Abstract
Although there have been reports about the role of erythrocyte membrane protein band 4.1 like 3 (EPB41L3) in several types of cancer, primarily in non-small-cell lung carcinoma, the molecular function and modulatory mechanisms of EPB41L3 remain unclear. In specific, the functional and clinical significance of EPB41L3 in esophageal squamous cell carcinoma (ESCC) has not been explored to date. In the present study, reduced EPB41L3 expression was demonstrated in ESCC cell lines and tissues, which was due to its high methylation rate. Ectopic expression of EPB41L3 in ESCC cells inhibited cell proliferation in vivo and in vitro. In addition, EPB41L3 overexpression induced apoptosis and G2/M cell cycle arrest by activating Caspase-3/8/9 and Cyclin-dependent kinase 1/Cyclin B1 signaling, respectively. Notably, patients with higher EPB41L3 expression had markedly higher overall survival rates compared with patients with lower EPB41L3 expression. In summary, the present results suggest that EPB41L3 may be a tumor suppressor gene in ESCC development, representing a potential therapeutic target and a prognostic indicator for ESCC.
Keywords: erythrocyte membrane protein band 4.1 like 3, esophageal squamous cell carcinoma, methylation, prognostic indicator, tumor suppressor
Introduction
Esophageal squamous cell carcinoma (ESCC) is one of the most malignant tumors worldwide (1–3) and the sixth most fatal cause of cancer-related death. Squamous cell carcinoma (SCC) is the main histological type of this cancer. Northern and central China is part of the 'Asian belt' which has a very high incidence of ESCC; there are >100 cases per 100,000 annually. The urgency of preventing and curing ESCC is obvious (4,5).
Similar to other types of cancer, the development of ESCC is believed to be a multi-step process caused by the accumulation of activated oncogenes and inactivated tumor suppressor genes (TSGs) (6). TSGs can be inactivated by both genetic and epigenetic mechanisms. Genetic deletions and point mutations disrupt TSG functions, and epigenetic mechanisms, including CpG island promoter methylation and histone modifications, frequently lead to the loss of TSG functions and are involved in tumor development and progression (7). The aberrant methylation of CpG islands leads to gene silencing, resulting in TSG inactivation, which can increase the rate of tumor formation by disabling multiple normal cellular processes, such as apoptosis and cell cycle progression (7). In breast cancer and renal clear cell carcinoma (8), methylation of erythrocyte membrane protein band 4.1 like 3 (EPB41L3) disables its tumor suppressive functions (9,10), suggesting that this gene is a potential example of TSG inactivation in human cancers. However, the role of EPB41L3 in ESCC remains unclear. EPB41L3, which belongs to the protein 4.1 family, is a membrane skeletal protein 4 that is commonly expressed in various human tissues. It is localized to sites of cell-cell contact and functions as an adapter protein, linking the plasma membrane to the cytoskeleton or associated cytoplasmic signaling effectors. Subsequently, EPB41L3 facilitates their activities in different pathways (11). Using a methylation microarray analysis, our group has demonstrated that EPB41L3 is methylated in ESCC tissue (12). Our subsequent study revealed that EPB41L3 suppressed tumor cell invasion and inhibited matrix metallopeptidase (MMP)2 and MMP9 expression in ESCC cells (13). However, the underlying mechanism by which EPB41L3 displays a tumor suppressive role has not been fully elucidated. Thus, aiming to improve clinical management and prolong the life expectancy of ESCC patients, the present study investigated the tumor suppressive functions of EPB41L3 in ESCC and its potential as a prognostic indicator. To explore the molecular function of EPB41L3 in ESCC, the expression of EPB41L3 in seven cell lines was measured. Then a tissue microarray with 97 pairs of tumor and paired normal tissues was used in order to assess its methylation status. The results demonstrated that expression of EPB41L3 was decreased in both ESCC cells and tissues and its methylation rate was increased compared with normal cells and tissues. Multiple tumor suppressors have been reported to function by inducing apoptosis, and loss of tumor suppression results in reduced apoptotic activity and increased tumor growth (14–17). Therefore the role of EPB41L3 in ESCC apoptosis and growth was examined. The results suggested that EPB41L3 may be a potential tumor suppressor and prognostic indicator in ESCC.
Materials and methods
Tissue microarray and immunohistochemistry
A ESCC tissue microarray was used to examine the expression of EPB41L3. The tissue microarray was purchased from Shanghai Outdo Biotech Co., Ltd. (Shanghai, China) and contained 97 pairs of ESCC samples and their para-carcinoma tissues. The patients were selected based on a clear pathological diagnosis of relatively early stage (Stages IA-IIIA) ESCC and follow-up data dated back to July 2007. Their clinical features were listed in Table I. None of the patients had received chemotherapy or radiotherapy prior to surgery. Immunohistochemistry on these tissues was performed following a previously published protocol (9). Scoring of EPB41L3 expression was based on the ratio of positive cells (score 0, 0–5% positive cells; 1, 6–35%; 2, 36–70%; 3, >70%) and staining intensity (score 0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining). The final score was calculated as follows: positive cell score x staining intensity score. The samples were marked as follows: '−' for a score of 0–1, '+' for a score of 2–3, '++' for a score of 4–6, and '+++' for a score of >6. Low expression was defined as a total score of <4 and high expression as a total score ≥4. For further analysis, '−' and '+' were considered as low expression, while '++' and '+++' as high expression. These scores were determined independently by two senior pathologists (Yu Wang and Jingyu Li; Department of Pathology, Zhujiang Hospital) in a blinded manner (18).
Table I.
Correlation between clinicopathological characteristics and EPB41L3 protein expression in esophageal squamous cell carcinoma.
| Characteristic | Low | High | Total | P-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 49 | 21 | 70 | 0.125 |
| Female | 23 | 4 | 27 | |
| Age (years) | ||||
| <65 | 34 | 12 | 46 | 0.947 |
| >65 | 38 | 13 | 51 | |
| Pathology | ||||
| G1-G2 | 55 | 22 | 77 | 0.216 |
| G3 | 17 | 3 | 20 | |
| Lymph node metastasis | ||||
| Negative | 39 | 11 | 50 | 0.381 |
| Positive | 33 | 14 | 47 | |
| Tumor infiltration depth | ||||
| T1-T2 | 12 | 10 | 22 | 0.016 |
| T3 | 60 | 15 | 75 | |
| TNM stage | ||||
| C1-C2 | 38 | 17 | 55 | 0.186 |
| C3 | 34 | 8 | 42 |
EPB41L3, erythrocyte membrane protein band 4.1 like 3; TNM, tumor-node-metastasis.
Cell lines
The non-neoplastic esophageal epithelial cell line Het-1a and six ESCC cell lines (Eca-109, TE-1, CaEs17, KYSE-150, KYSE-450 and KYSE-510) were obtained from the Research Center of Clinical Medicine at Nanfang Hospital in Guangzhou, China. Het-1a was cultured in RPMI-1640 medium (Life Technologies; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (Merck Millipore, Billerica, MA, USA) and 2 mM L-glutamine. CaEs17 and Eca-109 were cultured in DMEM (Life Technologies; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Merck Millipore), and the other four cell lines were cultured in RPMI-1640 supplemented with 10% fetal bovine serum.
DNA and RNA extraction
Total DNA and RNA were extracted using the Omega Tissue DNA kit and the Omega Total RNA kit I (both from Omega Bio-Tek, Inc., Norcross, GA, USA) respectively, according to the manufacturer's instructions.
Reverse transcription-polymerase chain reaction (RT-PCR) and reverse transcription -quantitative PCR (RT-qPCR)
Using the total RNA extracted from cells described above, reverse transcription was performed using the PrimeScript RT Reagent kit with gDNA Eraser (Takara Bio Inc., Otsu, Japan). qPCR was performed using SYBR Premix Ex Taq reagent (Takara Bio Inc.). The sequences of the reverse transcription-PCR primers were as follows: EPB41L3 forward, 5′-GTAGTGGTCCATAA AGAGACAGAGA-3 and reverse, 5′-GATACAAGTCAGTTGG GTTAGAAGA-3; GAPDH forward, 5′-TGCTGAGTATGTCGT GGAGTCT-3′ and reverse, 5′-CCCTGTTGCTGTAGCCATA TTC-3′. The gel concentration was 1% and the results were visualized under a UV lamp. qPCR was performed using a LightCycler 480 system (Roche, Basel, Switzerland). The qPCR reaction protocol was 30 sec at 95°C followed by 40 cycles of 5 sec at 95°C and 20 sec at 60°C. Quantification was performed using the 2−ΔΔCq method (19).
Methylation-specific PCR (MSP)
Total DNA was extracted as aforementioned, and bisulfite-modified using the BisulFlash DNA Modification kit (EpiGentek, Farmingdale, NY, USA) according to the manufacturer's protocol. The modified DNA was maintained at −20°C until PCR amplification. MSP was performed with 0.25 µl Taq HS, primers (500 nM), 50 ng total genomic DNA, and dNTP Mixture, and 10X PCR Buffer (both from Takara Biotechnology Co., Ltd., Dalian, China) in a total reaction volume of 20 µl. The primer pairs for EPB41L3 were as follows: to detect unmethylated sites, US, 5′-TTTGTGTATTGT TGTTGAGGAGTG-3′ and UAS, 5′-CACAATCCCCCACTCCA AAAAACA-3′; and to detect methylated sites, MS, 5′-GCAGTG CAAAGTGATACTTC-3′ and MAS, 5′-TCTGGTGGATAAA ATTTCACAT-3′. The thermocycling conditions for the MSP were: 95°C for 10 min, then 38 cycles of 94°C for 30 sec, 58°C or 60°C or 55°C for 30 sec and 72°C for 30 sec, followed by 72°C for 5 min, as previously described (20). The results were visualized using a BiQ Analyzer (Max Planck Institute for Informatics, Saarbrücken, Germany).
Bisulfite treatment and pyrosequencing analysis
Genomic DNA was extracted from seven cell lines using the Tissue DNA kit (Omega Bio-Tek, Inc.). Extracted DNA was bisulfite-modified using the BisulFlash DNA Modification kit (EpiGentek). The average methylation rates of 33 CpG sites that are located at −141551, −141494, −141439 and −141379 bp in the EPB41L3 promoter were detected. For EPB41L3, methylation rates of >20%, 10–20% and <10% were defined as hypermethylation, partial methylation, and non-methylation respectively (8).
For the methylation analysis, the extracted seven DNA samples were subjected to bisulfite conversion using the Bisulfite Conversion kit (Qiagen GmbH, Hilden, Germany), according to the manufacturer's instructions. Then, the purified bisulfate-converted DNA was used to perform MSP with biotinylated primers and Platinum Taq DNA polymerase (Kapa Biosystems, Inc., Wilmington, MA, USA). All the primers used for PCR were designed using PyroMark Assay Design 2.0 (Qiagen GmbH), synthesized by BGI (Shenzhen, China), and presented in Table II. The PCR products were purified using a Qiaquick PCR purification kit (Qiagen GmbH), and single-stranded DNA was prepared using Dynabeads M280 streptavidin (Thermo Fisher Scientific, Inc.). The samples were analyzed using PyroMark Q96 ID (Qiagen GmbH). Analysis of the results was performed with the PyroMark Q96-CpG software (Qiagen GmbH).
Table II.
Sequences of primers used for pyrosequencing.
| Primer | Sequence | 5′ labeling |
|---|---|---|
| 122-01-1F | AGTTTAGGGAGGAGGTTTGTAAG | |
| 122-01-1R | ACACCTCCAAATTACCACCTACAA | Biotin |
| 122-01-1S | GTTTGTAAGGAGATTTATATTTTG | |
| 122-01-2F | GGAGGAGGTTTGTAAGGAGAT | |
| 122-01-2R | CACCTCCAAATTACCACCTACA | Biotin |
| 122-01-2S | TTTAGGGAGGGGTAGA | |
| 122-01-3F | GAGGAGGTTTGTAAGGAGATTTAT | |
| 122-01-3R | ACCTCCAAATTACCACCTAC | Biotin |
| 122-01-3S | GGGTTAGAGAGGGTTGA | |
| 122-01-4F | GGGAGGAGGTTTGTAAGGAGATTT | |
| 122-01-4R | ATCCCTCCCCAAAAACTCTTTCCTT | Biotin |
| 122-01-4S | GAAGAGAGTTGTAGGTGGTAAT |
5-Aza-2-deoxygcytidine treatment
To determine whether demethylation could restore the expression of EPB41L3 in ESCC cells, 2×105 cells were seeded in each well of a 6-well plate and treated with 50 µmol/l 5-Aza-2′-deoxycytidine (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 3 days. The medium was changed with fresh medium containing 5-Aza-2′-deoxycytidine every 24 h. Total RNA and protein was then extracted, and EPB41L3 expression was measured by RT-PCR and western blotting (6).
Cell counting kit-8 (CCK-8) cell viability assay
Cell viability was assessed by a CCK-8 assay (Dojindo Molecular Technologies, Inc., Kumamoto, Japan). Vec-150, epb-150, vec-510, epb-510 cells, si-510 and si-NC (5×103 cells/well) were plated separately into 96-well plates. After being plated for 24, 48 or 72 h, the CCK-8 reagent was added to each well, and cells were incubated for 2 h at 37°C. The absorbance was measured at 450 nm by spectrophotometry.
Western blot analysis
Western blotting was performed according to the standard protocol (21) with the following antibodies: EPB41L3 (1:2,000; Abcam, Cambridge, MA, USA), caspase-8 (1:1,000), caspase-9 (1:1,000), caspase-3 (1:1,000), CyclinA (1:2,000), CyclinD (1:1,000), CyclinE (1:1,000) (all from Cell Signaling Technology, Inc., Danvers, MA, USA), CyclinB1 (1:1,000), Cyclin-dependent kinase 1 (CDK1, also known as Cdc2; 1:1,000) (both from ProteinTech Wuhan Sanying Biotechnology, Wuhan, China). GAPDH (1:8,000; ProteinTech Wuhan Sanying Biotechnology) was used as loading control.
Flow cytometry
To measure dividing cells by flow cytometry, KYSE-150 and KYSE-510 cells transfected with Vec-NC or Vec-epb were fixed in 70% ethanol overnight, stained with propidium iodide the next day, and DNA content was analyzed using an LSR FORTESSA (BD Biosciences, Franklin Lakes, NJ, USA). To measure apoptosis, these cells were further were stained and analyzed using the Annexin V-allophycocyanin (APC)/7-am inoactinomycin D (7AAD) apoptosis kit (KeyGen Biotech Co., Ltd., Nanjing, China), according to the manufacturer's instructions. Briefly, 2×106 cells were washed with PBS, stained with 7-AAD and Annexin V-APC for 15 min at room temperature and analyzed using an LSR FORTESSA. The results were analyzed using ModFit LT2.0 software (Verity Software House, Topsham, ME, USA).
EPB41L3 plasmid construction and cell transfection
The EPB41L3 expression vector pReceiver-M98-EPB41L3 was constructed by inserting the 417nt ORF sequence of EPB41L3 (MFIQIFPVIFLETSIAYSNVVWVYISYLHLLMKMFMR DHFGCLMNYLPCFSTETFSLTPTVLAVGWLLERREVS FSCSEWDKVASVVEQCLKYFFLSLFCSPFLSHLEHGIW TCQSSGNTLPLLVGTWIMWVSPEICV) into the pReceiver-M98 vector (Genecopoeia Inc., Rockville, MD, USA). One pair of specific oligonucleotides (EPB41L3 gene isoform 1) was annealed and then subcloned into the vector. Cell transfection was performed with Lipofectamine 2000 and KYSE-150 and KYSE-510 were selected with 500 µg/ml G418 sulfate (both from Thermo Fisher Scientific, Inc.) for 2–3 weeks, according to the manufacturer's protocol for stable transfection. The KYSE-150 and KYSE-510 cells transfected with the EPB41L3 overexpression plasmid were termed vec-150-epb and vec-510-epb respectively. The KYSE-150 and KYSE-510 cells transfected with the empty control plasmid were termed vec-150-nc and vec-510-nc respectively.
Small interefering (si) RNA vector construction and cell transfection
Three pairs of siRNA and a negative control siRNA were purchased from GenePharma Co., Ltd. (Shanghai, China) and subcloned into pcDNA6.2 GW/EmGFP vectors (Thermo Fisher Scientific, Inc.). KYSE-510 cells were transfected with the vectors carrying siRNA-EPB41L3 or the negative control siRNA. There were 3 siRNA-epb41l3 vectors were transfected separately. Silencing efficiency was measured at 48 h post-transfection by western blotting. The siRNA with the highest knockdown efficiency was selected for subsequent experiments (sense, GCUGCGAAUAAACAGAUUUTT and antisense, AAAUCUGUUUAUUCGCAGCTT). The negative control siRNA (sense, CUGCGGAAAUAAUUUCAGATT and antisense, AAUCAUUUUGACGUUAGCCTT). Cells with stable knockdown of EPB41L3 expression were established under selection with Blasticidin S HCl (Thermo Fisher Scientific, Inc.). KYSE-519 cells transfected with siRNA-EPB41L3 or the negative control siRNA were termed si-510-epb and si-510-nc respectively.
Clonogenic assays
The effects of EPB41L3 expression were analyzed by clonogenic assays using standard protocols. Eighteen h prior to transfection, cells were seeded in 6-well plates at a density of 2×105 per well in complete RPMI-1640 or DMEM (Thermo Fisher Scientific, Inc.). Cells were transfected with plasmid DNA using the Lipofectamine 2000 transfection reagent. Twenty-four h after transfection, cells were trypsinized and plated in 100 mm dishes at identical densities for each transfection. Transfected cells were selected in 500 µg/ml G418 for 10 to 14 days, and surviving colonies were stained with crystal violet and counted (22). Transgene expression was confirmed for each experiment. Each experiment was conducted at least three times.
In vivo experiments
To assess the role of EPB41L3 in inhibiting tumor growth in vivo, suspensions of 1×106 EPB41L3-overexpressing KYSE-150 cells in 0.1 ml of PBS were injected subcutaneously into the backs of 4–5-week-old male immunodeficient SCID-Beige mice weighing 18–20 g (obtained from the Laboratory Animal Center, Southern Medical University, Guangzhou, China). As a control, suspensions of 1×106 KYSE-150 cells transfected with control vector were injected into the same mice on a different site. To assess the role of EPB41L3 in promoting tumor growth in vivo, suspensions of 1×106 EPB41L3-knockdown or control KYSE-510 cells in 0.1 ml PBS were injected subcutaneously into two sites of the backs of 4–5-week-old male immunodeficient SCID-Beige mice. The slightly upper position of the waist was selected as the optimal injection site. Four mice were used in each experimental group. Tumor growth was assessed by measuring the xenografts in two dimensions once a week. Tumor volumes were calculated according to the following formula: (volume) = 1/2 × (long axis) × (short axis)2. At 28 days post-injection, mice were sacrificed and the tumors were carefully dissected. All animal experiments were performed in accordance with the principles and procedures outlined in the Southern Medical University Guide for the Care and Use of Animals under the assurance number SCXK (Guangdong) 2008–0002. Approval was obtained from the Nanfang Hospital Animal Ethics Committee.
Statistical analysis
Statistical analysis was performed using the SPSS version 13.0 software (SPSS, Inc., Chicago, IL, USA). Each experiment was performed in triplicate. The results were expressed as the mean ± standard deviation. Significant differences were determined using Student's t-test to compare two groups. The χ2 test was used to analyze the correlations between EPB41L3 expression and clinicopathological features in patients with ESCC. Survival curves were evaluated using the Kaplan-Meier method, and differences between survival curves were assessed by the log-rank test. The Cox proportional hazards regression model was used to examine univariate and multivariate hazard ratios for the study variables that were dichotomized. Only significantly different variables in univariate analysis were entered into the subsequent multivariate analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
EPB41L3 expression is downregulated in ESCC tissues
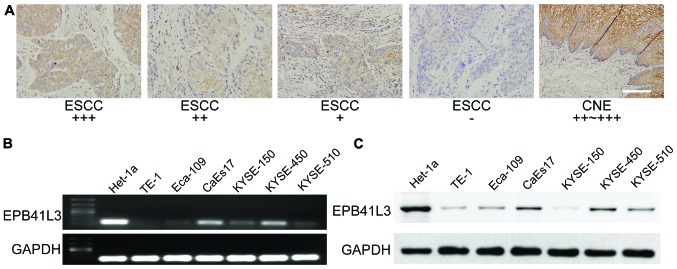
To explore the expression status of EPB41L3 in ESCC tissues, immunohistochemistry analysis was performed using a tissue microarray which contained 97 pairs of ESCC samples and their adjacent non-tumor tissue samples. In general, the results demonstrated that EPB41L3 expression was significantly lower in tumor tissues compared with their matched adjacent non-tumor tissues (Table III; Fig. 1A). High immune reactivity to EPB41L3 was detected along cell membranes and cytoplasm in 91 out of 97 non-tumor tissue samples, while only 25 out of 97 ESCC tissue samples revealed high expression. Only 6 out of 97 adjacent non-tumor tissue samples exhibited low EPB41L3 expression or no expression, while 72 out of 97 ESCC tissue samples exhibited low expression or no expression (Table III; Fig. 1A).
Table III.
EPB41L3 protein expression in NE and ESCC samples.
| Group | Cases | EPB41L3 protein expression
|
P-value | |
|---|---|---|---|---|
| Low | High | |||
| NE | 97 | 6 | 91 | <0.001 |
| ESCC | 97 | 72 | 25 | |
EPB41L3, erythrocyte membrane protein band 4.1 like 3; ESCC, esophageal squamous cell carcinoma; NE, non-cancerous esophagus.
Figure 1.
EPB41L3 expression is downregulated in ESCC tissue samples and cell lines compared with their para-carcinoma tissues and a non-neoplastic esophageal epithelium cell line, respectively. (A) Representative images of EPB41L3 immunohistochemistry staining in ESCC tissues compared with CNE. Scale bar, ×200. (B) Reverse transcription-polymerase chain reaction analyses of EPB41L3 mRNA expression in the ESCC cell lines TE-1, Eca-109, CaEs17, KYSE-150, KYSE-450 and KYSE-510 and one non-neoplastic esophageal epithelium cell line Het-1a. (C) Western blot analyses of EPB41L3 protein expression confirmed that EPB41L3 expression was lower in the ESCC cell lines compared with the non-neoplastic cell line. EPB41L3, erythrocyte membrane protein band 4.1 like 3; ESCC, esophageal squamous cell carcinoma; CNE, corresponding noncancerous esophagus.
EPB41L3 expression is lower in ESCC cells compared with Het-1a cells due to methylation
RT-PCR analysis was performed to dete2ct EPB41L3 mRNA expression in six ESCC cell lines and in one non-neoplastic esophagus cell line, Het-1a. The results revealed that the non-neoplastic Het-1a cell line had markedly higher expression of EPB41L3 compared with all six esophagus cancer cell lines (Fig. 1B). Western blotting was then used to detect EPB41L3 expression at the protein level. Similar to the RT-PCR results, higher EPB41L3 protein expression was observed in the normal esophagus cell line compared with the ESCC cell lines (Fig. 1C).
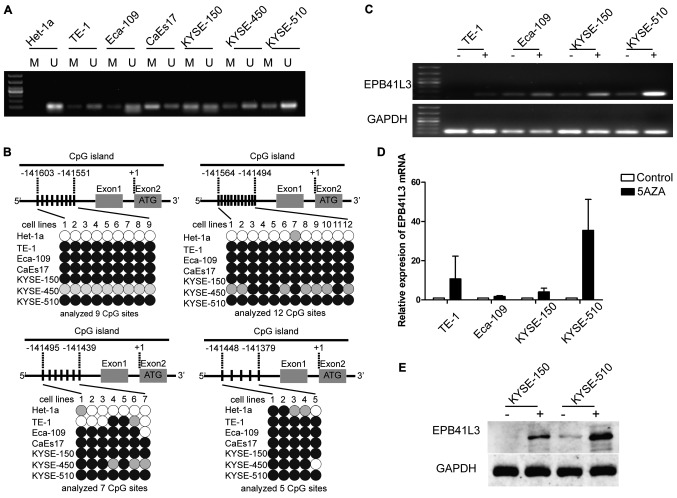
In order to explore the reason why EPB41L3 was down-regulated in ESCC cells, methylation patterns were examined using MSP in the six esophagus cancer cell lines and the non-neoplastic esophagus Het-1a cell line. All six esophagus cancer cell lines developed both methylated and unmethylated bands, suggesting that EPB41L3 had partial methylation in the cancer cell lines. By contrast, Het-1a developed only an unmethylated band, demonstrating that EPB41L3 was unmethylated in the normal esophageal cells (Fig. 2A).
Figure 2.
Reduced expression of EPB41L3 in ESCC is due to methylation. (A) Methylation-specific polymerase chain reaction analysis of the seven cell lines revealed that EPB41L3 was partially methylated in all six ESCC cell lines while it was unmethylated in the normal Het-1a cells. (B) Representation schematic of the pyrosequencing results for the methylation status of the CpG promoter in the seven cell lines tested. Gray boxes indicate exons. Vertical bars indicate CpG sites examined for methylation. Black, gray, and white circles represent hypermethylation, partial methylation and nonmethylation, respectively. The schematic illustrates the representative results of pyrosequencing of a cytosine residue(s) at −141551, −141494, −141439 and −141379 bp of the EPB41L3 promoter. (C) Treatment with 5-Aza-2-deoxygcitidine restored the expression of EPB41L3 in ESCC lines, suggesting that downregulation of EPB41L3 in ESCC is due to methylation. (D) Reverse transcription-quantitative polymerase chain reaction analyses revealed that EPB41L3 expression was increased in ESCC cell lines following 5-Aza-2-deoxygcitidine treatment. The increase was most evident in the KYSE-510 cells. In the other cell lines, although an increase was observed, this was not as evident as in the KYSE-510 cells. (E) Western blot analyses revealed that EPB41L3 expression was increased in KYSE-150 and KYSE-510 cells following 5-Aza-2-deoxygcitidine treatment. EPB41L3, erythrocyte membrane protein band 4.1 like 3; ESCC, esophageal squamous cell carcinoma; M, methylated primer; U, unmethylated primer; 5aza, 5-Aza-2-deoxygcitidine.
Next, the different promoter methylation levels were explored by pyrosequencing. A quantitative analysis of DNA methylation at 33 CpG sites of the EPB41L3 gene promoter was performed. The pyrosequencing results revealed high levels of EPB41L3 methylation in five cell lines, KYSE-150, KYSE-510, Eca-109, CaEs17 and TE-1, which were represented by dark circles in Fig. 2B. For Het-1a, the methylation rate was <20%, which was considered to be unmethylated (represented by white circles in Fig. 2B). These results were in accordance with the MSP results.
To identify whether the downregulated EPB41L3 mRNA and protein expression levels in ESCC were due to methylation, 5-aza-2-deoxycytidine, a potent inhibitor of DNA methyltransferase, was used to treat the TE-1, Eca-109, KYSE-150 and KYSE-510 cell lines. If the downregulation of EPB41L3 was due to methylation, treatment with 5-aza-2-deoxycytidine would be expected to increase EPB41L3 expression. Indeed, treating the cells with the inhibitor restored EPB41L3 mRNA expression, as identified by RT-PCR (Fig. 2C) and qPCR (Fig. 2D). Furthermore, western blotting confirmed restoration of EPB41L3 protein expression in KYSE-150 and KYSE-510 (Fig. 2E). These results indicated that the decreased EPB41L3 expression in esophageal cancer cell lines occurred via promoter methylation.
EPB41L3 inhibits ESCC cell proliferation and reduces colony formation in vitro
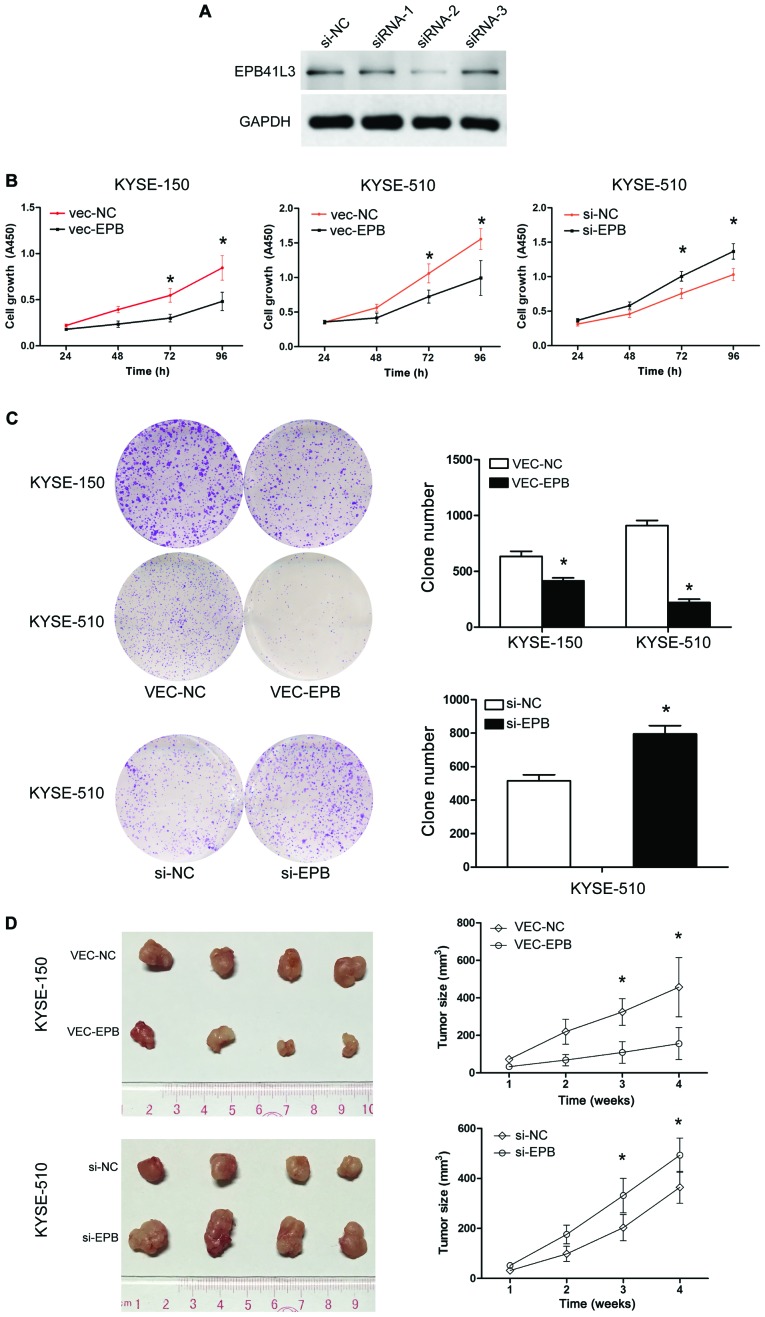
In order to examine the functional role of EPB41L4 in ECC, its expression was silenced in ESCC cells by siRNA. First, the knockdown efficiency of the siRNAs was confirmed by western blotting. The results demonstrated that siRNA2 resulted in the highest knockdown efficiency (Fig. 3A), and therefore siRNA2 as selected for subsequent experiments.
Figure 3.
EPB41L3 inhibits cell proliferation in ESCC in vitro and in vivo. (A) Western blot analysis of the siRNA transfection efficiency of 3 siRNAs. siRNA-2 resulted in the largest decrease in protein levels and was therefore selected for subsequent experiments. (B) Growth curves of cells transfected with either EPB41L3-ovexpression plasmid (vec-EBP), or siRNA (si-EBP) or respective controls by cell counting kit-8 assay. (C) Representative images and quantification of colony formation assays. (D) Photographic images of the xenograft tumors and quantification of tumor volumes in each experimental group. *P<0.01 compared with control. EPB41L3, erythrocyte membrane protein band 4.1 like 3; ESCC, esophageal squamous cell carcinoma; si, small interfering; nc, negative control.
To investigate the effects of overexpressing EPB41L3 on human ESCC cell growth in vitro, the KYSE-150 and KYSE-510 cell lines were used to perform CCK-8 assays. EPB41L3 overexpression in KYSE-150 and KYSE-510 cells resulted in significant inhibition of cell proliferation compared with control cells (P<0.05; Fig. 3B). By contrast, the transfection of EPB41L3 siRNA in KYSE-510 cells resulted in increased cell proliferation compared with control cells (P<0.05; Fig. 3B). The ability of these two cell lines to form colonies in soft agar was then examined for two weeks. Overexpression of EPB41L3 significantly reduced the cell colony numbers in KYSE-150 and KYSE-510 cells, while EPB41L3 knockdown by siRNA in KYSE-510 cells had the opposite effect (Fig. 3C). Collectively, these results indicated that EPB41L3 inhibited the growth of ESCC cells in vitro.
Epb41l3 inhibits tumor formation in vivo
KYSE-510 cells transfected with EPB41L3 overexpression plasmid or control plasmid were termed vec-510-epb and vec-510-nc, respectively. KYSE-510 cells transfected with siRNA-EPB41L3 or control siRNA were termed si-510-epb or si-510-nc, respectively. To further examine the role of EPB41L3 in tumor growth in vivo, tumor xenografts were generated in mice by injecting vec-510-epb, vec-510-nc, si-510-epb and si-510-nc cells (four nude mice per group). Visible tumors began to form at 10 days post-injection. The volume of the tumors was measured twice a week and the mice were sacrificed at day 28 post-injection. At the end of the experiment, the mice weighted 168–180 g. The maximum diameter of a single tumor was 1.5 cm. None of the mice developed multiple tumors. The group injected with vec-510-epb cells formed significantly smaller tumors compared with the group injected with the control vec-510-nc cells (Fig. 3D). The group injected with si-510-EPB cells formed significantly larger tumors compared with the group injected with the control si-510-NC cells (Fig. 3D). Overall, these results data demonstrated that increased expression of Epb41L3 inhibited tumor growth, while decreased expression of Epb41L3 promoted tumor growth in vivo.
Ectopic expression of EPB41L3 promotes apoptosis by activating the caspase-3/8/9 pathway
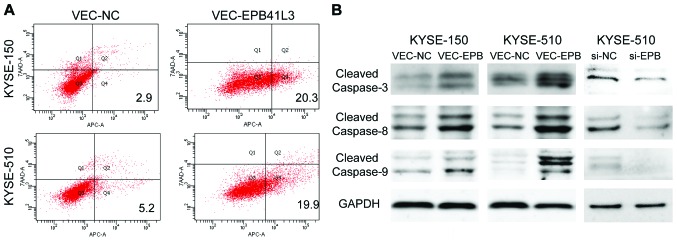
Apoptosis was detected using the 7-AAD/Annexin V-APC double staining method followed by flow cytometry analysis. Compared with the control group, KYSE-150 and KYSE-510 cells overexpressing EPB41L3 had significantly higher apoptotic rates compared with cells transfected with control plasmid (20.3 vs. 2.9%, and 19.9 vs. 5.2%, respectively) (Fig. 4A). To further investigate the mechanisms underlying the higher apoptotic rates following EPB41L3 overexpression, western blot analysis was performed to determine the expression levels of apoptosis-related proteins. The results demonstrated that the expression levels of caspase-8, caspase-9 and caspase-3 were elevated in KYSE-150 and KYSE-510 cells following EPB41L3 over-expression compared with control cells (Fig. 4B). Then, the expression levels of caspase-8, caspase-9 and caspase-3 were detected in KYSE-510 cells following EPB41L3 knockdown by siRNA. The results demonstrated that expression of caspase-8, caspase-9 and caspase-3 was markedly decreased following EPB41L3 knockdown compared with control cells (Fig. 4B).
Figure 4.
EPB41L3 has proapoptotic effects by activating the caspase-3/8/9 pathway. (A) Representative plots from the flow cytometry analysis following 7-AAD and Annexin V-APC double staining. (B) Western blot analysis for the expression levels of cleaved-caspase-3, cleaved-caspase-8 and cleaved-caspase-9 following EPB41L3 or knockdown in ESCC cells. GAPDH was used as a loading control. EPB41L3, erythrocyte membrane protein band 4.1 like 3; 7-AAD, 7-aminoactinomycin D; APC, allophycocyanin; ESCC, esophageal squamous cell carcinoma; si, small interfering; nc, negative control.
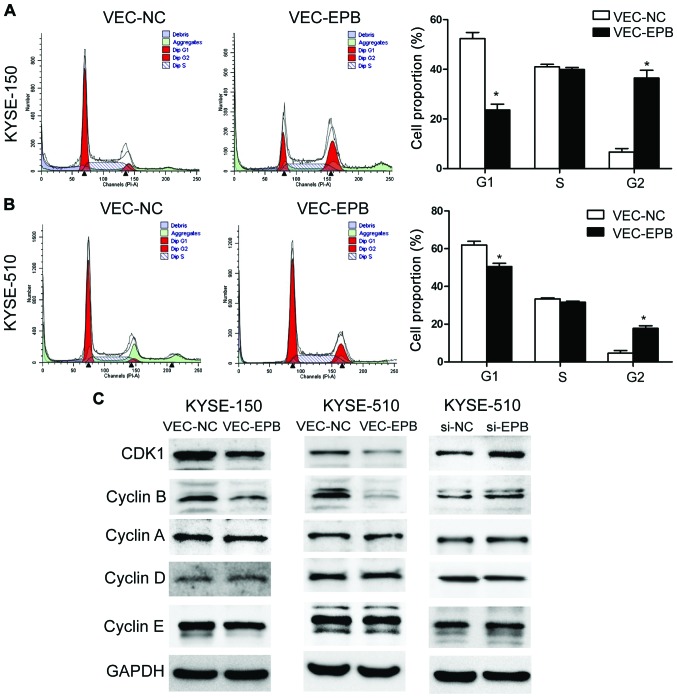
EPB41L3 causes G2/M cell cycle arrest by activating the CDK1 pathway
Flow cytometry experiments revealed that the % of cells in the G2 phase of the cell cycle was significantly increased in the EPB41L3-overexpressing group compared with the control group, in both the KYSE-150 (Fig. 5A) and KYSE-510 (Fig. 5B) cell lines. The % of cells in G2 was 36.43 and 6.77% for the EPB41L3-overexpressing and control KYSE150 cells respectively (Fig. 5A), and 39.35 and 8.93% for the EPB41L3-overexpressing and control KYSE-510 cells respectively (Fig. 5B). Western blotting was also performed in order to explore the mechanism of G2/M cell cycle arrest. The results demonstrated that overexpression of EPB41L3 decreased the protein expression levels of CDK1 and CyclinB1 in vec-150-epb and vec-510-epb cells, compared with their respective controls (Fig. 5C). In addition, the expression levels of these proteins were examined following EPB41L3 knockdown in KYSE-510 cells. The expression levels of CDK1 and CyclinB1 were significantly increased in the knockdown cells compared with control cells (Fig. 5C). Together with the flow cytometry results, these data suggested that inhibition of CDK1/CyclinB1 might be the main mechanism of the G2/M arrest induced by EPB41L3.
Figure 5.
EPB41L3 induces G2/M arrest by activating the CyclinB/CDK1 pathway. (A and B) Flow cytometry analysis of cell cycle phase distribution revealed a G2/M arrest following EPB41L3 overexpression. (C) Western blot analysis of cell cycle-related proteins following EPB41L3 or knockdown in ESCC cells. *P<0.05 compared with control. EPB41L3, erythrocyte membrane protein band 4.1 like 3; CDK1, Cyclin-dependent kinase 1; ESCC, esophageal squamous cell carcinoma; si, small interfering; nc, negative control.
EPB41L3 is negatively correlated with depth of tumor infiltration and may be a prognostic indicator
The relationship between clinicopathological characteristics and EPB41L3 expression levels in patients with ESCC is summarized in Table I. The clinicopathological characteristics contained age, sex, pathology classification, numbers of lymph nodes, tumor infiltration and tumor-node-metastasis (TNM) stage (based on the 7th edition of American Joint Committee on Cancer clinical staging (23). No significant association was observed between EPB41L3 expression levels and the patients' age, sex, pathologic type or lymph status in the 97 ESCC cases. However, the expression levels of EPB41L3 were significantly negatively correlated with depth of tumor infiltration (P=0.016; Table I). Next, univariate analysis was used to explore the association between clinicopathological characteristics and the overall survival. The results demonstrated that tumor infiltration depth (P=0.01), TNM stage (P<0.001) and EPB41L3 expression (P=0.004) were all positively correlated with overall survival (Table IV). These three factors were further investigated using multivariate analysis, and the results revealed that only TNM stage (P=0.004) and EPB41L3 expression (P=0.033) were statistically significant (Table IV). To investigate the prognostic value of EPB41L3 expression in ESCC, the association between the expression levels of EPB41L3 and the patients' survival was assessed using Kaplan-Meier analysis with the log-rank test. The results demonstrated that patients with lower expression of EPB41l3 had shorter survival time compared with the patients that had higher EPB41l3 expression (P=0.002; Fig. 6). These findings revealed that EPB41L3 may have the potential to serve as a prognosis predictor in ESCC.
Table IV.
Summary of univariate and multivariate Cox regression analyses of overall survival.
| Parameter | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| P-value | HR | 95% CI | P-value | HR | 95% CI | |
| Sex | 0.26 | 1.368 | 0.793–2.36 | |||
| Age (years) | 0.649 | 1.114 | 0.701–1.769 | |||
| Pathology | 0.91 | 0.968 | 0.548–1.71 | |||
| Lymph nodes involved | 0.071 | 0.651 | 0.409–1.037 | |||
| Tumor infiltration depth | 0.01 | 0.425 | 0.222–0.812 | 0.301 | 0.689 | 0.34–1.395 |
| TNM stage | <0.001 | 0.398 | 0.247–0.643 | 0.004 | 0.478 | 0.289–0.79 |
| EPB41L3 expression in cancer tissue | 0.004 | 2.392 | 1.329–4.304 | 0.033 | 1.942 | 1.054–3.577 |
| EPB41L3 expression in para-cancerous tissue | 0.327 | 1.521 | 0.657–3.524 | |||
HR, hazard ratio; CI, confidence interval; EPB41L3, erythrocyte membrane protein band 4.1 like 3.
Figure 6.
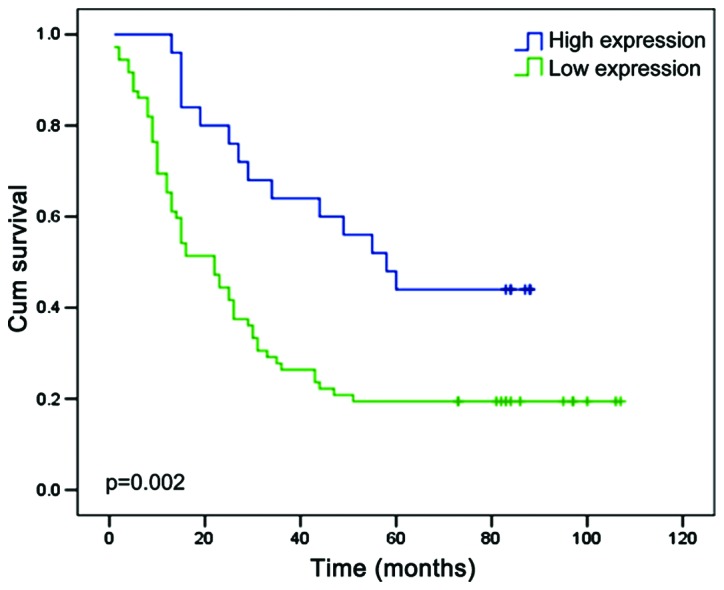
EPB41L3 expression predicts overall survival in patients with ESCC. Kaplan-Meier analysis with the log-rank test was used to assess the association between levels of tumor EPB41L3 expression and patients' survival time. EPB41L3, erythrocyte membrane protein band 4.1 like 3; ESCC, esophageal squamous cell carcinoma.
Discussion
Esophageal carcinoma is a common malignancy with poor prognosis. The main reason for low survival is that the majority of ESCC are asymptomatic and undetected until they have spread beyond the esophageal wall and thus become unresectable (24). EPB41L3, also known as 4.1B or DAL1, is located on chromosome 18p11.32, and it is expressed in multiple adult tissues, including the brain, lung, kidney, intestine and prostate. It has been demonstrated to serve as an antitumor factor in some cancers, primarily in non-small-cell lung carcinoma (NSCLC). Kikuchi et al (30) noted that EPB41L3 methylation was involved in the development and progression of NSCLC and served as a poor prognosis predictor. Nevertheless, previous studies have not focused on the mechanisms underlying the tumor suppressive function and the clinical significance of EPB41L3 in ESCC. Our group has been studying the role of EPB41L3 in ESCC with the aim to explore its methylation status and to identify potential plasma biomarkers for early diagnosis. By using an Infinium Methylation 450k array, the methylation frequency of EPB41L3 was demonstrated to be higher in tumor tissues compared with normal surrounding tissues, and this was correlated with large tumor size and advanced pT tumor stage (12). EPB41L3 has been demonstrated to be partly silenced due to hypermethylation (13). Other genetic and epigenetic mechanisms may result in the regulation of EPB41L3 expression, including histone modifications and microRNAs, and these will need further investigation in the future.
There has been evidence suggesting that hypermethylation has a critical role in the inactivation of EPB41L3. Demethylation treatments were able to restore EPB41L3 expression in several types of cancer (9,25–27). Aberrant DNA methylation is one of the best-characterized epigenetic modifications, contributing to tumor initiation and progression (28,29). This is because DNA methylation, which usually occurs on the cytosine residues of CpG dinucleotides, is key to tissue differentiation during early embryonic growth (30). The present data provided evidence that EPB41L3 expression was downregulated in ESCC cells due to promoter methylation. First, EPB41L3 expression was demonstrated to be lower in ESCC tissues and cell lines compared with adjacent normal tissues and a non-neoplastic cell line. Second, MSP and pyrosequencing confirmed higher promoter methylation rate in ESCC cell lines compared with the normal esophageal cell line. Notably, although methylation was evident in all six cell lines, they all exhibited partial methylation rather than full methylation of the promoter. The methylation rates were different in different ESCC cell lines, demonstrating heterogeneity in these cancer cells. Pyrosequencing has been recently regarded as a better method for testing methylation in large-scale validation studies, biomarker development and clinical diagnostics (31), while MSP has some disadvantages, including its inability of detecting mosaic DNA methylation (32,33).
DNA methylation provides an alternative way for tumor suppressor gene function loss (12). Having detected EPB41L3 methylation in ESCC cells, the present study aimed to explore the mechanisms underlying the role of EPB41L3 as a tumor suppressor. Thus, experiments related to proliferation, apoptosis and cell cycle phase distribution were performed. CCK-8 cell viability and clonogenic assay results indicated that EPB41L3 overexpression inhibited tumor cell proliferation in vitro. Tumor xenograft experiments revealed that EPB41L3 over-expression suppressed tumor growth in vivo. Flow cytometry assay demonstrated higher rates of apoptosis in EPB41L3-overexpressing cells compared with control cells. These results offered a foundation for mechanistic exploration. Previous study has revealed that apoptosis in MCF-7 cells was primarily associated with the activation of Caspase-8, and inhibition of this activation blocked the ability of EPB41L3 to induce cell death in MCF-7 (10). Cleaved-caspase-8 and cleaved-caspase-9 activate pro-caspase 3 into cleaved-caspase-3, resulting in apoptosis. Thus, the expression of the caspase family was detected by western blotting in order to explore the mechanism of apoptosis induction in ESCC. The results demonstrated that expression of caspase-8, caspase-9 and caspase-3 were elevated in the EPB41L3-overexpressing cells. By contrast, EPB41L3 knockdown by siRNA resulted in the opposite effects.
Kuns et al (31) reported that, during pregnancy, the overexpression of EPB41L3 results in reduced CyclinA expression and Rb phosphorylation, which is accompanied by decreased tyrosine kinase receptor ErbB2 phosphorylation, causing the mammary epithelial cells to be arrested in the G1 phase (34). The present data revealed no significant change in CyclinA expression, while expression of CyclinB1 and CDK1 decreased following EPB41L3 overexpression. High expression of CyclinB1-CDK1 only occurs in the M phase, not the G2 phase (35). CDK1 controls entry into and exit from the M phase of the cell cycle and is commonly accompanied by CyclinA and CyclinB1 (36). This is in accordance with the present cell cycle data. Cell death can occur during mitochondrial membrane permeabilization with the release of cell death effectors such as apoptosis-inducing factor (37). The G2/M phase arrest may limit the proliferation of chromosomally unstable, pre-cancerous cells. Downregulation of the G2/M checkpoint may lead to tumorigenesis (38). The present findings indicate that ectopic expression of EPB41L3 may inhibit cell proliferation by upregulating apoptosis and causing G2/M phase arrest.
To date, several genes have been reported to be hypermethylated in ESCC, including cyclin-dependent kinase inhibitor 2A (CDKN2A)/p16INK4a (39–41). These methylated genes have the potential to be used as diagnostic or prognostic molecular markers for ESCC. Recent study has focused on the correlation of epigenetic changes and ESCC patient survival. Zare et al (42) and Lee et al (43) separately reported that methylation of adenomatous polyposis coli (APC) or fragile histidine triad protein (FHIT) was closely correlated to poor outcome in patients with ESCC. The current study examined EPB41L3 expression in ESCC and its correlation with clinicopathological features and prognosis. A statistically significant difference was observed between EPB41L3 expression in ESCC tumor tissues and their matched adjacent non-tumor tissues. This was consistent in both RT-PCR and western blotting results. Survival analysis revealed that patients with higher expression of EPB41L3 had improved prognosis. The relationship between EPB41L3 expression levels and certain clinical features were then analyzed. Univariate analysis demonstrated that EPB41L3 expression was significantly correlated with tumor infiltration depth and TNM stage. These results indicate that EPB41L3 has a significant correlation with tumor progression. Through a tissue microarray, the current study revealed the clinical importance of EPB41L3 in ESCC patients, which may have the potential to be prognostic indicator. To date, only 20 types of tumor markers have been put into clinical use. No specific tumor markers exist to date for ESCC (44). The main obstacle to developing clinically useful indicators is the lack of large clinical trials in ESCC, which are hampered by a lack of sizeable esophageal tissue repositories that include complete clinical annotation (45). Furthermore, because chemotherapy and radiation remain important in ESCC treatment, predictive indicators may be critical for identifying patients who may benefit from molecular targeted agents or are more sensitive to radiotherapy (46). Further studies are warranted to probe the clinical significance of EPB41L3 in ESCC.
In summary, low levels of EPB41L3 may be an unfavorable indicator of ESCC prognosis. Overexpressing EPB41L3 inhibited proliferation, and induced apoptosis and G2/M arrest in ESCC cells through the Caspase-3/8/9 and CDK1/CyclinB1 pathways. Detection of the tumor EPB41L3 methylation status may aid in predicting prognosis in patients with ESCC.
Acknowledgments
Not applicable.
Footnotes
Funding
This study was supported by the Natural Science Foundation of Guangdong Province, China (grant no. 2015A030313273).
Availability of data and materials
The analyzed datasets generated during the study are available from the corresponding author on reasonable request.
Author's contributions
RZ performed the cell culture, MSP, western blotting, transfection, CCK-8, clonogenic assays and was involved in the writing of the article. YL performed qPCR analysis. ZJJ performed the flow cytometry experiment. JPH performed the statistical analysis; YW performed the IHC analysis; XFL, WBX and XCW were involved in the animal experiments; JRZ and QEW performed pyrosequencing; YFZ contributed to the whole design of this research project. JRZ, QEW and YFZ all contributed to the writing of the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All animal experiments were performed in accordance with the principles and procedures outlined by the Southern Medical University Guide for the Care and Use of Animals under the assurance number SCXK (Guangdong) 2008–0002. Approval was obtained from the Nanfang Hospital Animal Ethics Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zhu YH, Fu L, Chen L, Qin YR, Liu H, Xie F, Zeng T, Dong SS, Li J, Li Y, et al. Downregulation of the novel tumor suppressor DIRAS1 predicts poor prognosis in esophageal squamous cell carcinoma. Cancer Res. 2013;73:2298–2309. doi: 10.1158/0008-5472.CAN-12-2663. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 5.Offner FA. Etiology, molecular biology and pathology of squamous cell carcinoma of the esophagus. Pathologe. 2000;21:349–357. doi: 10.1007/s002920000414. In German. [DOI] [PubMed] [Google Scholar]
- 6.Kong KL, Kwong DL, Fu L, Chan TH, Chen L, Liu H, Li Y, Zhu YH, Bi J, Qin YR, et al. Characterization of a candidate tumor suppressor gene uroplakin 1A in esophageal squamous cell carcinoma. Cancer Res. 2010;70:8832–8841. doi: 10.1158/0008-5472.CAN-10-0779. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Li J, Cui Y, Li T, Ng KM, Geng H, Li H, Shu XS, Li H, Liu W, et al. CMTM3, located at the critical tumor suppressor locus 16q22.1, is silenced by CpG methylation in carcinomas and inhibits tumor cell growth through inducing apoptosis. Cancer Res. 2009;69:5194–5201. doi: 10.1158/0008-5472.CAN-08-3694. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi Y, Iwai M, Kawai T, Arakawa A, Ito T, Sakurai-Yageta M, Ito A, Goto A, Saito M, Kasumi F, et al. Aberrant expression of tumor suppressors CADM1 and 4.1B in invasive lesions of primary breast cancer. Breast Cancer. 2012;19:242–252. doi: 10.1007/s12282-011-0272-7. [DOI] [PubMed] [Google Scholar]
- 9.Yamada D, Kikuchi S, Williams YN, Sakurai-Yageta M, Masuda M, Maruyama T, Tomita K, Gutmann DH, Kakizoe T, Kitamura T, et al. Promoter hypermethylation of the potential tumor suppressor DAL-1/4.1B gene in renal clear cell carcinoma. Int J Cancer. 2006;118:916–923. doi: 10.1002/ijc.21450. [DOI] [PubMed] [Google Scholar]
- 10.Jiang W, Newsham IF. The tumor suppressor DAL-1/4.1B and protein methylation cooperate in inducing apoptosis in MCF-7 breast cancer cells. Mol Cancer. 2006;5:4. doi: 10.1186/1476-4598-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Zhang J, Ye M, Zhu M, Zhang B, Roy M, Liu J, An X. Tumor suppressor role of protein 4.1B/DAL-1. Cell Mol Life Sci. 2014;71:4815–4830. doi: 10.1007/s00018-014-1707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Zhou F, Jiang C, Wang Y, Lu Y, Yang F, Wang N, Yang H, Zheng Y, Zhang J. Identification of a DNA methylome profile of esophageal squamous cell carcinoma and potential plasma epigenetic biomarkers for early diagnosis. PLoS One. 2014;9:e103162. doi: 10.1371/journal.pone.0103162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng R, Huang JP, Li XF, Xiong WB, Wu G, Jiang ZJ, Song SJ, Li JQ, Zheng YF, Zhang JR. Epb41l3 suppresses esophageal squamous cell carcinoma invasion and inhibits MMP2 and MMP9 expression. Cell Biochem Funct. 2016;34:133–141. doi: 10.1002/cbf.3170. [DOI] [PubMed] [Google Scholar]
- 14.Saeki T, Mhashilkar A, Swanson X, Zou-Yang XH, Sieger K, Kawabe S, Branch CD, Zumstein L, Meyn RE, Roth JA, et al. Inhibition of human lung cancer growth following adenovirus-mediated mda-7 gene expression in vivo. Oncogene. 2002;21:4558–4566. doi: 10.1038/sj.onc.1205553. [DOI] [PubMed] [Google Scholar]
- 15.Ji L, Fang B, Yen N, Fong K, Minna JD, Roth JA. Induction of apoptosis and inhibition of tumorigenicity and tumor growth by adenovirus vector-mediated fragile histidine triad (FHIT) gene overexpression. Cancer Res. 1999;59:3333–3339. [PubMed] [Google Scholar]
- 16.Roz L, Gramegna M, Ishii H, Croce CM, Sozzi G. Restoration of fragile histidine triad (FHIT) expression induces apoptosis and suppresses tumorigenicity in lung and cervical cancer cell lines. Proc Natl Acad Sci USA. 2002;99:361–3620. doi: 10.1073/pnas.062030799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao X, Seidlitz E, Truant R, Hitt M, Ghosh HP. Re-expression of TSLC1 in a non-small-cell lung cancer cell line induces apoptosis and inhibits tumor growth. Oncogene. 2004;23:5632–5642. doi: 10.1038/sj.onc.1207756. [DOI] [PubMed] [Google Scholar]
- 18.Yang JY, Jiang SH, Liu DJ, Yang XM, Huo YM, Li J, Hua R, Zhang ZG, Sun YW. Decreased LKB1 predicts poor prognosis in pancreatic ductal adenocarcinoma. Sci Rep. 2015;5:10575. doi: 10.1038/srep10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Nagata M, Sakurai-Yageta M, Yamada D, Goto A, Ito A, Fukuhara H, Kume H, Morikawa T, Fukayama M, Homma Y, et al. Aberrations of a cell adhesion molecule CADM4 in renal clear cell carcinoma. Int J Cancer. 2012;130:1329–1337. doi: 10.1002/ijc.26160. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Zhang R, Yan K, Chen F, Huang W, Lv B, Sun C, Xu L, Li F, Jiang X. Mesenchymal stem cells inhibit lipopolysaccharide-induced inflammatory responses of BV2 microglial cells through TSG-6. J Neuroinflammation. 2014;11:135. doi: 10.1186/1742-2094-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerber MA, Bahr SM, Gutmann DH. Protein 4.1B/differentially expressed in adenocarcinoma of the lung-1 functions as a growth suppressor in meningioma cells by activating Rac1-dependent c-Jun-NH(2)-kinase signaling. Cancer Res. 2006;66:5295–5303. doi: 10.1158/0008-5472.CAN-05-1628. [DOI] [PubMed] [Google Scholar]
- 23.Rice TW, Rusch VW, Ishwaran H, Blackstone EH. Cancer of the esophagus and esophagogastric junction: Data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116:3763–3773. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 24.Al-Kaabi A, van Bockel LW, Pothen AJ, Willems SM. p16INK4A and p14A RF gene promoter hypermethylation as prognostic biomarker in oral and oropharyngeal squamous cell carcinoma: A review. Dis Markers. 2014;2014:260549. doi: 10.1155/2014/260549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baylin SB, Chen WY. Aberrant gene silencing in tumor progression: Implications for control of cancer. Cold Spring Harb Symp Quant Biol. 2005;70:427–433. doi: 10.1101/sqb.2005.70.010. [DOI] [PubMed] [Google Scholar]
- 26.Wong SY, Haack H, Kissil JL, Barry M, Bronson RT, Shen SS, Whittaker CA, Crowley D, Hynes RO. Protein 4.1B suppresses prostate cancer progression and metastasis. Proc Natl Acad Sci USA. 2007;104:12784–12789. doi: 10.1073/pnas.0705499104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Xu R, Li G, Xie X, Long J, Wang H. Loss of expression of the differentially expressed in adenocarcinoma of the lung (DAL-1) protein is associated with metastasis of non-small cell lung carcinoma cells. Tumour Biol. 2012;33:1915–1925. doi: 10.1007/s13277-012-0452-x. [DOI] [PubMed] [Google Scholar]
- 28.Ma X, Wang YW, Zhang MQ, Gazdar AF. DNA methylation data analysis and its application to cancer research. Epigenomics. 2013;5:301–316. doi: 10.2217/epi.13.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 30.Kikuchi S, Yamada D, Fukami T, Masuda M, Sakurai-Yageta M, Williams YN, Maruyama T, Asamura H, Matsuno Y, Onizuka M, et al. Promoter methylation of DAL-1/4.1B predicts poor prognosis in non-small cell lung cancer. Clin Cancer Res. 2005;11:2954–2961. doi: 10.1158/1078-0432.CCR-04-2206. [DOI] [PubMed] [Google Scholar]
- 31.Bock C, Halbritter F, Carmona FJ, Tierling S, Datlinger P, Assenov Y, Berdasco M, Bergmann AK, Booher K, Busato F, et al. BLUEPRINT consortium Quantitative comparison of DNA methylation assays for biomarker development and clinical applications. Nat Biotechnol. 2016;34:726–737. doi: 10.1038/nbt.3605. [DOI] [PubMed] [Google Scholar]
- 32.Reed K, Poulin ML, Yan L, Parissenti AM. Comparison of bisulfite sequencing PCR with pyrosequencing for measuring differences in DNA methylation. Anal Biochem. 2010;397:96–106. doi: 10.1016/j.ab.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 33.Hömig-Hölzel C, Savola S. Multiplex ligation-dependent probe amplification (MLPA) in tumor diagnostics and prognostics. Diagn Mol Pathol. 2012;21:189–206. doi: 10.1097/PDM.0b013e3182595516. [DOI] [PubMed] [Google Scholar]
- 34.Kuns R, Kissil JL, Newsham IF, Jacks T, Gutmann DH, Sherman LS. Protein 4.1B expression is induced in mammary epithelial cells during pregnancy and regulates their proliferation. Oncogene. 2005;24:6502–6515. doi: 10.1038/sj.onc.1208813. [DOI] [PubMed] [Google Scholar]
- 35.Deshpande A, Sicinski P, Hinds PW. Cyclins and cdks in development and cancer: A perspective. Oncogene. 2005;24:2909–2915. doi: 10.1038/sj.onc.1208618. [DOI] [PubMed] [Google Scholar]
- 36.Xiang Q, Zhen Z, Deng DY, Wang J, Chen Y, Li J, Zhang Y, Wang F, Chen N, Chen H, et al. Tivantinib induces G2/M arrest and apoptosis by disrupting tubulin polymerization in hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34:118. doi: 10.1186/s13046-015-0238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: A molecular definition. Oncogene. 2004;23:2825–2837. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- 38.Löbrich M, Jeggo PA. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat Rev Cancer. 2007;7:861–869. doi: 10.1038/nrc2248. [DOI] [PubMed] [Google Scholar]
- 39.Guo M, Ren J, House MG, Qi Y, Brock MV, Herman JG. Accumulation of promoter methylation suggests epigenetic progression in squamous cell carcinoma of the esophagus. Clin Cancer Res. 2006;12:4515–4522. doi: 10.1158/1078-0432.CCR-05-2858. [DOI] [PubMed] [Google Scholar]
- 40.Salam I, Hussain S, Mir MM, Dar NA, Abdullah S, Siddiqi MA, Lone RA, Zargar SA, Sharma S, Hedau S, et al. Aberrant promoter methylation and reduced expression of p16 gene in esophageal squamous cell carcinoma from Kashmir valley: A high-risk area. Mol Cell Biochem. 2009;332:51–58. doi: 10.1007/s11010-009-0173-7. [DOI] [PubMed] [Google Scholar]
- 41.Taghavi N, Biramijamal F, Sotoudeh M, Khademi H, Malekzadeh R, Moaven O, Memar B, A'rabi A, Abbaszadegan MR. p16INK4a hypermethylation and p53, p16 and MDM2 protein expression in esophageal squamous cell carcinoma. BMC Cancer. 2010;10:138. doi: 10.1186/1471-2407-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zare M, Jazii FR, Alivand MR, Nasseri NK, Malekzadeh R, Yazdanbod M. Qualitative analysis of Adenomatous Polyposis Coli promoter: Hypermethylation, engagement and effects on survival of patients with esophageal cancer in a high risk region of the world, a potential molecular marker. BMC Cancer. 2009;9:24. doi: 10.1186/1471-2407-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee EJ, Lee BB, Kim JW, Shim YM, Hoseok I, Han J, Cho EY, Park J, Kim DH. Aberrant methylation of Fragile Histidine Triad gene is associated with poor prognosis in early stage esophageal squamous cell carcinoma. Eur J Cancer. 2006;42:972–980. doi: 10.1016/j.ejca.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 44.Guo H, Zhou X, Lu Y, Xie L, Chen Q, Keller ET, Liu Q, Zhou Q, Zhang J. Translational progress on tumor biomarkers. Thorac Cancer. 2015;6:665–671. doi: 10.1111/1759-7714.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaz AM, Grady WM. Epigenetic biomarkers in esophageal cancer. Cancer Lett. 2014;342:193–199. doi: 10.1016/j.canlet.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fareed KR, Kaye P, Soomro IN, Ilyas M, Martin S, Parsons SL, Madhusudan S. Biomarkers of response to therapy in oesophago-gastric cancer. Gut. 2009;58:127–143. doi: 10.1136/gut.2008.155861. [DOI] [PubMed] [Google Scholar]