Abstract
Patient safety incidents involving insulin are frequent and cause considerable distress to people with diabetes and anxieties to their families and carers. This article describes an analysis of the National Reporting and Learning System database of patient safety incidents concerning insulin reported from NHS providers in England and Wales over six years. The main causes are discussed and the ongoing developments by the National Patient Safety Agency and partner organisations to reduce insulin errors are described.
Key Words: insulin, medication error, patient safety, prescribing error
Introduction
Serious patient safety incidents involving insulin frequently receive media attention and insulin errors are among the top high alert medicines worldwide.1–3 Hypoglycaemia resulting from insulin is an important cause of hospital admissions and is common in hospitalised patients.4 The National Patient Safety Agency (NPSA) is a special health authority working in the NHS in England and Wales and operates the National Reporting and Learning System (NRLS) which receives patient safety incidents reported from NHS organisations in all healthcare sectors. The NPSA reviews these incidents, identifies important risks to patients and issues guidance to the NHS to alert the service to these risks with recommended actions to minimise recurrence.
Method
All medication incident reports which included the term ‘insulin’ or the proprietary names of insulin products received between November 2003 and November 2009 were searched. Quantitative and qualitative methods were used to analyse the incident data.
Results
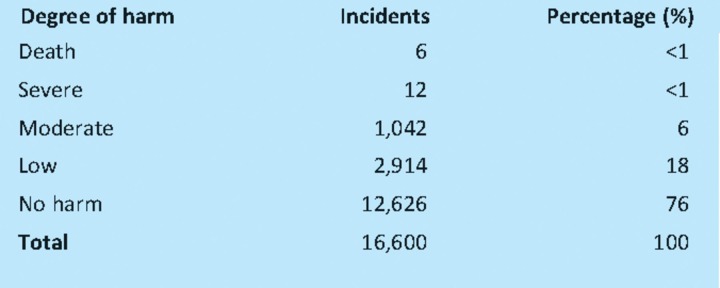
A total of 16,600 incidents involving insulin were identified and 24% reported harm to the patient. There were 18 incidents with fatal and severe outcomes and 1,042 incidents of moderate harm (Table 1).
Table 1.
The degree of harm from incidents involving insulin.
Incidents were reported at all stages of the medication process. The majority (61%) occurred during insulin administration with a further 17% caused by prescribing errors and 10% at dispensingn.
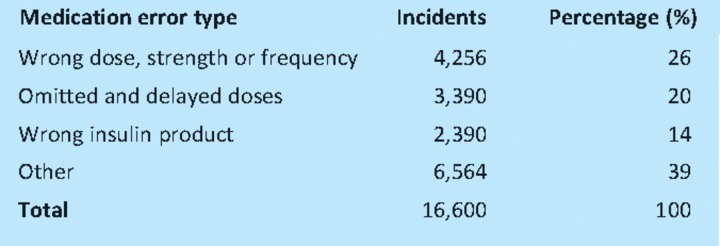
Qualitative analysis showed that the top three medication error types were wrong dose, omitted or delayed insulin, and wrong insulin product which together accounted for 60% of all incidents reported (Table 2).
Table 2.
The medication error type involving incidents with insulin.
The main causes of wrong dose incidents were:
incorrect prescription on admission
abbreviation of ‘units’. With poor handwriting the abbreviations ‘U’ ‘IU’ may be read as ‘0’ or 10' and lead to unintended 10 times and 100 times dose errors
incorrect monitoring of blood glucose and dose adjustment of insulin
poor documentation of dose administration on inpatient medicine charts
duplicate dose administration
errors in calculation of insulin doses for intravenous infusion
incorrect programming of electronic infusion devices.
Omitted and delayed insulin resulted from:
insulin not prescribed on hospital admission and transfer
failure to prescribe or supply the correct insulin preparation, vial, cartridge or pen
prescribed insulin product not available in the clinical area or following discharge
confusion over insulin dose administration when patients were ‘nil by mouth’
insulin dose not administered at an appropriate time to the administration of food (including enteral feeding).
Selection of the incorrect insulin product was described in 2,201 incidents (14% of the total) due in large part to the ‘look alike’ and ‘sound alike’ proprietary names, for example:
Novorapid and Novomix
Humalog and Humalog Mix
Humulin S, I and M3
Humalog and Humulin
Glulisine and Glargine
Lantus and Lente
Hypurine – neutral, isophane, lente
Discussion
Insulin is used by many thousands of patients in the UK and increasingly by those with type 2 diabetes to achieve recommended glycaemic control. Nearly all type 1 patients experience episodes of hypoglycaemia although only a small proportion will require assistance or need hospitalisation. Patients and their carers require careful training in the management and monitoring of their insulin therapy. The safety incidents reported here are almost entirely from acute secondary care and represent only a small fraction of the actual incidents that occur.
Despite considerable under reporting the NRLS database has received information on a large number of incidents described, nearly all of which took place in hospital under direct medical supervision. Similar findings have been reported elsewhere. Analysis of medication error reports to the US Pharmacopoeia MEDMARX reporting programme over a two-year period identified a total of 4,764 insulin errors with approximately 6.6% (n=320) of these causing harm to the patient.5 Omission errors (leading to hyperglycaemia) and improper dose/quantity (leading to hyper- or hypoglycaemia) were the two most frequently reported types of error associated with insulin in US hospitals.5 In a further study reviewing prescribing errors in a hospital in the USA, 9.2% of the errors involved insulin. Of the fatal and severe incidents 57% involved prescriptions for insulin.6
The risks are largely preventable but require action by several organisations. These include regulators, responsible for packaging and ‘look alike’ names of insulin products, as well as the healthcare providers who need to ensure a safer hospital environment for all people with diabetes including safer prescribing and use of insulin.
A number of organisations in the UK have developed initiatives to improve the safer use of insulin. In Northern Ireland, the Medicine Governance Team and the Clinical Resources Efficiency Support Team (CREST) have issued guidance on the safe use of insulin in hospitals.7–8 The NHS Institute For Innovation and Improvement has launched the ‘Think glucose programme’.9 This identified that the provision of consistent, effective and proactive inpatient care for people with diabetes is often inadequate, leaving patients with a poor experience in terms of their diabetes treatment.
National data confirm that, on average, a patient with diabetes spends longer in hospital than a patient without diabetes for the same procedure or condition. The programme provides a package of tried and tested products to support learning and improve awareness in the treatment of patients in whom diabetes is a secondary diagnosis. Implementing a clinical pathway will improve the patient experience and the quality of care, thereby reducing the length of stay.9 NHS Diabetes carried out a national inpatient audit in September 2009 and plan future audits.10 It has also published guidance on the safe and effective use of insulin in hospitalised patients.11 The National Patient Safety Campaigns in England and Wales have included the safe use of insulin in the programmes to make the use of high alert medicines safer.12–13 There are a range of other resources on the safe use of insulin on the NHS Diabetes website.
What the NPSA will do
The NPSA is working with the above programmes in 2010–11 to make the use of insulin safer. The agency has issued a Rapid response report on reducing harm from omitted and delayed medicines in hospitals – insulin was identified as a critical medicine in this report.14
A rapid response report recommending safer administration of insulin recommends that the term ‘unit’ is used to describe the dose and strength of insulin therapy is never abbreviated in practice, and that intravenous syringes are never used to measure and administer insulin doses as both these practices are error prone.15 The guidance will also emphasise the importance of clinical staff receiving adequate training on the safe measurement and administration of insulin products. NHS Diabetes is planning to issue an e-learning package on this topic. Longer term, the NPSA is preparing support materials to assist the NHS design inpatient insulin charts that, in practice, will be safer.
The agency is also working with patient groups and health professionals to develop a design for patient-held information that will better inform patients, carers and healthcare professionals about the insulin products that are being used by individual patients and to reduce errors in practice. The NPSA has published guidance on the design of the dispensing environment to reduce errors.16 The guidance recommends the use of barcode technology to minimise dispensing errors and the NPSA continues to promote the use of technology to help with distribution.
Finally, the NPSA intends to work with pharmaceutical manufacturers and regulators to introduce design changes to insulin products that will improve the safe use of these products in practice. The NPSA has already published a design for a patient safety guide to the labelling and packaging of injectable medicines.17
References
- 1. Institute of Safe Medication Practice (USA). List of high alert medicines 2008. www.ismp.org/Tools/highalertmedications.pdf.
- 2.National Patient Safety Agency . Safety in doses. London: NPSA; 2009. www.nrls.npsa.nhs.uk/resources/patient-safety-topics/medication-safety/?entryid45=61625&q=0%c2%acsafety+in+doses%c2%ac. [Google Scholar]
- 3.Cox AR, Ferner RE. Prescribing errors in diabetes. Brit J Diabet Vasc Dis. 2009;9:84–8. doi: 10.1177/1474651409103902. [DOI] [Google Scholar]
- 4.Van der Hooft CS, Dieleman JP, Siemes C, et al. Adverse drug reaction related hospitalisation: a population based cohort study. Pharmacoepidemiol Drug Saf. 2008;17:365–71. doi: 10.1002/pds.1565. [DOI] [PubMed] [Google Scholar]
- 5.United States Pharmacopoeia . Insulin errors a common problem. Patient Safety CAPS link. Section 1. USP medication analysis. Rockville MD: USP; 2003. www.usp.org/pdf/EN/patientSafety/capsLink2003-07-01.pdf. [Google Scholar]
- 6.Lesar LS. Prescribing errors involving medication dosage forms. J Gen Intern Med. 2002;17:579–87. doi: 10.1046/j.1525-1497.2002.11056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Northern Ireland Medicines Governance team Recommendations to improve the safe use of insulin in secondary care in Northern Ireland 2005 www.medicinesgovernanceteam.hscni.net/Recommendations/Safe%20use%20of%20insulin%20in%20Secondary%20Care.pdf.
- 8.Clinical Resources Efficiency Support Team Safe and effective use of insulin in secondary care 2006. www.gain-ni.org/guidelines/insulin.pdf.
- 9.NHS Institute for Innovation and Improvement Think glucose. www.institute.nhs.uk/quality_and_value/think_glucose/welcome_to_the_website_for_thinkglucose.html.
- 10.NHS Diabetes Inpatient audit. www.diabetes.nhs.uk/our_work_areas/inpatient_care/inpatient_audit/
- 11.NHS Diabetes The safe use of insulin. www.diabetes.nhs.uk/safe_use_of_insulin.
- 12.Patient Safety First Campaign High risk medicines 2008. www.patientsafetyfirst.nhs.uk/Content.aspx?path=/interventions/High-riskmedication/
- 13.Patient Safety First Campaign 1000 lives campaign. Improving medicines management 2008. www.patientsafetyfirst.nhs.uk/Content.aspx?path=/interventions/High-riskmedication/
- 14.National Patient Safety Agency Rapid response report on reducing harm from omitted and delayed medicines in hospitals 2010. www.nrls.npsa.nhs.uk/resources/?entryid45=66720.
- 15.National Patient Safety Agency Rapid response report on safer administration of insulin 2010. www.nrls.nhs.uk/alerts/?entryid45=74287. [DOI] [PubMed]
- 16.National Patient Safety Agency Design for patient safety. The dispensing environment 2007. www.nrls.npsa.nhs.uk/resources/?EntryId45=59830.
- 17.National Patient Safety Agency Design for patient safety. The labelling and packaging of injectable medicines 2008. www.nrls.npsa.nhs.uk/resources/?entryid45=59831.