Key Points
Prevention
Clostridium difficile infection (CDI) can be effectively reduced through a combination of prudent antimicrobial prescribing and infection control measures, including environmental decontamination, hand hygiene, isolation and use of personal protective equipment
Hand washing with soap and water is more effective than use of alcohol gel
Treatment
Oral vancomycin is the treatment of choice for severe CDI
Early surgical review is indicated for patients with fulminant colitis and those worsening on medical therapy
Treatment of recurrent CDI remains challenging
Clostridium difficile, an anaerobic spore-forming organism, is the leading cause of infective diarrhoea in hospitals1,2 and is responsible for a spectrum of illnesses ranging from mild diarrhoea to life-threatening pseudomembranous colitis and toxic megacolon (Fig 1). The severity of C. difficile infection (CDI) has increased since the emergence of epidemic strain BI/NAP1/027, initially in North America and subsequently in many European countries, including the UK where high-profile outbreaks have been reported.3 Apart from the associated mortality, CDI places a substantial burden on NHS resources, with an estimated increase in length of hospital stay of 21 days and an additional cost of $4,000 per case.4 These data were obtained in 1995 and current costs may be significantly higher.
Fig 1.
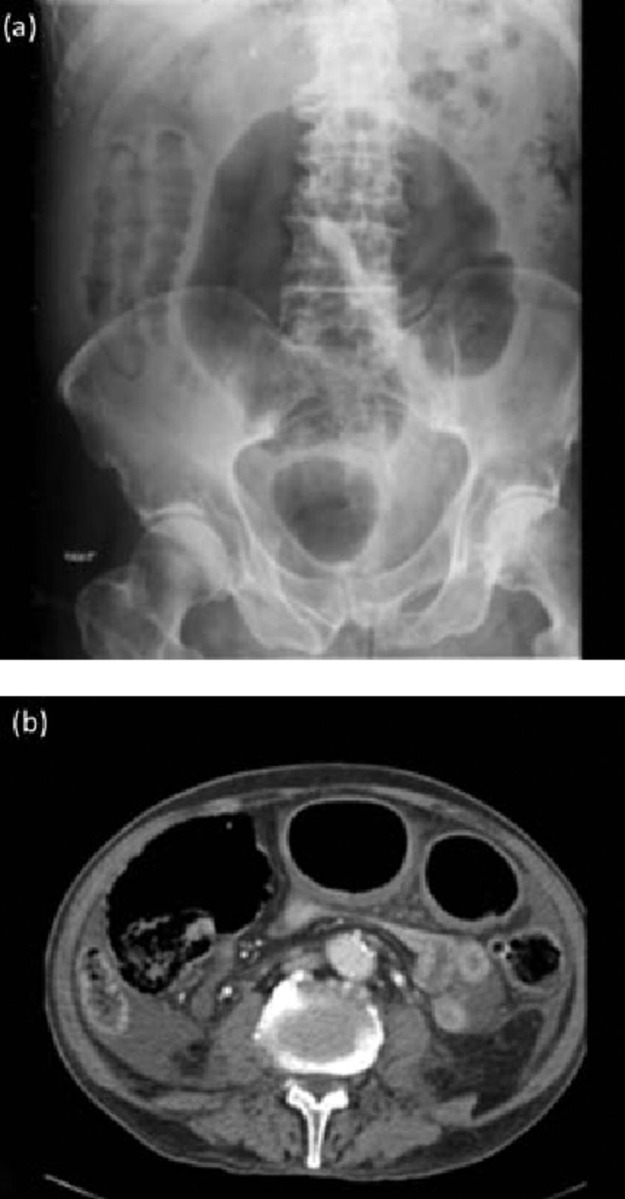
Toxic megacolon in a patient with Clostridium difficile infection: dilated loops of large bowel seen in (a) plain abdominal X-ray; (b) computed tomography scan.
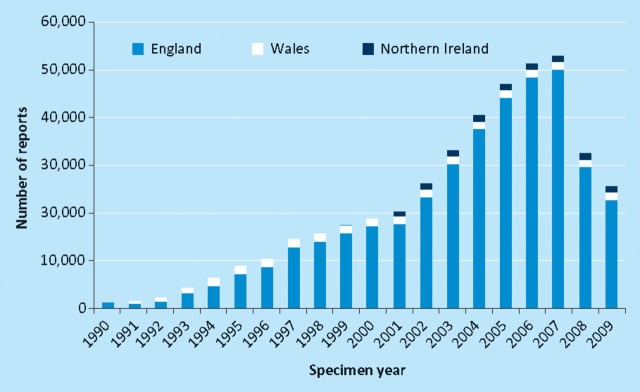
Health Protection Agency figures for England, Wales and Northern Ireland show a marked increase in reported cases over the last decade, peaking at approximately 57,000 cases per year in 2007. The widespread adoption of best practice for the prevention and management of CDI has led to an impressive reduction of CDI incidence to 28,000 cases per year for 2009 (Fig 2).5 The most commonly affected patients remain older people, with the highest rates observed in those aged over 75 years.5
Fig 2.
Health Protection Agency voluntary surveillance of Clostridium difficil e-positive laboratory reports for England, Wales and Northern Ireland (included since 2001). Reproduced with permission from the Health Protection Agency.5
Transmission and pathogenesis
C. difficile spores are excreted in large numbers from patients with diarrhoea and contaminate the environment in which they can survive for months or years. Spore ingestion can lead to asymptomatic colonisation, where the combined effect of normal gut microbiota and host immunity (particularly high antitoxin A antibody levels) protects against disease development. In susceptible people, particularly elderly individuals colonised with C. difficile, exposure to antibiotics leads to disruption of the normal protective intestinal flora, promoting the elaboration of toxins A and B1,3 and subsequently diarrhoea.
Prevention
Existing CDI prevention strategies can successfully prevent both colonisation and disease in individuals already colonised.6 In the UK, the Saving Lives campaign launched High Impact Intervention No. 7 in 2007, with the aim of reducing CDI rates through a care bundle approach. This was based on prudent antimicrobial prescribing, hand hygiene, isolation or cohort nursing, environmental decontamination and use of personal protective equipment.4 These measures are reiterated in UK,3 US7 and European guidelines.8 The evidence for each measure independently is presented below.
Antimicrobial stewardship
Prior antimicrobial use is the most important risk factor for CDI. It has been difficult to produce a ‘hit list’ of individual antimicrobials causing CDI based on risk, as published studies have methodological limitations and their conclusions are not necessarily applicable across institutions.9 Almost any antibiotic can predispose to CDI, but broad-spectrum cephalosporins and clindamycin are generally accepted as high-risk agents, with quinolones implicated in the recent BI/NAP1/027 strain outbreaks. Observational studies substituting other agents for broad- spectrum cephalosporins or clindamycin have resulted in reduction of CDI rates in endemic and outbreak situations.3,7,8 The use of narrow-spectrum agents for as short a duration as possible is encouraged.3,7
Hand hygiene and protective equipment
Many studies have shown that C. difficile is carried and transmitted via the hands of healthcare workers (HCWs).3,7,8 No antiseptic agent has reliable sporicidal activity. Water and soap are more effective than alcohol gel at decontaminating hands through the physical action of rubbing and rinsing.10 Existing guidelines recommend hand washing with soap and water before and after contact with patients infected with C. difficile.3
Glove use has been shown to significantly reduce CDI but does not completely prevent hand contamination.3 Current guidelines recommend the use of both gloves and gowns when dealing with patients with CDI.3
Isolation/cohort nursing
Prompt isolation of patients with infectious diarrhoea is an established infection control intervention. Guidance recommends patient isolation in a single room with toilet facility or dedicated commode as soon as CDI is suspected. Confirmed cases should remain isolated until there has been no diarrhoea (types 5–7 on the Bristol Stool Chart) for 48 hours and formed stool (Bristol types 1–4) has been passed.3
Isolation measures have been effective in controlling outbreaks of CDI as part of broader infection control interventions. Nevertheless, no randomised trials or systematic reviews of existing studies have assessed the value of isolation in controlling CDI. However, a systematic review of studies assessing the impact of isolation on methicillin-resistant Staphylococcus aureus (MRSA) colonisation and infection rates found such measures effective at controlling MRSA within the context of wider control measures.11 This has been quoted as indirect evidence for the value of isolation in CDI.3
Although current guidelines allow the use of cohort bays or dedicated wards, this should be in situations only when side room capacity is filled. The use of C. difficile isolation wards has increased in recent years in the UK following their successful use in outbreaks.3,8 Isolation wards offer a number of potential advantages, for example:
limiting environmental contamination to one part of the hospital
use of dedicated nursing staff
promotion of hand washing facilities as opposed to alcohol gels
optimisation of care by facilitating clinical review and management by dedicated multidisciplinary teams.
Environmental cleaning
Environmental sampling of areas around patients with CDI has revealed high levels of contamination, particularly on the floors, bedrails, commodes, fomites and medical equipment.8 Increasing levels of contamination correlate with higher levels of C. difficile carriage on the hands of HCWs. The spores are resistant to conventional cleaning agents (eg detergents), hence sporicidal agents, such as sodium hypochlorite (bleach) or hydrogen peroxide vapour, are recommended to ensure effective decontamination. Cleaning with hypochlorite at a concentration of at least 1,000 ppm has been shown to be effective in decreasing rates of CDI in several studies.3,7,8
UK guidance emphasises the need for at least daily cleaning of rooms hosting patients with CDI.3 Hydrogen peroxide vapour is an effective agent but its use is limited by cost and the requirement to vacate and seal the unit during cleaning. It may have a role in terminal disinfection of rooms.3
Decontamination of medical equipment is also important as outbreaks have been linked to instruments, such as rectal thermometers. Use of disposable or dedicated equipment is recommended, particularly in outbreak situations.3,7,8
Other measures
An increased incidence or outbreaks of CDI may indicate lapses in infection control practices. The use of real-time local surveillance of new cases and assessment of severity of illness will enable prompt recognition of potential outbreaks. Polymerase chain reaction ribotyping of C. difficile isolates and, more recently, enhanced fingerprinting to discriminate between similar ribotypes are valuable molecular tools in the investigation of potential outbreaks.
Rapid and accurate diagnostic tools are essential for the correct and timely institution of preventive measures. The currently available rapid tests for toxin detection, based on commercial immunoassay kits, have suboptimal sensitivity and specificity compared with the ‘gold-standard’ (but slower) cytotoxin assays.12 Research for new diagnostic methodologies is being actively pursued.
Treatment
Supportive measures, including rehydration, electrolyte correction and nutrition, are essential in the management of CDI. If deemed safe, discontinuation of offending antibiotics or substitution with lower risk agents is strongly encouraged. Antiperistaltic agents should be avoided due to the risk of precipitating toxic megacolon.
A treatment algorithm is presented in Fig 3.
Fig 3.
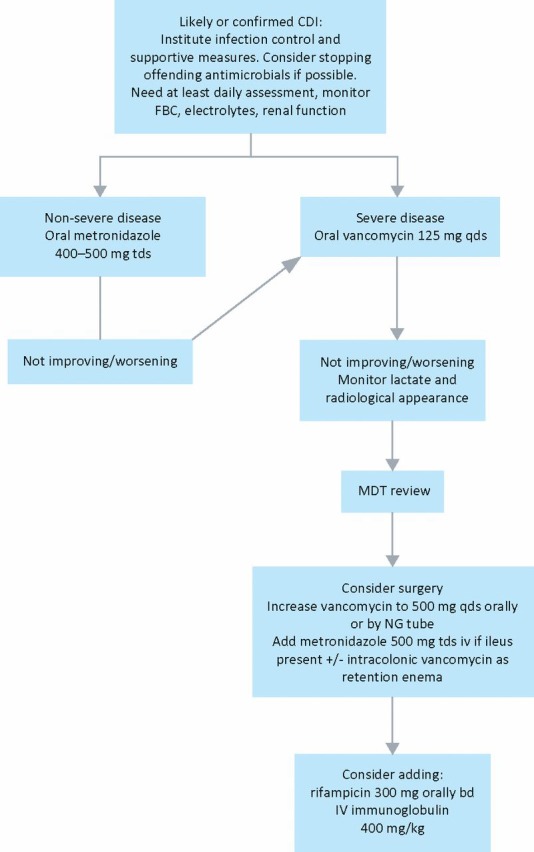
Suggested treatment algorithm based on Department of Health and Health Protection Agency guidelines.3 bd = 12-hourly; CDI = Clostridium difficile infection; FBC = full blood count; iv = intravenous; MDT = multidisciplinary team (microbiologists/infectious diseases specialists, gastroenterologists, colorectal surgeons); NG = nasogastric; qds = 6-hourly; tds = 8-hourly. (Severe disease: see text for definition.)
Metronidazole or vancomycin
The standard treatment of CDI is oral metronidazole 400–500 mg 8-hourly or oral vancomycin 125 mg 6-hourly for 10–14 days.3,7 In a recent randomised controlled trial (RCT), vancomycin treatment was associated with improved cure rates compared with metronidazole in severe cases of CDI. Both agents were equally effective in mild disease.13 UK guidelines recommend vancomycin in severe cases, as defined by any of the following:
temperature above 38.5 °C
an acute rise in serum creatinine (>50% from baseline)
leukocyte count above 15 × 109/l
evidence of severe colitis.3
However, a prospectively derived and validated severity score is lacking and definitions of severity are not uniform.7,14 Metronidazole is still recommended for mild to moderate cases, based on lower cost and the risk of selecting for vancomycin-resistant enterococci with vancomycin use.3,7
Management of infected patients
Infected patients should undergo daily review. In refractory or worsening cases, a multidisciplinary approach involving infection specialists, gastroenterologists and surgeons should be used. Recommendations suggest increasing the dose of vancomycin up to 500 mg 6-hourly and considering the addition of oral rifampicin or intravenous immunoglobulin (ivIg).3 Evidence is lacking for the effectiveness of these interventions. In the presence of ileus, iv metronidazole should be added and vancomycin may be administered as retention enemas to ensure effective delivery of these agents intraluminally.3,7,15 There is no role for iv vancomycin in the treatment of CDI.
Urgent surgical evaluation is indicated in patients with radiological signs of toxic megacolon, perforation or colonic wall thickening as subtotal colectomy can be life saving in selected patients with fulminant colitis.3 A retrospective analysis suggested the highest benefit is derived in immunocompetent patients, those aged over 65 years and those with a leukocytosis of 20 × 109/l or above and a serum lactate level of 2.2–4.9 mmol/l. A leukocyte count over 50 × 109/l and especially a lactate level of 5 mmol/l or higher are associated with very poor prognosis.16
Recurrent infection
The risk of recurrence is estimated at 20%.3 Risk factors for recurrence include:
new antimicrobial exposure
older age
duration of hospitalisation
low serum albumin concentration
low levels of antitoxin A antibody
previous recurrence.
First recurrence
Treatment with the agent used for the initial episode is recommended for a first recurrence (following appropriate severity stratification–as shown in figure 3).3,7,17
Second recurrence
Treatment of a second or further recurrent episodes is challenging. Oral vancomycin is the agent of choice. Both pulsed and tapering regimens have been described.3,7,14,17 Large epidemiological studies have linked the use of acid suppression therapy with CDI.18 Discontinuation of this therapy can be considered in recurrent infections.3
Immunotherapy
Immunotherapy and biotherapy are two non-antimicrobial strategies for the treatment of recurrent CDI, aimed at boosting immunity and increasing colonisation resistance, respectively.
Passive immunotherapy using pooled ivIg at a dose of 400 mg/kg has been beneficial in uncontrolled studies and is recommended in guidelines, but strong evidence to support its use is lacking.3 A recent phase 2 RCT evaluating the use of monoclonal antibodies against toxins A and B as adjunctive therapy for the treatment of CDI resulted in decreased recurrence rates compared with placebo (from 25% to 7%). The benefit persisted in the subgroup of patients with previous recurrent infection.19 Active immunisation with a toxoid vaccine is undergoing phase 2 trials.
Faecal transplantation
A number of small uncontrolled studies have reported successful treatment in 90% of cases with recurrent CDI using faecal transplantation either via enema or nasogastric tube.17 An RCT is underway to assess this practice formally.
Probiotic therapy
A Cochrane review evaluated the effect of probiotic preparations in the treatment of CDI and concluded that current evidence is insufficient to recommend their routine use.20 An RCT reported that the use of a proprietary yoghurt containing three different gut organisms was effective in the primary prevention of CDI, but the study design has been extensively criticised.3,7
The future
Newer agents for the treatment of CDI are being evaluated and are reviewed elsewhere.3,14,17
Conflicts of interest
NMB has received lecture honoraria, consultancy fees or sponsorship to attend conferences from a number of pharmaceutical companies, including Gilead Sciences, Astra Zeneca, Pfizer, Janssen Cilag, Merck Sharp & Dohme and Wyeth. SHA has received sponsorship to attend conferences from Gilead Sciences, Schering Plough and Wyeth. TG has no conflicts of interest to declare.
References
- 1.Shannon-Lowe J, Matheson NJ, Cooke FJ, Aliyu SH. Prevention and medical management of Clostridium difficile infection. BMJ. 2010;340:641–6. doi: 10.1136/bmj.c1296. [DOI] [PubMed] [Google Scholar]
- 2.Kelly CP, LaMont JT. Clostridium difficile – more difficult than ever. N Engl J Med. 2008;359:1932–40. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 3.Department of Health and Health Protection Agency . Clostridium difficile infection: how to deal with the problem. 2008. www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1232006607827. [Google Scholar]
- 4.Saving Lives: reducing infection delivering clean and safe care . High impact intervention No 7: Care bundle to reduce the risk from Clostridium difficile. 2007. www.clean-safe-care.nhs.uk/toolfiles/79_SL_HII_7_v2.pdf. [Google Scholar]
- 5.Health Protection Agency . Voluntary surveillance of Clostridium difficile in England, Wales and Northern Ireland, 2009. www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1266228040791. [Google Scholar]
- 6.Muto CA, Blank MK, Marsh JW, et al. Control of an outbreak of infection with the hypervirulent Clostridium difficile BI strain in a university hospital using a comprehensive ‘bundle’ approach. Clin Infect Dis. 2007;45:1266–73. doi: 10.1086/522654. [DOI] [PubMed] [Google Scholar]
- 7.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–55. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 8.Vonberg RP, Kuijper EJ, Wilcox MH, et al. Infection control measures to limit the spread of Clostridium difficile. Clin Microbiol Infect. 2008;14((Suppl 5)):2–20. doi: 10.1111/j.1469-0691.2008.01992.x. [DOI] [PubMed] [Google Scholar]
- 9.Owens RC, Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial- associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008;46((Suppl 1)):S19–31. doi: 10.1086/521859. [DOI] [PubMed] [Google Scholar]
- 10.Oughton MT, Loo VG, Dendukuri N, Fenn S, Libman MD. Hand hygiene with soap and water is superior to alcohol rub and antiseptic wipes for removal of Clostridium difficile. Infect Control Hosp Epidemiol. 2009;30:939–44. doi: 10.1086/605322. [DOI] [PubMed] [Google Scholar]
- 11.Cooper BS, Stone SP, Kibbler CC, et al. Isolation measures in the hospital management of methicillin resistant Staphylococcus aureus (MRSA): systematic review of the literature. BMJ. 2004;329:533. doi: 10.1002/bjs.1800830445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Planche T, Aghaizu A, Holliman R, et al. Diagnosis of Clostridium difficile infection by toxin detection kits: a systematic review. Lancet Infect Dis. 2008;8:777–84. doi: 10.1016/S1473-3099(08)70233-0. [DOI] [PubMed] [Google Scholar]
- 13.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–7. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 14.Gerding DN, Muto CA, Owens RC., Jr Treatment of Clostridium difficile infection. Clin Infect Dis. 2008;46((Suppl 1)):S32–42. doi: 10.1086/521860. [DOI] [PubMed] [Google Scholar]
- 15.Apisarnthanarak A, Razavi B, Mundy LM. Adjunctive intracolonic vancomycin for severe Clostridium difficile colitis: case series and review of the literature. Clin Infect Dis. 2002;35:690–6. doi: 10.1086/342334. [DOI] [PubMed] [Google Scholar]
- 16.Lamontagne F, Labbé AC, Haeck O, et al. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann Surg. 2007;245:267–72. doi: 10.1097/01.sla.0000236628.79550.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Nood E, Speelman P, Kuijper EJ, Keller JJ. Struggling with recurrent Clostridium difficile infections: is donor faeces the solution? Euro Surveill. 2009;14:pii–19316. doi: 10.2807/ese.14.34.19316-en. www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19316. [DOI] [PubMed] [Google Scholar]
- 18.Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170:784–90. doi: 10.1001/archinternmed.2010.89. [DOI] [PubMed] [Google Scholar]
- 19.Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362:197–205. doi: 10.1056/NEJMoa0907635. [DOI] [PubMed] [Google Scholar]
- 20.Pillai A, Nelson RL. Probiotics for the treatment of Clostridium difficile- associated colitis in adults. Cochrane Database Syst Rev. 2008;1:CD004611. doi: 10.1002/14651858.CD004611.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]