Abstract
This study aimed to compare interventions made by pharmacists attending consultant-led ward rounds in addition to providing a ward pharmacy service, with those made by pharmacists providing a ward pharmacy service alone. A prospective non-randomised controlled study on five inpatient medical wards was carried out at two teaching hospitals. A mean of 1.73 physician-accepted interventions were made per patient for the study group, compared to 0.89 for the control (Mann Whitney U, p<0.001) with no difference between groups in the nature or clinical importance of the interventions. One physician-accepted intervention was made every eight minutes during the consultant-led ward rounds, compared to one every 63 minutes during a ward pharmacist visit. Pharmacists attending consultant-led ward rounds in addition to undertaking a ward pharmacist visit make significantly more interventions per patient than those made by pharmacists undertaking a ward pharmacist visit alone, rectifying prescribing errors and optimising treatment.
Key Words: consultant ward rounds, pharmacists, prescribing errors
Introduction
In UK hospitals, ward pharmacy services are traditionally provided by pharmacists undertaking daily visits to their allocated ward(s).1 During these ward visits, pharmacists discuss medication-related issues and make recommendations to medical staff, nursing staff and patients as necessary; these contributions to patient care are typically termed ‘interventions’.
With this retrospective ward pharmacy service, there can be delays between prescriptions being written and pharmacists' intervention taking place.2 As many pharmacists' interventions are made to resolve prescribing errors or improve quality of care, it is important that interventions are made as soon as possible after prescribing, or preferably, at the point of prescribing.
Adverse drug events (ADEs) are a well known cause of mortality and morbidity. Most preventable ADEs originate in prescribing errors.3 It has been suggested that insufficient information about the medication or patient can contribute to such errors.4 If pharmacists attend ward rounds, detailed information and advice on medication can be provided at the point of prescribing, leading to a reduction in preventable ADEs.5
There are various US studies evaluating the impact of pharmacists attending ‘rounds’.4–7 However, there are many differences between UK and US healthcare systems1 leading to differences in the way that pharmacists practise medicine,8 and therefore studies of pharmacy services cannot be generalised between the USA and UK.8
Within the UK, there is evidence of the benefits of pharmacists attending post-take ward rounds,9–11 but less evidence relating to other types of ward round. One UK study12 found that having a pharmacist attending daily rounds led by the senior house officer (SHO) or registrar doubled the number of interventions made by the pharmacist. However, attending daily ward rounds is potentially very time consuming for the pharmacist. Another UK study retrospectively reviewed interventions made by four pharmacists on consultant-led ward rounds, focusing on clinically significant interventions.13 One significant intervention was recorded for every two patients. However, at the time of this study, pharmacists at the study hospital rarely saw patients on their usual ward visit. This differs to the current ward pharmacy service in most UK trusts, where ward pharmacists see their patients most days, limiting this study's generalisability. The aim of this study was therefore to explore the effects of including ward pharmacists on weekly consultant-led ward rounds in addition to providing their usual ward pharmacy visits. The number, nature and clinical importance of physician accepted interventions made by pharmacists attending consultant-led ward rounds in addition to providing a conventional ward pharmacy service were compared with those made by pharmacists providing a conventional ward pharmacy service alone.
Specific objectives were to compare the following:
number of interventions made per patient seen by a pharmacist at least once during their inpatient admission
type and clinical importance of interventions
grade of physician with whom the intervention was discussed.
Methods
Setting
The study took place in five inpatient medical wards at two teaching hospitals in one NHS trust. All wards were visited every weekday by a ward pharmacist, who reviewed medication prescribed on paper drug charts. A pharmacist provided the ward pharmacy service for all patients on a particular ward, irrespective of the physician team involved. During their ward visit, pharmacists assessed drug charts to ensure that prescribing was safe, effective, economical and legal; ensured sufficient supplies of all medication were available; provided advice, information and recommendations to healthcare professionals; established medication histories; performed therapeutic drug monitoring; and counselled patients on medication as required.
The study was approved by the Hammersmith and Queen Charlotte's and Chelsea Research Ethics Committee (reference 08/H0707/52).
Design
This was a prospective non-randomised controlled study. The control group comprised patients who were seen by pharmacists during conventional ward pharmacist visits, but pharmacists did not attend the doctors' ward rounds. The study group consisted of patients under the care of a physician who had a pharmacist routinely attending weekly consultant-led ward rounds, in addition to providing a conventional ward pharmacy service. Data collection for the control and study groups was undertaken simultaneously.
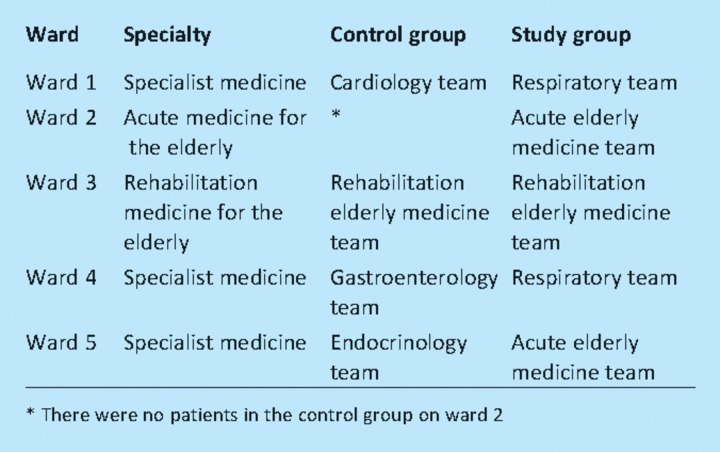
Physician teams in the control and study groups were chosen so that wherever possible, wards had patients in both the control and study groups (Table 1); this was to minimise any effect of ward, nursing staff or ward pharmacist. The only exception was Ward 2, which did not have patients in the control group. The primary outcome measure was the number of physician-accepted interventions made for each patient seen by a pharmacist at least once during their inpatient admission. Secondary outcome measures were the intervention acceptance rate, clinical importance of the interventions, types of intervention made, grade of physician with whom the interventions were discussed, and pharmacists' time required to undertake ward pharmacy visits and attend ward rounds.
Table 1.
Wards and specialty for the control and study groups.
Definitions
A pharmacist intervention was defined as ‘any verbal or written communication between a pharmacist and a physician, undertaken with the intention of influencing prescribing’.10 Reactive and proactive interventions were included in this definition, however, pharmacists' standard endorsements made on drug charts were excluded. A physician-accepted intervention was an intervention which was then acted upon by the physician.
A patient was considered to have been seen by a pharmacist if the pharmacist had seen and reviewed their drug chart at least once during their admission to a study ward. Each admission to a study ward during the data collection period was counted as a separate episode, if the pharmacist had seen and reviewed the drug chart at least once during each episode.
Data collection
Based on published data,13 it was anticipated that the mean number of physician-accepted interventions made per patient on a consultant-led ward round would be about 0.45, compared to approximately 0.045 on a ward pharmacist visit. Using these figures, 200 patients were needed for the study (100 in each group), with a probability of 80% that the study would detect a difference between the two groups at a 5% level of significance, based on a difference between the means of 0.4 standard deviations.14 This assumed that the distribution of interventions per patient would follow a normal distribution. Using the admissions and discharge book as a guide for the number of patients likely to be admitted to the wards included in this study, it was estimated that at least seven weeks of data collection would be needed to achieve this sample size. Therefore an eight-week data collection period was chosen.
Pharmacists in both control and study groups collected data on the interventions made and patients seen, using a standard data collection form. Pharmacists provided written consent to take part in the study and were briefed on the data to be collected. All the consultants whose teams would be involved were informed of the study.
Data analysis
Any patient on a study ward who was under the care of a physician team other than those included in the study (‘outliers’) were excluded from analysis.
Each physician-accepted intervention was classified according to type by the investigator and a senior pharmacist, who were blinded as to whether each intervention was from the control or study group. Any intervention that was categorised differently by the two pharmacists was then classified by a third pharmacist to obtain agreement. Interventions were also classified according to grade of physician, based on the grades in use at the time of the study.
Clinical importance of the interventions was assessed using a method developed and validated to assess the severity of medication errors,15 and subsequently adapted to evaluate pharmacists' interventions.16 A panel of healthcare professionals made up of two physicians, two nurses and two pharmacists individually scored each physician-accepted intervention, in terms of potential clinical importance to the patient. This was undertaken on a scale of zero to 10, where zero meant that no potential harm would have occurred to the patient had the intervention not been made, and 10 represented potential death to the patient if the intervention had not been made.
Differences between control and intervention groups were assessed using appropriate statistical tests for the type of data, with the level of significance set as p≤0.05.
Results
Data were collected for eight weeks beginning 22 October 2007. Two days data were missing. Sixteen pharmacists undertook between one and 38 ward pharmacist visits and/or consultant-led ward rounds (mean 12.6, standard deviation 11.2) during the study period. Pharmacist grade ranged from band 6 (newly qualified) to band 8b (senior lead pharmacist). Band 6 pharmacists undertook 100 ward pharmacist visits and/or consultant-led ward rounds, band 7 pharmacists conducted 60 visits, band 8a pharmacists made 40 visits and there was one visit by an 8b pharmacist. Over the course of the study, 524 patients were seen at least once during their admission by a pharmacist. Of these, 20 (4%) were excluded as they were outliers. Of the 504 remaining patients, 186 (37%) were in the control group and 318 (63%) in the study group. A total of 38 consultant-led ward rounds were attended by a pharmacist during the study period (range 5–8 for each consultant). On these ward rounds 232 patients were seen and 433 patient consultations carried out (mean number of patient consultations per ward round was 11.4, standard deviation 1.25).
Pharmacists recorded 807 interventions. Of these, 165 (89.7%) were accepted by a physician for the control group, compared to 551 (88.4%) for the study group (p=0.643; χ2 test). For the control group 0.89 physician-accepted interventions were made per patient seen by a pharmacist at least once during their inpatient admission, compared to 1.73 for the study group (p≤0.001; Mann Whitney U test).
Table 2 presents the grade of medical staff with whom the intervention was made. The difference between the groups was statistically significant (p<0.001; χ2 test). For the control group, all the physician-accepted interventions were made during a ward pharmacist visit. In comparison, only 260 (47.2%) of physician-accepted interventions for the study group were made during a ward pharmacist visit and 291 (52.8%) during the ward round.
Table 2.
The grade of medical staff with whom the accepted interventions were discussed.
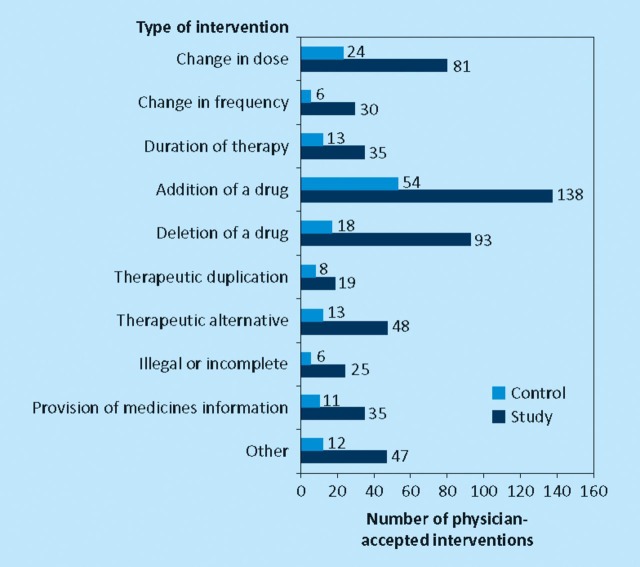
For both groups, the three most frequent types of physician-accepted interventions were addition of a drug, deletion of a drug and change in dose (Fig 1). There was no difference between the two groups (p=0.705; χ2 test).
Fig 1.
Types of interventions made.
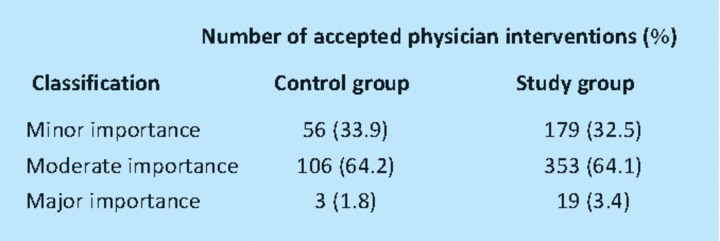
For the control group, the mean clinical importance of the physician-accepted interventions was 4.5 (standard deviation 1.38), compared to 4.5 (standard deviation 1.33) for the study group. The difference between the groups was not statistically significant (unpaired t-test, p=0.992). For both groups, 64% of physician-accepted interventions were of moderate importance (Table 3). The mean duration of a ward pharmacist visit was 68 minutes (standard deviation 40 minutes). The mean duration of a consultant-led ward round was 115 minutes (standard deviation 39 minutes). During a ward pharmacist visit, one physician-accepted intervention was made every 63 minutes compared to one every eight minutes during a consultant-led ward round with a pharmacist in attendance.
Table 3.
Classification of clinical importance of the physician accepted interventions.
Discussion
Pharmacists attending consultant-led ward rounds in addition to undertaking ward pharmacist visits made significantly more physician-accepted interventions per patient, compared to pharmacists who only undertook ward pharmacist visits. Although the number of physician-accepted interventions in the study group doubled compared to the control group, there was no significant change in the nature or clinical importance of the interventions.
The proportion of interventions that were accepted by a physician was similar in control and study groups. The high percentage of interventions that were accepted by a physician showed that the vast majority were appropriate. The acceptance rate was similar to that reported in the USA,17 and higher than a UK study,18 where 83% of interventions made by a pharmacist were accepted, with the prescription altered in 75% of cases.
The most senior member of the physician team, the consultant, was involved in the greatest number of discussions about interventions for the study group, even though they were present on the ward for only a few hours each week. For the control group, the SHO was the physician most commonly involved, probably because they were the most senior member of the team regularly present on the ward. These results suggest that pharmacists discuss interventions with the most senior member of the team available on the ward at the time of making the intervention.
Implications for practice
Based on the results of this study, pharmacists should attend weekly consultant-led ward rounds for medical specialties in addition to undertaking their usual ward pharmacist visits. Further research is needed to evaluate the benefit of pharmacists attending consultant-led ward rounds for other specialties.
Interventions made by pharmacists during consultant-led ward rounds reduces the amount of time that pharmacists spend during ward pharmacist visits on subsequently days. However, further work is needed to assess the overall time implications. Attending consultant-led ward rounds should complement the activities undertaken during ward pharmacist visits rather than replace ward pharmacist visits, as other tasks are carried out in addition to making interventions.
Strengths of the study
This is the first UK study to have assessed the contribution of pharmacists on consultant-led ward rounds in a range of medical specialties. Data were collected simultaneously for the control and study groups, minimising any effect that differences in nursing staff, physicians or pharmacists may have had on the results. All but one ward had patients in both control and study groups.
The data collection period was chosen so that the study would encompass a range of physicians, allowing for greater generalisability. The consultants in charge of some physician teams rotate on a regular basis, so the data collection period encompassed two consultants per specialty where the consultants rotated on a three-monthly basis and three consultants for a specialty where the consultants rotated monthly. In addition, the foundation year 1 (FY1) and SHOs also changed specialties during the data collection period.
Limitations
Ward 2 did not have any patients in the control group. However, a sub-analysis excluding this ward enhanced rather than diminished the difference between the control and study groups.
Neither patients nor consultant teams could be randomised to control and study groups. Patients are assigned to the most appropriate specialty based upon their presenting complaint and likely diagnosis. Different physicians may make different prescribing errors. However, previous work suggests that there are no obvious differences in prescribing error rates between clinical specialties19 and it is believed that this is unlikely to have affected the study's conclusions.
Using the rate of physician-accepted interventions to assess the effect of pharmacists on consultant-led ward rounds has a number of limitations. Firstly, pharmacists may not have documented all interventions made. To maximise documentation of interventions by pharmacists, training sessions were held prior to the start of data collection and pharmacists were frequently reminded to document interventions. Secondly, pharmacists may vary in their approach towards making interventions, with some making more than others.20 Thirdly, many interventions are made after a prescribing error has been made or if a patient's therapy has not already been optimised, meaning that a low intervention rate may be due to good prescribing rather than a poor pharmacist. Future studies could explore the feasibility of reviewing all drug charts to assess the number of interventions that should have been made but were not. A much larger study would be needed to assess any impact on ADE.
This study only included interventions made by the pharmacists during their daily ward visit. Any interventions made by pharmacists outside the daily visits, both within and outside normal working hours, were excluded. Although this is a possible limitation, this applied equally to control and study groups.
Conclusion
Pharmacists attending consultant-led ward rounds in addition to undertaking ward pharmacist visits make significantly more interventions per patient. These are of a similar nature and clinical importance to those made by pharmacists undertaking ward pharmacist visits alone. On average, one physician-accepted intervention was made every eight minutes during a consultant-led ward round, compared to one every 63 minutes during a ward pharmacist visit. Medical consultants and pharmacy departments should work together to include pharmacists on their multidisciplinary ward rounds.
Acknowledgements
The Centre for Medication Safety and Service Quality is affiliated with the Centre for Patient Safety and Service Quality at Imperial College Healthcare NHS Trust which is funded by the National Institute of Health Research. This work was carried out in part fulfilment of the requirements for the Master of Science Degree in Pharmacy Practice, University of London.
References
- 1.Stebbing C, Jacklin A, Barber N, Bates D. A comparison of the US and UK inpatient medication systems. Eur J Hosp Pharm Practice. 2006;12:36–40. [Google Scholar]
- 2.Farrar K, Stoddart M, Slee A. Clinical pharmacy and reactive prescription review–time for a change? Pharm J. 1998;260:759–61. [Google Scholar]
- 3.Bates D, Cullen D, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995;274:29–34. doi: 10.1001/jama.1995.03530010043033. [DOI] [PubMed] [Google Scholar]
- 4.Kucukarslan S, Peters M, Mlynarek M, Nafziger D. Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units. Arch Intern Med. 2003;163:2014–8. doi: 10.1001/archinte.163.17.2014. [DOI] [PubMed] [Google Scholar]
- 5.Leape L, Cullen D, Clapp M, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;281:267–70. doi: 10.1001/jama.282.3.267. [DOI] [PubMed] [Google Scholar]
- 6.Scarsi K, Fotis M, Noskin G. Pharmacists participation in medical rounds reduces medication errors. Am J Health-Syst Pharm. 2002;59:2089–92. doi: 10.1093/ajhp/59.21.2089. [DOI] [PubMed] [Google Scholar]
- 7.Rollins G. Having pharmacists participate in medical rounds reduces medication errors. Rep Med Guidel Outcomes Res. 2002;13:9–10, 12. [PubMed] [Google Scholar]
- 8.Brock T, Franklin BD. Differences in pharmacy terminology and practice between the United Kingdom and the United States. Am J Health Syst Pharm. 2007;64:1541–6. doi: 10.2146/ajhp060444. [DOI] [PubMed] [Google Scholar]
- 9.Bednall R, McRobbie D, Russell S, West T. A prospective evaluation of pharmacy contributions to post-take ward rounds. Pharm J. 2003;271:22–3. [Google Scholar]
- 10.Brady D, Franklin BD. An evaluation of the contribution of the medical admissions pharmacist at a London teaching hospital. Int J Pharm Pract. 2004;12:1–6. doi: 10.1211/0022357023213. [DOI] [Google Scholar]
- 11.Fertleman M, Barnett N, Patel T. Improving medication management for patients: the effect of a pharmacist on post-admission ward rounds. Qual Saf Health Care. 2005;14:207–11. doi: 10.1136/qshc.2004.011759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boorman S, Cairns C. Another way forward for pharmaceutical care: a team-based clinical pharmacy service. Pharm J. 2000;264:343–6. [Google Scholar]
- 13.Gibson P, Freeborn S. Are pharmacists effective on clinical rounds? Pharm J. 1985;234:201–2. [Google Scholar]
- 14.Schoenfeld D. Statistical considerations for a parallel trial where the outcome is a measurement. 1995. http://hedwig.mgh.harvard.edu/sample_size/size.html.
- 15.Dean B, Barber N. A validated, reliable method of scoring the severity of medication errors. Am J Health-Syst Pharm. 1999;56:57–62. doi: 10.1093/ajhp/56.1.57. [DOI] [PubMed] [Google Scholar]
- 16.Kollo A, Dean B. The development of a method to assess the severity of prescribing errors and the effect of related pharmacists' interventions. 2000. Abstract presented at the Sixth Heath Services Research and Pharmacy Practice Conference, Aberdeen.
- 17.Kallail J, Stanton S. Pharmacy-physician communications: potential to reduce medication errors. J Am Pharm Assoc. 2006;46:618–20. doi: 10.1331/1544-3191.46.5.618.Kallail. [DOI] [PubMed] [Google Scholar]
- 18.Hawkey S, Hodgson S, Norman A, Daneshmend T, Garner S. Effect of reactive pharmacy interventions on quality of hospital prescribing. BMJ. 1990;300:989–90. doi: 10.1136/bmj.300.6730.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franklin BD, O'Grady K, Paschalides C, et al. Providing feedback to hospital doctors about prescribing errors; a pilot study. Pharm World Sci. 2007;29:213–20. doi: 10.1007/s11096-006-9075-x. [DOI] [PubMed] [Google Scholar]
- 20.Barber N, Batty R, Ridout D. Predicting the rate of physician-accepted interventions by hospital pharmacists in the United Kingdom. Am J Health-Syst Pharm. 1997;54:397–405. doi: 10.1093/ajhp/54.4.397. [DOI] [PubMed] [Google Scholar]