP90 expression and Hsp90 represses Hsf1 activity[15]. Hsp90 activity is under intensive regulation by co-chaperones and enzymes that post-translationally modify Hsp90[15], and we postulate that Hsf1 could alter Hsp90 function by controlling the expression of these critical regulators. Our findings that loss of any single Hsf1-dependent Hsp90 co-chaperone is insufficient to induce filamentation (S9 Fig), suggests that misregulation of multiple Hsp90 regulatory proteins, including co-chaperones, lysine deacetylases or kinases in concert could be necessary to compromise Hsp90 function to induce filamentation. It is also possible HSF1 depletion modulates Hsp90 function through a mechanism independent of its transcriptional activity, by altering complex formation between Hsp90 and its clients or regulators. This is consistent with studies in mammalian cells, which demonstrated that HSF1 depletion impairs Hsp90 function in chaperoning kinase client proteins through reduced associations between Hsp90, Cdc37, and kinase clients[48, 49], as well as the reduced association between Hsp90 and the deacetylase HDAC6[49]. It is also possible that HSF1 depletion affects Hsp90 function indirectly. In S. cerevisiae it has been shown that loss of Hsf1 function leads to an accumulation of misfolded proteins due to the downregulation of Hsp90 and Hsp70 [41]. Since Hsf1 is a key regulator of many proteostasis genes, we hypothesize that HSF1 depletion would also lead to an accumulation of misfolded proteins, which would exceed the functional capacity of Hsp90. These possibilities are not mutually exclusive, and HSF1 depletion may induce filamentation through multiple of these mechanisms, as well as through an Hsp90-independent mechanism that requires the terminal transcription factor of the cAMP-PKA pathway, Efg1.
In contrast, HSF1 overexpression induces filamentation independent of Hsp90 (Fig 6), but in an exquisitely temperature-dependent manner (Fig 1 and Fig 2). Our transcriptional analyses revealed that upon overexpression, Hsf1 engages with an expanded target set, thereby directly activating a filamentation transcriptional program by up-regulating positive regulators of filamentation including HGC1, HWP1, UME6 and BRG1, and down-regulating repressors such as NRG1 (Fig 7). We previously established that ~80% of C. albicans promoters contain at least one Hsf1 consensus sequence[4], although Hsf1 was not bound to most of these promoters under basal or heat shock conditions, suggesting that there might be extensive environmentally contingent Hsf1 binding. Increased levels of Hsf1 protein due to HSF1 overexpression or elevated temperature could allow Hsf1 to bind to additional targets. Even when overproduced, the transcriptional output of Hsf1 is modulated by the environment, as filamentation in response to HSF1 overexpression was abolished at 23°C as was up-regulation of target genes involved in filamentation (Fig 2A and S10B Fig). This suggests that the transcriptional activity of Hsf1 is affected by additional factors that are dependent on environmental temperatures and might include nucleosome occupancy or post-translational modifications. Nucleosome occupancy at stress responsive genes is modulated by Hsp90[4], and nucleosome positioning may also be modulated by temperature, thereby altering accessibility of Hsf1 consensus sequences. Moreover, Hsf1 activity is regulated by phosphorylation, which increases upon heat shock[3, 25], and the phosphorylated form of S. cerevisiae Hsf1 recruits the Mediator complex to the promoters of its target genes, mediating transcription[31]. These results suggest a model in which Hsf1 may be bound to its target promoters in an unphosphorylated state at lower temperatures and therefore be unable to recruit the Mediator to influence gene expression. Taken together, our findings illuminate a new facet to repertoire of mechanisms that microbial pathogens use to couple sensing the environment to activation of a developmental program important for virulence.
By providing the first example of a protein that acts both as a positive and negative regulator of filamentation, this work challenges the classical model of morphogenetic regulation in which overexpression and depletion of key regulators have opposing effects on filamentation. As a classic example, Ume6 is a positive regulator of filamentation that promotes polarized growth in a dose-dependent manner[10, 11]. In contrast, our studies demonstrate that homeostasis of HSF1 levels is required to maintain the yeast state, and either overexpression or depletion of HSF1 induces filamentation. The filaments induced by overexpression of HSF1 differ considerably from those induced by depletion of HSF1 in terms of both morphology, temperature dependence, and mechanisms involved. Given that HSF1 expression and activity are exquisitely sensitive to the environment (Fig 1B, Fig 2A and S4 Fig), it is poised to sense and transduce environmental signals to orchestrate morphogenetic programs.
This work highlights Hsf1 as an environmentally contingent regulator for which tuning levels has a profound impact on diverse aspects of cellular biology, including response to temperature, morphogenesis, proteostasis, cell cycle, host cell adhesion and damage[4], drug sensitivity[50] and iron homeostasis[51]. Beyond these processes, Hsf1 may also influence additional C. albicans phenotypic states such as the white, grey, opaque and gastrointestinally induced transition (GUT) cell morphotypes, which are intimately connected with mating and host interactions[52]. Consistent with this expectation, HSF1 levels vary within cells of these states[53–55], and the master regulators of these transitions, Wor1 and Efg1[52], are targets of Hsf1 upon overexpression (S2 Data). We also observed that HSF1 levels were dramatically increased in biofilm growth conditions (S4B Fig), suggesting that cellular morphologies in this host-relevant state may be influenced by elevated HSF1 levels (S4 Fig). Hsf1 may also have a much broader role in governing virulence traits of diverse fungi such as thermally dimorphic fungi or C. neoformans for which HSF1 overexpression enables thermotolerance[27]. With Hsf1 and Hsp90 as core hubs of cellular circuitry in diverse cells types, there has been a growing appreciation of the therapeutic potential of targeting these regulators in treating cancer[56, 57], neurodegenerative disorders[58], and infectious disease[21]. Realizing this potential will require the development of novel chemical matter that can selectively perturb proteostasis and exploit differences between mammals and fungi in structures and functional relationships of Hsf1 and Hsp90. Exploring this core cellular circuitry creates unprecedented opportunity for understanding mechanisms governing environmentally responsive developmental programs and for developing new therapeutic strategies for devastating diseases.
Materials and methods
Strains and culture conditions
All strains used in this study are listed in S1 Table. All plasmids used to create strains are included in S2 Table. All oligonucleotide sequences used in this study are included in S3 Table. Strains were grown in rich YPD medium (1% yeast extract, 2% bactopeptone, 2% glucose) or YPM medium (1% yeast extract, 2% bactopeptone, 2% maltose) at 30°C, unless indicated otherwise. Archives of C. albicans strains were maintained at -80°C in YPD with 25% glycerol. For solid medium, 2% agar was used. Strains that were auxotrophic for uridine were grown with 80 mg/L uridine added to the growth medium. When indicated, strains were grown in the presence of doxycycline (Doxycycline Hydrochloride, BioBasic, DB0889) dissolved in water.
Since the tetO-HSF1/hsf1Δ and tetO-HSF1/tetO-HSF1 strains have different levels of HSF1 overexpression when grown in the absence of DOX (Fig 1A), the strains were grown for different lengths of time to deplete HSF1 and observe the relevant phenotypes. For experiments with tetO-HSF1/hsf1Δ strains looking at HSF1 depletion, unless indicated otherwise, overnights of relevant strains and controls were subcultured in the absence or presence of 20 μg/mL DOX and grown overnight. The cells were subcultured into the same conditions and grown to mid-log phase for quantitative reverse transcription PCR (qRT-PCR) experiments and western blot analysis or were grown overnight for microscopy analysis. For experiments with tetO-HSF1/tetO-HSF1 strains looking at HSF1 depletion, unless indicated otherwise, overnights of relevant strains and controls were subcultured in the absence or presence of DOX (at the indicated concentrations) and grown overnight. The cells were subcultured into the same conditions and grown for an additional overnight. Then, cells were subcultured into the same conditions and grown to mid-log phase for qRT-PCR experiments and western blot analysis or were grown overnight for microscopy analysis. For experiments monitoring HSF1 overexpression only, unless indicated otherwise, overnights were subcultured and grown in the absence of DOX to mid-log phase for qRT-PCR experiments and western blot analysis or were grown overnight for microscopy analysis.
To overexpress LEU3, UME6 or BRG1 using the tetON strains, overnights were subcultured in the absence or presence of 50 μg/mL DOX and grown overnight. The cells were subcultured into the same conditions for an additional overnight to achieve sufficient overexpression of UME6 and BRG1 to induce filamentation, allowing for microscopy analysis. For qRT-PCR experiments, cells were subcultured into the same conditions and grown to mid-log phase.
In order to monitor HSF1 levels in biofilm growth conditions, biofilms were grown in multi-well six well plates (Falcon). An overnight culture of the wild-type strain (HSF1-TAP/HSF1) was grown at 30°C in rich medium and diluted to an optical density at 600 nm of 0.5 in Spider medium (1% nutrient broth, 1% mannitol, 0.2% K2HPO4). The suspension was inoculated into sterile, six-well plates that had been preincubated with bovine serum (Gibco Life Technologies, 16170078) for 90 minutes and washed once with PBS. Cells were incubated for 90 minutes at 37°C in static conditions. Non-adherent cells were washed away once with PBS and fresh Spider medium was added. Plates were incubated for 48 hours at 37°C in static conditions. The supernatant was removed and the wells were washed once with PBS before the biofilm cells were collected by scraping. The pelleted cells were washed with cold, distilled water before being flash-frozen on a dry ice ethanol bath.
In order to monitor HSF1 levels upon heat shock, an overnight culture of the wild-type strain (HSF1-TAP/HSF1) was grown at 30°C in rich medium, diluted to an optical density at 600 nm of 0.1 and grown at 30°C until mid-log phase. The cells underwent a 30°C to 42°C heat shock, by diluting the mid-log phase cells two-fold in medium prewarmed at 54°C and incubating the cells at 42°C for 10 minutes before pelleting. The pelleted cells were washed with cold, distilled water before being flash-frozen on a dry ice ethanol bath.
Quantitative reverse transcription-PCR (qRT-PCR)
To monitor gene expression changes, strains were grown to mid-log phase, pelleted at 3000 rpm at 4°C and washed with cold, distilled water before being flash-frozen on a dry ice ethanol bath. The pellets were stored at -80°C. Cells were lysed by bead beating, six times for 30 seconds each, with one minute on ice between. RNA was extracted from the lysed cells using the QIAGEN RNeasy kit and DNase treated using the QIAGEN RNase free DNAase Set. Complementary DNA synthesis was performed using the AffinityScript Multi Temperature cDNA Synthesis Kit (Agilent Technologies). qRT-PCR was performed using in a 384-well plate, with a 10 μL reaction volume using Fast SYBR Green Master Mix (Applied Biosystems) and the BioRad CFX-384 Real Time System with the following cycling conditions: 95°C for 3 minutes, then 95°C for 10 seconds and 60°C for 30 seconds, for 40 cycles. The melt curve was completed with the following cycle conditions: 95°C for 10 seconds and 65°C for 10 seconds with an increase of 0.5°C per cycle up to 95°C. Reactions were performed in technical triplicate using the primer pairs oLC2285/oLC2286 for ACT1, oLC752/oLC753 for GPD1, oLC6472/oLC6473 for PMA1, oLC2451/oLC2452 for HSF1, oLC756/oLC757 for HSP90, oLC1460/oLC1461 for UME6, and oLC2635/oLC2636 for BRG1. Primer sequences are included in S3 Table. Data were analyzed using the BioRad CFX Manager 3.1. Error bars depict standard error of the means of technical triplicates.
Microscopy
Cells cultured in liquid medium were imaged using differential interference contrast (DIC) microscopy with a Zeiss Axio Imager.MI (Carl Zeiss) and an X-cite series 120 light source for fluorescence. ET green fluorescent protein (GFP), 4’,6-diamidino-2-phenylindole (DAPI) hybrid, and ET HQ tetramethylrhodamine isothiocyanate (TRITC)/DsRED filter sets from Chroma Technology (Bellows Falls, VT) were used to visualize Nop1-GFP, calcofluor white, and propidium iodide respectively. Propidium iodide (Sigma-Aldrich P4170, dissolved in water) was added to cells at a final concentration of 25 μg/mL and incubated in the dark for 5 minutes before imaging. Calcofluor white (Fluorescent Brightener 28, Sigma-Aldrich F3543, dissolved in water) was added to cells at a final concentration of 5 μg/mL. Quantification of nuclei was performed by counting the number of nuclei per cellular compartment in at least 300 compartments per condition for two biological replicates, using Image J software. Statistical significance was determined using an unpaired t test using GraphPad software (https://www.graphpad.com/quickcalcs/ttest1.cfm).
Growth curves
Sensitivity to geldanamycin was determined using minimum inhibitory concentration assays in 96-well microtiter plates (Sarstedt) as previously described[59–61]. Assays were performed in YPD with a total volume of 200 μL/well with two-fold dilutions of geldanamycin (LC Laboratories, G-4500, dissolved in DMSO). Strains were grown in the absence or presence of 20 μg/mL doxycycline dissolved in water. Strains were grown in a TECAN GENios plate reader at 30°C, with orbital shaking on high. Absorbance readings were measured using the XFluor4 software at an optical density of 595 nm every 15 minutes for 24 hours. Data is displayed for the growth in 3.13 μM geldanamycin, where there was minimal growth inhibition of the wild-type strain. To monitor filamentation in response to geldanamycin, minimum inhibitory concentration assays were performed as above but incubated at 30°C in static conditions. Filamentation was monitored at the highest concentration of geldanamycin that did not cause substantial growth inhibition upon HSF1 depletion.
Microarray analysis
Three independent overnights of the tetO-HSF1/hsf1Δ strain were grown in YPD and subcultured to an optical density of 600 nm (OD600) of 0.2 in the absence or presence of 20 μg/mL DOX overnight. The cells were subcultured into the same conditions for an additional overnight to achieve sufficient HSF1 depletion to induce filamentation. Finally, the cells were subcultured to OD600 of 0.05 into the same conditions in the absence or presence of DOX and grown to mid-log phase. At this time point, the HSF1 depleted cells were robustly filamentous. Cells were spun down at 4000 rpm for 5 minutes at 4°C, washed once with cold water, and spun again. All liquid was removed and the pellet was frozen in liquid nitrogen before storage at -80°C. RNA was extracted from the pellets by bead beating for 30 seconds, six times, with one minute on ice between. Pellets were split into multiple bead beating tubes for a higher efficiency of breaking open the cell. RNA was extracted using the Qiagen RNeasy Mini Kit. RNA purity and integrity was assessed by nanodrop and electrophoresis on a 1% agarose gel. Microarray analysis was performed as previously[20, 62]. Briefly, RNA was reverse transcribed using Superscript III Reverse Transcriptase (Invitrogen) and oligo(dT)21 in the presence of Cy3- or Cy5-dCTP (Invitrogen). The samples were treated with 2.5 units RNase H (USB) and 1 μg RNase A (Pharmacia) at 37°C for 15 minute to degrade template RNA. Labeled cDNA was purified with a QIAquick PCR Purification Kit (QIAGEN). Hybridization was performed using DIG Easy Hyb Solution (Roche Diagnostics) containing 0.45% salmon sperm DNA and 0.45% yeast tRNA at 42°C for 24 hours in a SlideBooster Hyb chamber SB 800 (Advalytix, Brunnthal, Germany) with regular microagitation. The slides were washed once in 1.0% SSC (0.15 M NaCl and 0.015 M sodium citrate) with 0.2% SDS at 42°C for 5 minutes and twice in 0.1% SSC with 0.2% SDS at 42°C for 5 minutes. The slides were additionally washed once in 0.1% SSC at 24°C for 5 minutes, followed by four rinses in 0.1% SSC. Microarray slides were air dried before being scanned using a ScanArray Lite microarray scanner (Perkin Elmer-Cetus; versions 2.0 and 3.0). The microarray data was analyzed with GeneSpring GX v7.3 (Agilent Technologies). Raw intensities were normalized with a Lowess curve using 20% of the data to fit each point. To identify transcripts with a significant change in abundance, the fluorescence ratios were compared using the Welch t-test and the Benjamini and Hochberg False Discovery Rate. Volcano plots were used to identify genes with statistically significant (P<0.5) changes in transcript abundance of greater than 1.5 fold. Gene Set Enrichment Analysis was performed as described previously[63], with details provided at http://www.broadinstitute.org/gsea/.
ChIP-seq and RNA-seq
Four independent overnights of the wild-type (CaLC2302), HSF1-TAP/HSF1 (CaLC5012), and tetO-HSF1-TAP/tetO-HSF1 (CaLC5014) strains were subcultured to an OD600 of 0.1 and grown to mid-log phase, approximately 4 hours. The culture was split so that ~45mL was spun down for RNA-seq and ~45mL was prepared for ChIP-seq. The samples for ChIP-seq were prepared as described previously[4, 64]. Briefly, 40 mL of culture was combined with 1.2 mL of 37% formaldehyde and incubated with gentle rocking for 20 minutes at room temperature. 10 mL of 2.5M glycine was then added to stop the crosslinking and cells were incubated with gentle rocking for 10 minutes. Cells were harvested by spinning at 3000 rpm for 2 minutes at 4°C, washing twice in 20 mL ice-cold Tris buffered saline (TBS). Pellets were frozen in liquid nitrogen and kept at -80°C until chromatin preparation. Cell pellets were lysed in ice-cold FA lysis buffer in a Bullet Blender for three times five minutes at setting 12. Chromatin was pelleted, resuspended in fresh FA lysis buffer and fragmented in a Qsonica Q800 sonicator with sonication cycles of 10 seconds on and 10 seconds off for a total sonication time of 20 minutes. Immunoprecipitation was carried out using ~15 μl IgG Sepharose (GE Healthcare) for Hsf1-TAP on an end-to-end rotator for at least 3 hours at room temperature before washing with 2 x FA lysis buffer, 1 x FA lysis buffer with 500 mM NaCl, 1 x LiCl buffer and 1 x TE buffer, as described previously[65]. Library preparation of ChIP DNA samples were carried out as described previously[66]. Single end 60 bp sequencing was performed on the Illumina 2500 platform. The samples for RNA-seq were prepared as described previously[4]. Briefly, cells were pelleted at 3000 rpm for 5 minutes at 4°C, washed with ice cold TBS, at which point supernatant was removed and the pellets were frozen in liquid nitrogen. All pellets were stored at -80°C. RNA was extracted using the Qiagen RNeasy Mini Kit and DNase treated using the Ambion DNA free kit. Quality was assessed by monitoring the 260/280 and 260/230 ratios and using Agilent RNA Bioanalyzer assay. When necessary, the RNA was cleaned up using the Qiagen RNeasy MinElute Cleanup column kit. Library preparation was carried out using TruSeq Stranded mRNA Sample Prep kit and TruSeq DNA Sample Prep PCR kit according to manufacturer’s instruction protocol version revision E. Pair-end 150 sequencing was done by the Illumina technology.
ChIP-seq data analysis
De-multiplexing and processing of ChIP-seq data was performed according to previous studies[66, 67]. Barcodes were removed from the 5’ end of each reads and mapped to the C. albicans A21 reference genome sequences using Bowtie2 with default parameters. Hsf1 binding peaks were identified by Model-based Analysis of ChIP-seq (MACS)[68] using standard parameters with -log10(q-value) cutoff of 50. De novo motif discovery was performed using MEME-ChIP on 200 bp sequences spanning Hsf1 summits reported by MACS (i.e. 100 bp on each side of summits)[69]. Hsf1 target genes were identified by mapping genes with ATG closest to Hsf1 binding sites using an in-house R-script. To identify Hsf1 motifs at promoters, canonical Hsf1 binding sites as well as motifs reported by the MEME analysis were searched and counted within 1 kb promoter sequence of Hsf1 target genes using an in-house R-script. ChIP-seq data are available at SRA under the accession number SRP119587.
RNA-seq data analysis
Raw data was mapped to the C. albicans reference genome (A21) using Tophat2[70] and gene expression values (fragments per kilobase of exon per million fragments mapped–FPKM) were calculated using Cuffdiff2[71] and genes with expression values larger than 1.5 are classified as differential expression genes. RNA-seq data are available at SRA under the accession number SRP119587.
Western blot analysis
Proteins were extracted for western blot analysis by two different methods. For western blots monitoring HSF1 overexpression alone, samples were prepared using a quick, protein extraction method. Briefly, cells were grown to mid-log phase and the equivalent of 1 mL of each culture at an OD600 of 1 was pelleted, spinning for 15 seconds on high. The supernatant was removed and the cells were spun for 15 seconds again, to remove all remaining liquid. The pellet was resuspended in 60 μL of 1X sample buffer (one-sixth volume of 6x sample buffer containing 0.35 M Tris-HCl, 10% (w/w) SDS, 36% glycerol, 5% β-mercaptoethanol, and 0.012% bromophenol blue). Samples were boiled for 5 minutes at 100°C. Cell debris was pelleted by spinning for 5 minutes at 14,000 rpm. Supernatant was loaded onto an 8% SDS-PAGE gel. For western blots monitoring HSF1 depletion, cells were grown overnight, subcultured to an OD600 of 0.1 in the absence or presence of the indicated concentrations of DOX and grown again. Cells were subcultured to an OD600 of 0.1 in the same conditions and grown until mid-log phase. Cells were pelleted at 3000 rpm at 4°C and washed with cold, distilled water before being flash-frozen on a dry ice ethanol bath. The pellets were stored at -80°C. For western blot analysis to detect pHog1, cells were treated for 10 minutes with 5 mM hydrogen peroxide (Sigma-Aldrich) before pelleting. Cells were lysed by bead beating, six times for 30 seconds each, with one minute on ice between. For all westerns, separated proteins were electrotransferred to polyvinylidene difluoride (PVDF) membrane (Bio-Rad Laboratories, Inc.). Blots were blocked with 5% skim milk in phosphate-buffered saline with 0.2% Tween 20 (PBS-T), except for pHog1 blots which were blocked in 5% Bovine Serum Albumin in TBS with 0.2% Tween 20 (TBS-T). Blots were hybridized with primary antibodies against the TAP epitope (1:3000, Thermo Fisher Scientific, CAB1001), pHog1 (1:1000, Phospho-p38 MAPK from Cell Signaling, 92155), Hog1 (1:1000–1:2500, Santa Cruz Biotechnology, SC 9079), Hsp90 (1:10,000, Bryan Larson), β-Actin (1:5000, Santa Cruz Biotechnology, SC 47778) or Tubulin (1:1000–1:5000, AbDseroTec, MCA78G) in block solution. Blots were washed with PBS-T or TBS-T and incubated with FITC-conjugated secondary antibodies diluted 1:5000 in the block solution. Blots were washed with PBS-T or TBS-T before detecting the signals using an ECL western blotting kit as per the manufacturer’s instructions (Pierce).
Strain construction
Strains were constructed according to standard protocols. To select for mutants prototrophic for arginine or histidine, synthetic defined medium plates (0.17% yeast nitrogen base without ammonium sulfate, 0.1% glutatmic acid, 2% glucose, 2% agar) were supplemented with arginine HCl (50 mg/L) or histidine HCl (20mg/L) as required. To select for nourseothricin (NAT)- resistant mutants, nourseothricin (Jena Bioscience) was solubilized in water and supplemented into YPD plates at a final concentration of 150 μg/mL.
CaLC970: To construct the HSF1/hsf1Δ strain, the plasmid pLC478, which contains the HSF1 knockout construct, was digested with BssHII and transformed into CaLC239 (SN95). NAT-resistant transformants were PCR tested for proper integration of the construct using primer pairs oLC989/oLC275 and oLC274/oLC990. The SAP2 promoter was induced to drive expression of FLP recombinase to excise the NAT marker cassette.
CaLC2928: To construct the tetO-HSF1/hsf1Δ strain, the plasmid pLC551 was digested with SapI and KpnI to liberate the TAR-tetO-HSF1 construct. This construct was transformed into CaLC970 (HSF1/hsf1Δ). NAT-resistant transformants were PCR tested for proper integration of the cassette using primer pairs oLC274/oLC996 and oLC275/oLC995. The SAP2 promoter was induced to drive expression of FLP recombinase to excise the NAT marker cassette. NAT-sensitive colonies were additionally PCR tested with the primer pairs oLC985/oLC988 to verify the presence of the deleted allele of HSF1, oLC991/oLC994 to ensure there were no remaining wild-type alleles of HSF1 present, and oLC300/oLC994 to verify the presence of the tetO promoter.
CaLC3017: To construct the tetO-HSF1/hsf1Δ ACT1p-HSP90/ACT1p-HSP90 strain the ACT1p-HSP90 construct with homology to the PHO23 locus was liberated by digesting the plasmid pLC755 with BssHII. The digested plasmid was transformed into CaLC2928 (tetO-HSF1/hsf1Δ). NAT-resistant transformants were PCR tested with the primer pairs oLC275/oLC376 and oLC381/oLC324 to verify the integration of the ACT1p-HSP90 cassette at the PHO23 locus. The SAP2 promoter was induced to drive expression of FLP recombinase to excise the NAT marker cassette. NAT-sensitive colonies were additionally PCR tested with the primers oLC1482/oLC609 to verify the presence of the ACT1p-HSP90. To put a second copy of HSP90 under the control of ACT1p in the genome, an ACT1p-HSP90 cassette with homology to the ACT1 locus was liberated by digesting the plasmid pLC757 with BsrGI. The digested plasmid was transformed into the strain. NAT-resistant transformants were PCR tested with the primers oLC3022/oLC199 to verify integration of the ACT1p-HSP90 cassette into the ACT1 locus.
CaLC3384 and CaLC3385: To construct tetO-HSP90/hsp90Δ strains, the plasmid pLC62 containing the HSP90 knockout construct was digested with KpnI-HF and SacII and transformed into CaLC239 (SN95). NAT-resistant transformants were PCR tested for proper integration using the primer pairs oLC275/oLC276 and oLC277/oLC274. The SAP2 promoter was induced to drive expression of FLP recombinase to excise the NAT marker cassette. The tetO promoter replacement construct was PCR amplified from pLC605 using the primers oLC3390 and oLC3220 and transformed into the heterozygous mutant strain. NAT-resistant transformants were PCR tested for proper integration of the tetO promoter at the HSP90 locus using the primers oLC294/oLC534 and oLC300/oLC408. Transformants were PCR tested for the absence of a wild-type promoter of HSP90 using the primers oLC294/oLC297. The SAP2 promoter was then induced to drive expression of FLP recombinase to excise the NAT marker cassette.
CaLC2995: To construct the tetO-HSF1-TAP/hsf1Δ strain, the TAP-ARG4 cassette was PCR amplified from pLC573 using the primers oLC2950 and oLC2922 and transformed into CaLC239 (SN95). ARG4+ transformants were PCR tested for correct integration of TAP at the C-terminus of HSF1 using the primer pairs oLC1597/oLC1593 and oLC1598/oLC1594.
CaLC3890: The tetO-HSF1-TAP/hsf1Δ ACT1p-HSP90/ACT1p-HSP90 strain was constructed using the same method as CaLC2995, except that the TAP-ARG4 cassette was transformed into CaLC3017 (tetO-HSF1/hsf1Δ ACT1p-HSP90/ACT1p-HSP90).
CaLC3786: The tetO-HSP90/hsp90Δ HSF1-TAP/HSF1 strain was constructed using the same method as CaLC2995, except that the TAP-ARG4 cassette was transformed into CaLC3384 (tetO-HSP90/hsp90Δ).
CaLC5012: The HSF1-TAP/HSF1 strain in the SN250 background was constructed using the same method as CaLC2995, except that the TAP-ARG4 cassette was transformed into CaLC2302 (SN250). CaLC974: To construct the MAL2p-HSF1/hsf1Δ strain, the plasmid pLC481, which contains the MAL2 promoter and sequence homologous to HSF1, was digested with BssHII and transformed into CaLC970 (HSF1/hsf1Δ). NAT-resistant transformants were PCR tested for proper integration of the construct using primer pairs oLC995/oLC275 and oLC274/oLC996. Transformants were additionally PCR tested with the primers oLC985/oLC988 to verify the presence of the deleted allele of HSF1 and oLC991/oLC994 to ensure there were no additional wild-type alleles of HSF1. The SAP2 promoter was induced to drive expression of FLP recombinase to excise the NAT marker cassette.
CaLC3171: To construct the cpr6Δ/cpr6Δ strain, the NAT knockout cassette was PCR amplified from pLC49 using primers oLC3062 and oLC3063 and transformed into CaLC239 (SN95). NAT-resistant transformants were PCR tested for proper integration using the primer pairs oLC274/oLC3065 and oLC3064/oLC275. The SAP2 promoter was induced to drive expression of FLP recombinase to excise the NAT marker. To delete the second allele of CPR6, the NAT knockout cassette was again amplified from pLC49 using oLC3062 and oLC3063 and transformed into the NAT-sensitive heterozygous strain. NAT-resistant transformants were PCR tested for proper integration using the same primers as above, and with the primers oLC3064/oLC3065 to ensure no wild-type alleles of CPR6 remained. The SAP2 promoter was then induced to drive expression of FLP recombinase to excise the NAT marker cassette.
CaLC3172: To construct the aha1Δ/aha1Δ strain, the NAT knockout cassette was PCR amplified from pLC49 using primers oLC3058 and oLC3059 and transformed into CaLC239 (SN95). NAT-resistant transformants were PCR tested for proper integration using the primer pairs oLC274/oLC2678 and oLC3172/oLC275. The SAP2 promoter was induced to drive expression of FLP recombinase to excise the NAT marker. To delete the second allele of AHA1, the NAT knockout cassette was again amplified from pLC49 using oLC3058 and oLC3059 and transformed into the NAT-sensitive heterozygous strain. NAT-resistant transformants were PCR tested for integration using the same primers as above, and with the primers oLC2677/oLC2678 to ensure no wild-type alleles of AHA1 remained. The SAP2 promoter was then induced to drive expression of FLP recombinase to excise the NAT marker cassette.
CaLC3193: To construct the hch1Δ/hch1Δ strain, the NAT knockout cassette was PCR amplified from pLC49 using primers oLC3086 and oLC3087 and transformed into CaLC239 (SN95). NAT-resistant transformants were PCR tested for proper integration using the primers oLC274/oLC3089 and oLC3088/3089 to ensure the presence of a deleted allele. The SAP2 promoter was induced to drive expression of FLP recombinase to excise the NAT marker. To delete the second allele of HCH1, the NAT knockout cassette was again PCR amplified from pLC49 using the primers oLC3086 and oLC3087, and transformed into the NAT-sensitive heterozygous strain. NAT-resistant transformants were PCR tested for integration using the primers oLC274/oLC3089 to test for proper integration and oLC3088/oLC3089 to ensure no wild-type alleles of HCH1 remained. The SAP2 promoter was then induced to drive expression of FLP recombinase to excise the NAT marker cassette.
CaLC3310: To construct the sti1Δ/sti1Δ strain, the NAT knockout cassette was PCR amplified from pLC49 using the primers oLC3074 and oLC3075 and transformed into CaLC239 (SN95). NAT-resistant transformants were tested for proper integration using the primer pairs oLC274/oLC2704 and oLC275/oLC3076. The SAP2 promoter was induced to drive expression of FLP recombinase to excise the NAT marker. To delete the second allele of STI1, the NAT knockout cassette was again PCR amplified from pLC49 using oLC3074 and oLC3075, and transformed into the NAT-sensitive heterozygous strain. NAT-resistant transformants were PCR tested for integration using the same primers as above, and with the primers oLC2703/oLC2704 to ensure no wild-type alleles of STI1 remained. The SAP2 promoter was then induced to drive expression of FLP recombinase to excise the NAT marker cassette.
CaLC3797: To construct the sba1Δ/sba1Δ strain, the NAT knockout cassette was PCR amplified from pLC49 using primers oLC3748 and oLC3067 and transformed into CaLC239 (SN95). NAT-resistant transformants were PCR tested for proper integration using the primer pairs oLC3170/oLC275 and oLC274/oLC3747. The SAP2 promoter was then induced to drive expression of FLP recombinase to excise the NAT marker cassette. To delete the second allele of SBA1, the NAT knockout cassette was again PCR amplified from pLC49 using oLC3748 and oLC3067, and transformed into the NAT-sensitive heterozygous strain. NAT-resistant transformants were PCR tested for integration using the same primers as above and the primers oLC3170 and oLC3747 to ensure that no wild-type alleles of SBA1 remained. The SAP2 promoter was then induced to drive expression of FLP recombinase to excise the NAT marker cassette.
CaLC3991: To construct the tetO-CDC37/cdc37Δ strain, the plasmid pLC506 was digested with BssHII to liberate the MAL2p-CDC37 cassette and the digested plasmid was transformed into CaLC239 (SN95). The NAT-resistant transformants were PCR tested for proper integration using the primer pairs oLC1093/oLC275 and oLC274/oLC1097. The SAP2 promoter was then induced to drive expression of FLP recombinase to excise the NAT marker cassette. The plasmid pLC505 was digested with BssHII to liberate the CDC37 knockout cassette and the digested plasmid was transformed into the NAT-sensitive MAL2p-CDC37/CDC37 strain. The NAT-resistant transformants were PCR tested for proper integration using the primers oLC275/oLC1093 and oLC274/oLC1094. Transformants were additionally PCR tested with oLC441/oLC1097 to verify the presence of the MAL2p-CDC37 allele and with oLC1093/oLC1097 to ensure that there were no remaining wild-type alleles of CDC37. The SAP2 promoter was then induced to drive expression of FLP recombinase to excise the NAT marker cassette, and the NAT-sensitive colonies were again PCR tested for the presence of the deleted allele using oLC1093/oLC1094, presence of the MAL2 promoter using oLC441/oLC1097, and the absence of a wild-type allele using oLC1093/ oLC1097. The TAR-tetO cassette was amplified from pLC605 using oLC2208 and oLC2291 and transformed into the MAL2p-CDC37/cdc37Δ strain, which was grown in YPM before transformation. NAT-resistant and glucose-insensitive colonies were PCR tested for proper integration using oLC300/oLC705. The SAP2 promoter was then induced to drive expression of FLP recombinase to excise the NAT marker cassette.
CaLC4760: The tetO-HSF1/tetO-HSF1 strain in the SN250 background was generated using CRISPR technology adapted from Vyas et al, 2015[72]. The plasmid pLC970 containing the CRISPR machinery and guide directed to the promoter of HSF1 was digested with KpnI-HF and SacI-HF to liberate the cassette. A repair template containing the TAR-tetO construct was PCR amplified from genomic DNA of the strain CaLC3786 (tetO-HSP90/hsp90Δ HSF1-TAP/HSF1) using the primers oLC4725 and oLC4726 which contain upstream and downstream homology to the promoter of HSF1. The digested plasmid and repair were transformed into CaLC2302 (SN250). NAT-resistant transformants were PCR tested for proper integration of the tetO promoter with the primers oLC4714/oLC1676 and PCR tested for the absence of wild-type alleles of HSF1 using oLC989/oLC1676. The SAP2 promoter was induced to drive expression of FLP recombinase to excise the NAT marker cassette. In order to reduce filamentation induced by HSF1 overexpression to allow for more efficient excising of the NAT marker cassette, 20 μg/mL DOX was included in the YNB-BSA cultures. NAT-sensitive colonies were PCR tested with the primers oLC4714/oLC1676 to confirm integration of the tetO promoter and oLC989/oLC1676 to ensure that there were no wild-type alleles of HSF1 remaining.
CaLC5014: The tetO-HSF1-TAP/tetO-HSF1 strain in the SN250 background was constructed using the same method as CaLC2995, except that the TAP-ARG4 cassette was transformed into CaLC4760 (SN250 tetO-HSF1/tetO-HSF1). Transformants were also PCR tested with the primers oLC4714/oLC1676 to confirm the presence of the tetO promoter and oLC989/oLC1676 to ensure that there were no wild-type alleles of the HSF1 promoter remaining.
CaLC4822: The tetO-HSF1/tetO-HSF1-TAP strain was constructed using the same method as CaLC4760, except that the CRISPR machinery/sgRNA to HSF1 and TAR-tetO repair were transformed into CaLC2993 (HSF1-TAP/HSF1).
CaLC4916: The tetO-HSF1/tetO-HSF1 NOP1-GFP strain was constructed using the same method as CaLC4760, except that the CRISPR machinery/sgRNA to HSF1 and TAR-tetO repair were transformed into CaLC4506 (NOP1-GFP/NOP1).
CaLC4931: The tetO-HSF1/tetO-HSF1 kex2Δ/kex2Δ strain was constructed using the same method as CaLC4760, except that the CRISPR machinery/sgRNA to HSF1 and TAR-tetO repair were transformed into CaLC3055 (kex2Δ/kex2Δ).
CaLC4958: The tetO-HSF1/tetO-HSF1 efg1Δ/efg1Δ strain was constructed using the same method as CaLC4760, except that the CRISPR machinery/sgRNA to HSF1 and TAR-tetO repair were transformed into CaLC563 (efg1Δ/efg1Δ).
CaLC4961: The tetO-HSF1/tetO-HSF1 strain in the CAI4 background was constructed using the same method as CaLC4760, except that the CRISPR machinery/sgRNA to HSF1 and TAR-tetO repair were transformed into CaLC75 (CAI4).
CaLC4963: The tetO-HSF1/tetO-HSF1 rob1Δ/rob1Δ strain was constructed using the same method as CaLC4760, except that the CRISPR machinery/sgRNA to HSF1 and TAR-tetO repair were transformed into CaLC2738 (rob1Δ/rob1Δ).
CaLC5039: The tetO-HSF1/tetO-HSF1 ume6Δ/ume6Δ (preflip) strain was constructed using the same method as CaLC4760, except that the CRISPR machinery/sgRNA to HSF1 and TAR-tetO repair were transformed into CaLC1566 (ume6Δ/ume6Δ). Multiple attempts at excising the CRISPR machinery/sgRNA out of the strain were unsuccessful, so the strain remains NAT resistant.
CaLC5275: The tetO-HSF1/tetO-HSF1 (preflip) strain was constructed using the same method as CaLC4760, except that the CRISPR machinery and sgRNA to HSF1 were not excised from the strain. This was done to have a marker-matched control to compare with CaLC5039. This strain has the same phenotype as CaLC4760.
CaLC4762: The tetO-HSF1/tetO-HSF1 brg1Δ/brg1Δ strain was constructed using the same method as CaLC4760, except that the CRISPR machinery/sgRNA to HSF1 and TAR-tetO repair were transformed into CaLC2736 (brg1Δ/brg1Δ).
CaLC5277: The tetO-HSF1/tetO-HSF1 brg1Δ/brg1Δ (preflip) strain was constructed using the same method as CaLC4762, except that the CRISPR machinery and sgRNA to HSF1 were not excised from the strain. This was done to have a marker-matched strain to compare with CaLC5039. This strain has the same phenotype as CaLC4762.
CaLC5042: The tetO-HSF1/tetO-HSF1 strain in the CAF2-1 background was constructed using the same method as CaLC4760, except that the CRISPR machinery/sgRNA to HSF1 and TAR-tetO repair were transformed into CaLC2742 (CAF2-1).
CaLC5077: The tetO-HSF1/tetO-HSF1 bub2Δ/bub2Δ strain was constructed using the same method as CaLC4760, except that the CRISPR machinery/sgRNA to HSF1 and TAR-tetO repair were transformed into CaLC1589 (bub2Δ/bub2Δ).
CaLC5079: The tetO-HSF1/tetO-HSF1 ras1Δ/ ras1Δ strain was constructed using the same method as CaLC4760, except that the CRISPR machinery/sgRNA to HSF1 and TAR-tetO repair were transformed into CaLC564 (ras1Δ/ ras1Δ).
CaLC4892: The tetO-HSF1/tetO-HSF1-TAP ACT1p-HSP90/ACT1p-HSP90 strain was generated using CRISPR technology adapted from Vyas et al, 2015[72]. The plasmid pLC976 containing the CRISPR machinery and guide directed to the promoter of HSP90 was digested with KpnI-HF and SacI-HF to liberate the CRISPR components. A repair template containing the ACT1p was PCR amplified from genomic DNA from the strain CaLC4828 (ACT1p-HSP90) using primers oLC5002 and oLC5003. The digested plasmid and repair were transformed into CaLC4822 (tetO-HSF1/tetO-HSF1-TAP). NAT-resistant transformants were PCR tested for proper integration using the primers oLC1850/oLC199 and for lack of wild-type HSP90 promoter with the primers oLC198/oLC199. The SAP2 promoter was induced to drive expression of FLP recombinase to excise the NAT marker cassette. NAT-sensitive colonies were additionally PCR tested with the primers oLC198 and oLC199 to ensure that there were no wild-type alleles of the HSP90 promoter remained.
CaLC4828: To construct the ACT1p-HSP90 strain the ACT1 promoter with a NAT cassette was PCR amplified from plasmid pLC620 using the primers oLC5002 and oLC5003 and transformed into CaLC239 (SN95). NAT-resistant transformants were PCR tested for proper integration of the cassette using the primer pairs oLC274/oLC199 and oLC3116/oLC268. The SAP2 promoter was induced to drive expression of FLP recombinase to excise the NAT marker cassette. NAT-sensitive colonies were additionally tested with oLC3116/oLC268 to verify the presence of the ACT1 promoter.
CaLC4855: The tetO-HSP90/tetO-HSP90 strain was generated using CRISPR technology adapted from Vyas et al, 2015[72]. The plasmid pLC976 containing the CRISPR machinery and guide directed to the promoter of HSP90 was digested with KpnI-HF and SacI-HF to liberate the CRISPR components. A repair template containing the TAR-tetO promoter was PCR amplified from genomic DNA from the CaLC3786 strain (tetO-HSP90/hsp90Δ HSF1-TAP/HSF1) using primers oLC4980 and oLC4981 with homology to HSP90. The digested plasmid and repair were transformed into CaLC239 (SN95). NAT-resistant transformants were PCR tested for proper integration using the primers oLC4714/oLC199 and for lack of wild-type HSP90 promoter with oLC268/oLC199. The SAP2 promoter was induced to drive expression of FLP recombinase to excise the NAT marker cassette. NAT-sensitive colonies were additionally PCR tested with oLC4714/oLC199 and oLC268/oLC199.
CaLC4790: The tetO-orf19.4021/tetO-orf19.4021 strain was generated using CRISPR technology adapted from Vyas et al, 2015[72]. The plasmid pLC971 containing the CRISPR machinery and guide directed to the promoter of orf19.4021 was digested with KpnI-HF and SacI-HF to liberate the CRISPR components. A repair template containing the TAR-tetO promoter was PCR amplified from genomic DNA from the CaLC3786 strain (tetO-HSP90/hsp90Δ HSF1-TAP/HSF1) using primers oLC4810 and oLC4811 with homology to orf19.4021. The digested plasmid and repair were transformed into CaLC239 (SN95). NAT-resistant transformants were PCR tested for proper integration using the primers oLC300/oLC4813 and for lack of wild-type orf19.4021 promoter with the primers oLC4812/oLC4813. The SAP2 promoter was induced to drive expression of FLP recombinase to excise the NAT marker cassette.
CaLC4791: The tetO-FOX2/tetO-FOX2 strain was generated using CRISPR technology adapted from Vyas et al, 2015[72]. The plasmid pLC972 containing the CRISPR machinery and guide directed to the promoter of FOX2 was digested with KpnI-HF and SacI-HF to liberate the CRISPR components. A repair template containing the TAR-tetO promoter was PCR amplified from genomic DNA from the CaLC3786 strain (tetO-HSP90/hsp90Δ HSF1-TAP/HSF1) using primers oLC4802 and oLC4803 with homology to FOX2. The digested plasmid and repair were transformed into CaLC239 (SN95). NAT-resistant transformants were PCR tested for proper integration using the primers oLC300/ oLC4806 and for lack of wild-type FOX2 promoter with oLC4807/oLC4806. The SAP2 promoter was induced to drive expression of FLP recombinase to excise the NAT marker cassette.
Plasmid construction
pLC335: Sequence homologous to the upstream region of PHO23 was PCR amplified from SC5314 genomic DNA (CaLC155) using the primers oLC396/oLC397, digested with KpnI andApaI, and ligated into pLC49. Integration was PCR tested using the primers oLC275/oLC396. Sequence homologous to the downstream region of PHO23 was PCR amplified from SC5314 genomic DNA using the primers LC398/oLC399, digested with SacI and SacII, and ligated into pLC49 containing the sequence homologous to the upstream region of PHO23. Integration was PCR tested using the primersoLC274/oLC399. The construct can be liberated using KpnI andSacI.
pLC478: Sequence homologous to the upstream region of HSF1 was PCR amplified from SC5314 genomic DNA using the primers oLC985/oLC986, digested with ApaI, and ligated into pLC49. Integration was PCR tested using the primers oLC275/oLC985. Sequence homologous to the downstream region of HSF1 was PCR amplified from SC5314 genomic DNA using the primers oLC987/oLC988, digested with SacI and SacII, and ligated into pLC49 containing the sequence homologous to the upstream region of HSF1. Integration was PCR tested using the primers oLC274/oLC988. The construct can be liberated using BssHII.
pLC481: Sequence homologous to the upstream region of HSF1 was PCR amplified from SC5314 genomic DNA using the primers oLC991/oLC992, digested with ApaI, and ligated into pLC49. Integration was PCR tested using the primers oLC275/oLC991. Sequence homologous to the beginning of the HSF1 open reading frame (beginning with ATG) was PCR amplified from SC5314 genomic DNA using the primers oLC993/oLC994, digested with SacI and SacII, and ligated into pLC49 containing the sequence homologous to the upstream region of HSF1. Integration was PCR tested using the primers oLC274/oLC994. The MAL2 promoter was liberated from pLC50 using NotI and SacII and cloned into the plasmid containing both pieces of sequence homologous to HSF1. Integration was PCR tested using the primer pairs oLC441/oLC994 and oLC274/oLC994. The construct can be liberated using BssHII.
pLC551: The plasmid pLC330, containing the tetO promoter and sequence homologous to HSP90, was digested with SapI and ApaI to remove the sequence homologous to the upstream region of HSP90. Sequence homologous to the upstream region of HSF1 was PCR amplified from SC5314 genomic DNA using the primers oLC1368/oLC1370, digested with SapI and ApaI, and ligated into pLC330 with the region of HSP90 sequence removed. Next, the plasmid pLC52, containing the tetracycline-repressible transactivator TAR, was digested with ApaI to liberate TAR and ligated into the plasmid containing sequence homologous to HSF1 and the tetO promoter. This plasmid was then digested with KpnI and SacII to remove the sequence homologous to the downstream region of HSP90. Sequence homologous to the downstream region of HSF1 was PCR amplified from SC5314 genomic DNA using the primers oLC993/oLC1369, digested with KpnI and SacII, and ligated into the plasmid. The construct can be liberated using SapI/KpnI.
pLC605: The plasmid pLC52, containing the tetracycline-repressible transactivator and the FLP-NAT cassette, was digested with NotI and SacII. The tetO promoter was PCR amplified from pLC46 using the primers oLC300 and oLC301, digested with NotI and SacII and ligated into the plasmid.
pLC755: The plasmid pLC335, containing sequence homologous to upstream and downstream of PHO23 was digested with NotI and SacII. HSP90 was PCR amplified from pLC455 using the primers oLC1493 and oLC1485, digested with NotI and SacII and ligated into the pLC335 vector backbone. The plasmid containing HSP90 was then digested with NotI. The ACT1 promoter was PCR amplified from pLC430 using the primers oLC1482 and oLC1492, digested with NotI, and ligated into the plasmid. The PHO23-ACT1p-HSP90-PHO23 cassette can be liberated using BssHII.
pLC757: The plasmid pLC430 containing the ACT1 promoter, was digested with XmaI. HSP90 was PCR amplified from pLC455 using the primers oLC1429 and oLC1430, digested with XmaI and ligated into the vector backbone. The entire integrating vector can be linearized with BsrGI to target it for integration at the ACT1 locus.
pLC963: The sequences for two adjacent regions at a neutral locus on the left arm of chromosome 5 (Neut5L) (5-Neut: Ca22chr5A_C_albicans_SC5314:232638–233017 and 3-Neut: Ca22chr5A_C_albicans_SC5314:233018–233358) were synthesized by IDT. These fragments were cloned into pLC933 (pV1093[72]) at the KpnI and SacI/SacII sites, respectively, by Gibson Assembly. The NatR containing ApaI and NotI fragment was replaced with a FLP-NatR amplicon generated using the primers NatFLP-fwd and NatFLP-rev and pJK863[73] as a template.
pLC970: The plasmid pLC963 was digested with BsmBI and the sgRNA directed towards the HSF1 promoter was inserted by ligating the annealed primers, oLC4723 and oLC4724. Proper integration was confirmed using Sanger sequencing with the primer oLC4609.
pLC971: The plasmid pLC963 was digested with BsmBI and the sgRNA directed towards the orf19.4021 promoter was inserted by ligating the annealed primers, oLC4808 and oLC4809. Proper integration was confirmed using Sanger sequencing with the primer oLC4609.
pLC972: The plasmid pLC963 was digested with BsmBI and the sgRNA directed towards the FOX2 promoter was inserted by ligating the annealed primers, oLC4804 and oLC4805. Proper integration was confirmed using Sanger sequencing with the primer oLC4609.
pLC976: The plasmid pLC963 was digested with BsmBI and the sgRNA directed towards the HSP90 promoter was inserted by ligating the annealed primers, oLC4978 and oLC4979. Proper integration was confirmed using Sanger sequencing with the primer oLC4609.
Supporting information
(XLSX)
List of basal and overexpression targets, GO terms associated with the targets, and all of the ChIP-Seq peaks.
(XLSX)
(XLSX)
List of the differentially expressed genes upon HSF1 overexpression and all of the expression data.
(XLSX)
(DOCX)
(DOCX)
(DOCX)
a) Strains were grown in rich medium for 6 hours at 30°C in the absence of DOX. Overexpression of HSF1, but not orf19.4021, FOX2 or HSP90, induces filamentation. b) Overnights of the wild-type strain or tetO-HSF1/tetO-HSF1 strain were subcultured to an OD of 0.1 in rich medium in the absence or presence of the indicated concentrations of DOX and grown overnight. Cultures were subcultured to an OD of 0.1 in the same conditions and grown for an additional overnight before imaging analysis. Heat killed cells were prepared by treating the wild-type strain at 100°C for five minutes. Cell death was assayed by treating 20 μL samples with 25 μg/mL of the membrane impermeable dye propidium iodide for 5 minutes in the dark before imaging. Images were all taken at the same fluorescence intensity.
(TIF)
Strains were grown in rich medium in the presence of no DOX, 0.01 μg/mL DOX, 0.1 μg/mL DOX, 1 μg/mL DOX or 10 μg/mL DOX at 30°C. a) Quantitative RT-PCR analysis to determine the levels of HSF1 when grown in varying levels of DOX. HSF1 transcript levels were normalized to ACT1 and GPD1. Data are means +/- standard error of the means for triplicate samples. *** indicates P value <0.005, ** indicates P value <0.01, * indicates P value <0.05, ns indicates no significant difference, unpaired t test. b) HSF1 overexpression induces filamentation when the tetO-HSF1-TAP/tetO-HSF1 strain is grown in the presence of no DOX or 0.01 μg/mL DOX. When grown in 0.1 μg/mL DOX, where the HSF1 levels are close to wild-type levels (no significant difference, P value 0.1773, unpaired t test), the strain grows in the yeast form. HSF1 depletion induces filamentation when grown in the presence of 1 μg/mL DOX or 10 μg/mL DOX. The morphology of the control strain (HSF1-TAP/HSF1) is unaffected by growth in varying concentrations of DOX.
(TIF)
Strains were grown in the presence of no DOX, 0.1 μg/mL DOX, or 20 μg/mL DOX at 30°C. Strains in the CAI4 background were grown with 80 mg/L uridine added to the growth medium.
(TIF)
a) A scatterplot showing the Fragments Per Kilobase of transcript per Million mapped reads (FPKM) values for HSF1 gene expression in 169 published RNA-seq datasets of wild-type strains of C. albicans grown under different conditions. HSF1 expression levels vary significantly under diverse experimental conditions. b) Quantitative RT-PCR analysis comparing the levels of HSF1 in the control strain (HSF1-TAP/HSF1) grown in HSF1 inducing experimental conditions and in the HSF1 overexpression strains. HSF1 expression was significantly induced in the wild-type strain upon a 30°C to 42°C heat shock or when grown in biofilms formed in Spider medium. While the levels of HSF1 in these experimental conditions did not reach the levels of the tetO-HSF1-TAP/tetO-HSF1 strain where filamentation is observed, the levels were comparable to the tetO-HSF1-TAP/hsf1Δ strain which filaments upon elevated temperature. Strains were grown in the presence of no DOX or 0.01 μg/mL DOX, as indicated. HSF1 transcript levels were normalized to ACT1 and PMA1. Data are means +/- standard error of the means for triplicate samples. *** indicates P value <0.005, ns indicates no significant difference, unpaired t test.
(TIF)
a) Filamentation induced by depletion of HSP90 is not blocked at 23°C. Strains were grown in the absence or presence of 20 μg/mL DOX at the indicated temperatures. b) Filamentation in response to overexpression of positive filamentation regulators BRG1 or UME6 is not blocked at 23°C. Overexpression of LEU3 serves as a control that has no impact on filamentation. Strains were grown at the indicated temperatures in the absence or presence of 50 μg/mL DOX to induce expression of the tetON promoter. c) Filamentation in response to homozygous deletion of TUP1 or NRG1 is not blocked at 23°C. Strains were grown in the absence of DOX at the indicated temperatures.
(TIF)
Strains were grown in rich medium with 80 mg/L uridine added, in the presence of no DOX, 0.1 μg/mL DOX, 20 μg/mL DOX or 50 μg/mL DOX at 30°C.
(TIF)
a) The impact of HSF1 depletion was assessed using a strain where the only allele of HSF1 is under the control of the tetO promoter (left panels), allowing for transcriptional repression with DOX treatment. The effect of HSF1 depletion was also monitored using a strain where the only allele of HSF1 was under the control of the MAL2 promoter (right panels), allowing for induction of HSF1 expression in rich medium with maltose (YPM) and repression in rich medium with glucose (YPD). Filamentation is observed when HSF1 expression is repressed through growth in 20 μg/mL DOX or 50% YPM/YPD, respectively. b) Microarray analysis identified transcriptional changes in response to HSF1 depletion in the tetO-HSF1/hsf1Δ strain. GO terms associated with the genes misregulated upon HSF1 depletion by greater than 2-fold are shown. c) Gene Set Enrichment Analysis identified transcriptional profiles that are significantly correlated to the transcriptional profile of genes up-regulated in response to HSF1 depletion. Of note, the profile of genes upregulated in response to geldanamycin treatment was similar to the genes upregulated in response to HSF1 depletion. d) Enrichment plot for geldanamycin.
(TIF)
Western blot analysis was performed to assay if HSF1 depletion compromises Hsp90 function by monitoring the phosphorylation of the Hsp90 client protein Hog1. Strains were grown in the absence or presence of 20 μg/mL DOX. Cells were treated with 5 mM hydrogen peroxide (H2O2) for 10 minutes to induce oxidative stress before protein extraction. Depletion of Hsf1 reduces the levels of phosphorylated Hog1 (pHog1), even in the isogenic strain with constitutive HSP90 expression. Tubulin or actin levels serve as loading controls. WT indicates the wild type, untagged control. Three biological replicates are shown.
(TIF)
Deletion or depletion mutants were made for the six Hsf1-dependent Hsp90 co-chaperone genes in C. albicans. Strains were grown in the absence or presence of high DOX (20 μg/mL) at 30°C as indicated. Scale bar represents 10 μm.
(TIF)
a) Quantitative RT-PCR analysis to validate the overexpression of UME6 or BRG1 in the tetracycline-inducible promoter (tetON) strains. Strains were grown in the absence or presence of 50 μg/mL DOX at 30°C to induce overexpression. Overexpression of LEU3 acts as a negative control. b) Overexpression of HSF1 no longer drives the overexpression of UME6 or BRG1 when grown at lower temperatures. The wild-type and tetO-HSF1/tetO-HSF1 strains were grown at 30°C or 23°C, in the absence of DOX, until mid-log phase. UME6, BRG1 and HSF1 transcript levels were normalized to ACT1 and GPD1. Data are means +/- standard error of the means for triplicate samples.
(TIF)
Acknowledgments
We thank all the members of the Cowen lab for helpful discussions. We thank the Donnelly Sequencing Centre at the University of Toronto for their help with sample preparation for RNA-seq, and Dr. Christophe D’Enfert for providing us with tetracycline-inducible conditional expression strains. We thank Jacky Chan and William Pang for their expert support on the High Performance Computer. This work was performed in part at the High Performance Computing Cluster (HPCC), which is supported by Information and Communication Technology Office (ICTO) of the University of Macau.
Data Availability
All relevant data are within the paper and its Supporting Information files or available at SRA under the accession number SRP119587. This data is available at https://www.ncbi.nlm.nih.gov/sra/?term=SRP119587.
Funding Statement
AOV is generously supported by a Canadian Institutes of Health Research Frederick Banting and Charles Best Canada Graduate Scholarship and by Ontario Graduate Scholarships. TRO is supported by a National Institutes of Health Ruth L. Kirschstein National Research Service Award (NRSA, AI115947-01) from the NIAID. MW is supported by Canadian Institutes of Health Research (MOP-42516) and a Tier 1 Canada Research Chair. KHW is supported by the Science and Technology Development Fund of Macau SAR (Grant number: FDCT-085/2014/A2). LEC is supported by Canadian Institutes of Health Research Operating Grants (MOP-86452 and MOP-119520) and Foundation Grant (FDN-154288), the Natural Sciences and Engineering Council (NSERC) of Canada Discovery Grants (06261 and 462167), an NSERC E.W.R. Steacie Memorial Fellowship (477598), and a National Institutes of Health NIAID R01 (1R01AI127375-01). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Leach MD, Cowen LE. Surviving the heat of the moment: A fungal pathogens perspective. PLoS Pathogens. 2013;9:e1003163 doi: 10.1371/journal.ppat.1003163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein BS, Tebbets B. Dimorphism and virulence in fungi. Current Opinion in Microbiology. 2007;10(4):314–9. doi: 10.1016/j.mib.2007.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholls S, Leach MD, Priest CL, Brown AJP. Role of the heat shock transcription factor, Hsf1, in a major fungal pathogen that is obligately associated with warm-blooded animals. Molecular Microbiology. 2009;74:844–61. doi: 10.1111/j.1365-2958.2009.06883.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leach MD, Farrer RA, Tan K, Miao Z, Walker LA, Cuomo CA, et al. Hsf1 and Hsp90 orchestrate temperature-dependent global transcriptional remodelling and chromatin architecture in Candida albicans. Nature Communications. 2016;7:11704 doi: 10.1038/ncomms11704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro RS, Robbins N, Cowen LE. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiology and Molecular Biology Reviews. 2011;75:213–67. doi: 10.1128/MMBR.00045-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sudbery PE. Growth of Candida albicans hyphae. Nature Reviews Microbiology. 2011;9:737–48. doi: 10.1038/nrmicro2636 [DOI] [PubMed] [Google Scholar]
- 7.Noble SM, French S, Kohn La, Chen V, Johnson AD. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nature Genetics. 2010;42:590–8. doi: 10.1038/ng.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Meara TR, Veri AO, Ketela T, Jiang B, Roemer T, Cowen LE. Global analysis of fungal morphology exposes mechanisms of host cell escape. Nature Communications. 2015;6:e1006142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee M, Kadosh D, Thompson DS, Lazzell A, Carlisle PL. UME6, a novel filament-specific regulator of Candida albicans hyphal extension and virulence. Molecular Biology of the Cell. 2008;19:1354–65. doi: 10.1091/mbc.E07-11-1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlisle PL, Banerjee M, Lazzell A, Monteagudo C, López-Ribot JL, Kadosh D. Expression levels of a filament-specific transcriptional regulator are sufficient to determine Candida albicans morphology and virulence. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:599–604. doi: 10.1073/pnas.0804061106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleary IA, Lazzell AL, Monteagudo C, Thomas DP, Saville SP. BRG1 and NRG1 form a novel feedback circuit regulating Candida albicans hypha formation and virulence. Molecular Microbiology. 2012;85(3):557–73. doi: 10.1111/j.1365-2958.2012.08127.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nantel A, Dignard D, Bachewich C, Harcus D, Marcil A, Bouin A- P, et al. Transcription profiling of Candida albicans cells undergoing the yeast-to-hyphal transition. Molecular Biology of the Cell. 2002;13:3452–65. doi: 10.1091/mbc.E02-05-0272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadosh D, Johnson AD. Induction of the Candida albicans filamentous growth program by relief of transcriptional repression: a genome-wide analysis. Molecular Biology of the Cell. 2005;16(6):2903–12. doi: 10.1091/mbc.E05-01-0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homann OR, Dea J, Noble SM, Johnson AD. A phenotypic profile of the Candida albicans regulatory network. PLoS Genetics. 2009;5(12):e1000783 doi: 10.1371/journal.pgen.1000783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leach MD, Klipp E, Cowen LE, Brown AJP. Fungal Hsp90: a biological transistor that tunes cellular outputs to thermal inputs. Nature Reviews Microbiology. 2012;10:693–704. doi: 10.1038/nrmicro2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro RS, Zaas AK, Betancourt-Quiroz M, Perfect JR, Cowen LE. The Hsp90 co-chaperone Sgt1 governs Candida albicans morphogenesis and drug resistance. PloS One. 2012;7:e44734 doi: 10.1371/journal.pone.0044734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shapiro RS, Uppuluri P, Zaas AK, Collins C, Senn H, Perfect JR, et al. Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Current Biology. 2009;19:621–9. doi: 10.1016/j.cub.2009.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapiro RS, Cowen LE. Coupling temperature sensing and development. Virulence. 2010;1:45–8. doi: 10.4161/viru.1.1.10320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senn H, Shapiro RS, Cowen LE. Cdc28 provides a molecular link between Hsp90, morphogenesis, and cell cycle progression in Candida albicans. Molecular Biology of the Cell. 2012;23:268–83. doi: 10.1091/mbc.E11-08-0729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro RS, Sellam A, Tebbji F, Whiteway M, Nantel A, Cowen LE. Pho85, Pcl1, and Hms1 signaling governs Candida albicans morphogenesis induced by high temperature or Hsp90 compromise. Current Biology. 2012;22:461–70. doi: 10.1016/j.cub.2012.01.062 [DOI] [PubMed] [Google Scholar]
- 21.Cowen LE. The fungal Achilles' heel: targeting Hsp90 to cripple fungal pathogens. Current Opinion in Microbiology. 2013;16:377–84. doi: 10.1016/j.mib.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 22.Li X, Robbins N, O'Meara TR, Cowen LE. Extensive functional redundancy in the regulation of Candida albicans drug resistance and morphogenesis by lysine deacetylases Hos2, Hda1, Rpd3 and Rpd31. Molecular Microbiology. 2017;103(4):635–56. doi: 10.1111/mmi.13578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leach MD, Tyc KM, Brown AJP, Klipp E. Modelling the regulation of thermal adaptation in Candida albicans, a major fungal pathogen of humans. PLoS One. 2012;7:e32467 doi: 10.1371/journal.pone.0032467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahn JS, Hu Z, Thiele DJ, Iyer VR. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Molecular and Cellular Biology. 2004;24(12):5249–56. doi: 10.1128/MCB.24.12.5249-5256.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leach MD, Budge S, Walker L, Munro C, Cowen LE, Brown AJP. Hsp90 orchestrates transcriptional regulation by Hsf1 and cell wall remodelling by MAPK signalling during thermal adaptation in a pathogenic yeast. PLoS Pathogens. 2012;8:e1003069 doi: 10.1371/journal.ppat.1003069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholls S, MacCallum DM, Kaffarnik FA, Selway L, Peck SC, Brown AJP. Activation of the heat shock transcription factor Hsf1 is essential for the full virulence of the fungal pathogen Candida albicans. Fungal Genetics and Biology. 2011;48:297–305. doi: 10.1016/j.fgb.2010.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang DH, Jung KW, Bang S, Lee JW, Song MH, Floyd-Averette A, et al. Rewiring of signaling networks modulating thermotolerance in the human pathogen Cryptococcus neoformans. Genetics. 2017;205(1):201–19. doi: 10.1534/genetics.116.190595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perisic O, Xiao H, Lis JT. Stable binding of Drosophila heat shock factor to head-to-head and tail-to-tail repeats of a conserved 5 bp recognition unit. Cell. 1989;59:797–806. [DOI] [PubMed] [Google Scholar]
- 29.Trinklein ND, Murray JI, Hartman SJ, Botstein D, Myers RM. The role of heat shock transcription factor 1 in the genome-wide regulation of the mammalian heat shock response. Molecular Biology of the Cell. 2004;15(3):1254–61. doi: 10.1091/mbc.E03-10-0738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sopko R, Huang D, Preston N, Chua G, Papp B, Kafadar K, et al. Mapping pathways and phenotypes by systematic gene overexpression. Molecular Cell. 2006;21(3):319–30. doi: 10.1016/j.molcel.2005.12.011 [DOI] [PubMed] [Google Scholar]
- 31.Zheng X, Krakowiak J, Patel N, Beyzavi A, Ezike J, Khalil AS, et al. Dynamic control of Hsf1 during heat shock by a chaperone switch and phosphorylation. eLife. 2016;5:e18638 doi: 10.7554/eLife.18638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie JL, O'Meara TR, Polvi EJ, Robbins N, Cowen LE. Staurosporine induces filamentation in the human fungal pathogen Candida albicans via signaling through Cyr1 and Protein Kinase A. mSphere. 2017;2:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mnaimneh S, Davierwala AP, Haynes J, Moffat J, Peng WT, Zhang W, et al. Exploration of essential gene functions via titratable promoter alleles. Cell. 2004;118(1):31–44. doi: 10.1016/j.cell.2004.06.013 [DOI] [PubMed] [Google Scholar]
- 34.Yu L, Pena Castillo L, Mnaimneh S, Hughes TR, Brown GW. A survey of essential gene function in the yeast cell division cycle. Molecular Biology of the Cell. 2006;17(11):4736–47. doi: 10.1091/mbc.E06-04-0368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng Q, Summers E, Guo B, Fink G. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. Journal of Bacteriology. 1999;181:6339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bachewich C, Nantel A, Whiteway M. Cell cycle arrest during S or M phase generates polarized growth via distinct signals in Candida albicans. Molecular Microbiology. 2005;57:942–59. doi: 10.1111/j.1365-2958.2005.04727.x [DOI] [PubMed] [Google Scholar]
- 37.Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. Crystal structure of an Hsp90–geldanamycin complex: Targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–50. [DOI] [PubMed] [Google Scholar]
- 38.Diezmann S, Michaut M, Shapiro RS, Bader GD, Cowen LE. Mapping the Hsp90 genetic interaction network in Candida albicans reveals environmental contingency and rewired circuitry. PLoS Genetics. 2012;8:e1002562 doi: 10.1371/journal.pgen.1002562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheetham J, Smith DA, da Silva Dantas A, Doris KS, Patterson MJ, Bruce CR, et al. A single MAPKKK regulates the Hog1 MAPK pathway in the pathogenic fungus Candida albicans. Molecular Biology of the Cell. 2007;18(11):4603–14. doi: 10.1091/mbc.E07-06-0581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Meara TR, Veri AO, Polvi EJ, Li X, Valaei SF, Diezmann S, et al. Mapping the Hsp90 genetic network reveals ergosterol biosynthesis and phosphatidylinositol-4-kinase signaling as core circuitry governing cellular stress. PLoS Genetics. 2016;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solis EJ, Pandey JP, Zheng X, Jin DX, Gupta PB, Airoldi EM, et al. Defining the essential function of yeast Hsf1 reveals a compact transcriptional program for maintaining eukaryotic proteostasis. Molecular Cell. 2016;63(1):60–71. doi: 10.1016/j.molcel.2016.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoldt VR, Sonneborn A, Leuker CE, Ernst JF. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO Journal. 1997;16:1982–91. doi: 10.1093/emboj/16.8.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newport G, Agabian N. KEX2 influences Candida albicans proteinase secretion and hyphal formation. Journal of Biological Chemistry. 1997;272(46):28954–61. [DOI] [PubMed] [Google Scholar]
- 44.Polvi EJ, Averette AF, Lee SC, Kim T, Bahn YS, Veri AO, et al. Metal chelation as a powerful strategy to probe cellular circuitry governing fungal drug resistance and morphogenesis. PLoS Genetics. 2016;12(10):e1006350 doi: 10.1371/journal.pgen.1006350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Znaidi S, Nesseir A, Chauvel M, Rossignol T, d'Enfert C. A comprehensive functional portrait of two heat shock factor-type transcriptional regulators involved in Candida albicans morphogenesis and virulence. PLoS Pathogens. 2013;9:e1003519 doi: 10.1371/journal.ppat.1003519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basso V, Znaidi S, Lagage V, Cabral V, Schoenherr F. The two-component response regulator Skn7 belongs to a network of transcription factors regulating morphogenesis in Candida albicans and independently limits morphogenesis-induced ROS accumulation. Molecular Microbiology. 2017;106(1):157–82. doi: 10.1111/mmi.13758 [DOI] [PubMed] [Google Scholar]
- 47.Chauvel M, Nesseir A, Cabral V, Znaidi S, Goyard S, Bachellier-Bassi S, et al. A versatile overexpression strategy in the pathogenic yeast Candida albicans: Identification of regulators of morphogenesis and fitness. PLoS One. 2012;7(9):e45912 doi: 10.1371/journal.pone.0045912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xi C, Hu Y, Buckhaults P, Moskophidis D, Mivechi NF. Heat shock factor Hsf1 cooperates with ErbB2 (Her2/Neu) protein to promote mammary tumorigenesis and metastasis. Journal of Biological Chemistry. 2012;287(42):35646–57. doi: 10.1074/jbc.M112.377481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ganguly S, Home T, Yacoub A, Kambhampati S, Shi H, Dandawate P, et al. Targeting HSF1 disrupts HSP90 chaperone function in chronic lymphocytic leukemia. Oncotarget. 2015;6:31767–79. doi: 10.18632/oncotarget.5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhamgaye S, Devaux F, Vandeputte P, Khandelwal NK, Sanglard D, Mukhopadhyay G, et al. Molecular mechanisms of action of herbal antifungal alkaloid berberine in Candida albicans. PLoS One. 2014;9:e104554 doi: 10.1371/journal.pone.0104554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nair R, Shariq M, Dhamgaye S, Mukhopadhyay CK, Shaikh S, Prasad R. Non-heat shock responsive roles of HSF1 in Candida albicans are essential under iron deprivation and drug defense. Biochimica et Biophysica Acta. 2017;1864(2):345–54. doi: 10.1016/j.bbamcr.2016.11.021 [DOI] [PubMed] [Google Scholar]
- 52.Noble SM, Gianetti BA, Witchley JN. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nature Reviews Microbiology. 2016;15:96–108. doi: 10.1038/nrmicro.2016.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tao L, Du H, Guan G, Dai Y, Nobile CJ, Liang W, et al. Discovery of a "white-gray-opaque" tristable phenotypic switching system in Candida albicans: roles of non-genetic diversity in host adaptation. PLoS Biology. 2014;12(4):e1001830 doi: 10.1371/journal.pbio.1001830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lan C- Y, Newport G, Murillo LA, Jones T, Scherer S, Davis RW, et al. Metabolic specialization associated with phenotypic switching in Candida albicans. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14907–12. doi: 10.1073/pnas.232566499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pande K, Chen C, Noble SM. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nature Genetics. 2013;45:1088–91. doi: 10.1038/ng.2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitesell L, Lindquist S. Inhibiting the transcription factor HSF1 as an anticancer strategy. Expert Opinion on Therapeutic Targets. 2009;13:469–78. doi: 10.1517/14728220902832697 [DOI] [PubMed] [Google Scholar]
- 57.Dai C, Sampson SB. HSF1: Guardian of proteostasis in cancer. Trends in Cell Biology. 2016;26(1):17–28. doi: 10.1016/j.tcb.2015.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neef DW, Turski ML, Thiele DJ. Modulation of heat shock transcription factor 1 as a therapeutic target for small molecule intervention in neurodegenerative disease. PLoS Biology. 2010;8:e1000291 doi: 10.1371/journal.pbio.1000291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh SD, Robbins N, Zaas AK, Schell Wa, Perfect JR, Cowen LE. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathogens. 2009;5:e1000532 doi: 10.1371/journal.ppat.1000532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.LaFayette SL, Collins C, Zaas AK, Schell Wa, Betancourt-Quiroz M, Gunatilaka aaL, et al. PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathogens. 2010;6:e1001069 doi: 10.1371/journal.ppat.1001069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie JL, Singh-Babak SD, Cowen LE. Minimum Inhibitory Concentration (MIC) assay for antifungal drugs. Bio-Protocol. 2012;2:1–6. [Google Scholar]
- 62.Sellam A, Hogues H, Askew C, Tebbji F, van Het Hoog M, Lavoie H, et al. Experimental annotation of the human pathogen Candida albicans coding and noncoding transcribed regions using high-resolution tiling arrays. Genome Biology. 2010;11:R71 doi: 10.1186/gb-2010-11-7-r71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sellam A, Tebbji F, Whiteway M, Nantel A. A novel role for the transcription factor Cwt1p as a negative regulator of nitrosative stress in Candida albicans. PLoS One. 2012;7(8):e43956 doi: 10.1371/journal.pone.0043956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie JL, Qin L, Miao Z, Grys BT, Diaz JC, Ting K, et al. The Candida albicans transcription factor Cas5 couples stress responses, drug resistance and cell cycle regulation. Nature Communications. 2017;8(1):499 doi: 10.1038/s41467-017-00547-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fan X, Lamarre-Vincent N, Wang Q, Struhl K. Extensive chromatin fragmentation improves enrichment of protein binding sites in chromatin immunoprecipitation experiments. Nucleic Acids Research. 2008;36(19):e125 doi: 10.1093/nar/gkn535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong KH, Jin Y, Moqtaderi Z, Wong KH, Jin Y, Moqtaderi Z. Multiplex Illumina Sequencing using DNA barcoding Current Protocols in Molecular Biology. Hoboken, NJ, USA: John Wiley & Sons, Inc; 2013. p. 7.11.1–7. [DOI] [PubMed] [Google Scholar]
- 67.Wong KH, Struhl K. The Cyc8-Tup1 complex inhibits transcription primarily by masking the activation domain of the recruiting protein. Genes and Development. 2011;25:2525–39. doi: 10.1101/gad.179275.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, et al. Model-based analysis of ChIP-Seq (MACS). Genome Biology. 2008;9:R137 doi: 10.1186/gb-2008-9-9-r137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Machanick P, Bailey TL. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics. 2011;27(12):1696–7. doi: 10.1093/bioinformatics/btr189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biology. 2013;14:R36 doi: 10.1186/gb-2013-14-4-r36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature Protocols. 2012;7(3):562–78. doi: 10.1038/nprot.2012.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vyas VK, Barrasa MI, Fink GR. A Candida albicans CRISPR system permits genetic engineering of essential genes and gene families. Science Advances. 2015;1:e1500248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shen J, Guo W, Ko JR. CaNAT1, a heterologous dominant selectable marker for transformation of Candida albicans and other pathogenic Candida species. Infection and Immunity. 2005;73:1239–42. doi: 10.1128/IAI.73.2.1239-1242.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
List of basal and overexpression targets, GO terms associated with the targets, and all of the ChIP-Seq peaks.
(XLSX)
(XLSX)
List of the differentially expressed genes upon HSF1 overexpression and all of the expression data.
(XLSX)
(DOCX)
(DOCX)
(DOCX)
a) Strains were grown in rich medium for 6 hours at 30°C in the absence of DOX. Overexpression of HSF1, but not orf19.4021, FOX2 or HSP90, induces filamentation. b) Overnights of the wild-type strain or tetO-HSF1/tetO-HSF1 strain were subcultured to an OD of 0.1 in rich medium in the absence or presence of the indicated concentrations of DOX and grown overnight. Cultures were subcultured to an OD of 0.1 in the same conditions and grown for an additional overnight before imaging analysis. Heat killed cells were prepared by treating the wild-type strain at 100°C for five minutes. Cell death was assayed by treating 20 μL samples with 25 μg/mL of the membrane impermeable dye propidium iodide for 5 minutes in the dark before imaging. Images were all taken at the same fluorescence intensity.
(TIF)
Strains were grown in rich medium in the presence of no DOX, 0.01 μg/mL DOX, 0.1 μg/mL DOX, 1 μg/mL DOX or 10 μg/mL DOX at 30°C. a) Quantitative RT-PCR analysis to determine the levels of HSF1 when grown in varying levels of DOX. HSF1 transcript levels were normalized to ACT1 and GPD1. Data are means +/- standard error of the means for triplicate samples. *** indicates P value <0.005, ** indicates P value <0.01, * indicates P value <0.05, ns indicates no significant difference, unpaired t test. b) HSF1 overexpression induces filamentation when the tetO-HSF1-TAP/tetO-HSF1 strain is grown in the presence of no DOX or 0.01 μg/mL DOX. When grown in 0.1 μg/mL DOX, where the HSF1 levels are close to wild-type levels (no significant difference, P value 0.1773, unpaired t test), the strain grows in the yeast form. HSF1 depletion induces filamentation when grown in the presence of 1 μg/mL DOX or 10 μg/mL DOX. The morphology of the control strain (HSF1-TAP/HSF1) is unaffected by growth in varying concentrations of DOX.
(TIF)
Strains were grown in the presence of no DOX, 0.1 μg/mL DOX, or 20 μg/mL DOX at 30°C. Strains in the CAI4 background were grown with 80 mg/L uridine added to the growth medium.
(TIF)
a) A scatterplot showing the Fragments Per Kilobase of transcript per Million mapped reads (FPKM) values for HSF1 gene expression in 169 published RNA-seq datasets of wild-type strains of C. albicans grown under different conditions. HSF1 expression levels vary significantly under diverse experimental conditions. b) Quantitative RT-PCR analysis comparing the levels of HSF1 in the control strain (HSF1-TAP/HSF1) grown in HSF1 inducing experimental conditions and in the HSF1 overexpression strains. HSF1 expression was significantly induced in the wild-type strain upon a 30°C to 42°C heat shock or when grown in biofilms formed in Spider medium. While the levels of HSF1 in these experimental conditions did not reach the levels of the tetO-HSF1-TAP/tetO-HSF1 strain where filamentation is observed, the levels were comparable to the tetO-HSF1-TAP/hsf1Δ strain which filaments upon elevated temperature. Strains were grown in the presence of no DOX or 0.01 μg/mL DOX, as indicated. HSF1 transcript levels were normalized to ACT1 and PMA1. Data are means +/- standard error of the means for triplicate samples. *** indicates P value <0.005, ns indicates no significant difference, unpaired t test.
(TIF)
a) Filamentation induced by depletion of HSP90 is not blocked at 23°C. Strains were grown in the absence or presence of 20 μg/mL DOX at the indicated temperatures. b) Filamentation in response to overexpression of positive filamentation regulators BRG1 or UME6 is not blocked at 23°C. Overexpression of LEU3 serves as a control that has no impact on filamentation. Strains were grown at the indicated temperatures in the absence or presence of 50 μg/mL DOX to induce expression of the tetON promoter. c) Filamentation in response to homozygous deletion of TUP1 or NRG1 is not blocked at 23°C. Strains were grown in the absence of DOX at the indicated temperatures.
(TIF)
Strains were grown in rich medium with 80 mg/L uridine added, in the presence of no DOX, 0.1 μg/mL DOX, 20 μg/mL DOX or 50 μg/mL DOX at 30°C.
(TIF)
a) The impact of HSF1 depletion was assessed using a strain where the only allele of HSF1 is under the control of the tetO promoter (left panels), allowing for transcriptional repression with DOX treatment. The effect of HSF1 depletion was also monitored using a strain where the only allele of HSF1 was under the control of the MAL2 promoter (right panels), allowing for induction of HSF1 expression in rich medium with maltose (YPM) and repression in rich medium with glucose (YPD). Filamentation is observed when HSF1 expression is repressed through growth in 20 μg/mL DOX or 50% YPM/YPD, respectively. b) Microarray analysis identified transcriptional changes in response to HSF1 depletion in the tetO-HSF1/hsf1Δ strain. GO terms associated with the genes misregulated upon HSF1 depletion by greater than 2-fold are shown. c) Gene Set Enrichment Analysis identified transcriptional profiles that are significantly correlated to the transcriptional profile of genes up-regulated in response to HSF1 depletion. Of note, the profile of genes upregulated in response to geldanamycin treatment was similar to the genes upregulated in response to HSF1 depletion. d) Enrichment plot for geldanamycin.
(TIF)
Western blot analysis was performed to assay if HSF1 depletion compromises Hsp90 function by monitoring the phosphorylation of the Hsp90 client protein Hog1. Strains were grown in the absence or presence of 20 μg/mL DOX. Cells were treated with 5 mM hydrogen peroxide (H2O2) for 10 minutes to induce oxidative stress before protein extraction. Depletion of Hsf1 reduces the levels of phosphorylated Hog1 (pHog1), even in the isogenic strain with constitutive HSP90 expression. Tubulin or actin levels serve as loading controls. WT indicates the wild type, untagged control. Three biological replicates are shown.
(TIF)
Deletion or depletion mutants were made for the six Hsf1-dependent Hsp90 co-chaperone genes in C. albicans. Strains were grown in the absence or presence of high DOX (20 μg/mL) at 30°C as indicated. Scale bar represents 10 μm.
(TIF)
a) Quantitative RT-PCR analysis to validate the overexpression of UME6 or BRG1 in the tetracycline-inducible promoter (tetON) strains. Strains were grown in the absence or presence of 50 μg/mL DOX at 30°C to induce overexpression. Overexpression of LEU3 acts as a negative control. b) Overexpression of HSF1 no longer drives the overexpression of UME6 or BRG1 when grown at lower temperatures. The wild-type and tetO-HSF1/tetO-HSF1 strains were grown at 30°C or 23°C, in the absence of DOX, until mid-log phase. UME6, BRG1 and HSF1 transcript levels were normalized to ACT1 and GPD1. Data are means +/- standard error of the means for triplicate samples.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files or available at SRA under the accession number SRP119587. This data is available at https://www.ncbi.nlm.nih.gov/sra/?term=SRP119587.