Abstract
Schizophrenia involves abnormalities in the medial frontal cortex that lead to cognitive deficits. Here we investigate a novel strategy to normalize medial frontal brain activity by stimulating cerebellar projections. We used an interval timing task to study elementary cognitive processing that requires both frontal and cerebellar networks that are disrupted in patients with schizophrenia. We report three novel findings. First, patients with schizophrenia had dysfunctional delta rhythms between 1–4 Hz in the medial frontal cortex. We explored cerebellar-frontal interactions in animal models and found that both frontal and cerebellar neurons were modulated during interval timing and had delta-frequency interactions. Finally, delta-frequency optogenetic stimulation of thalamic synaptic terminals of lateral cerebellar projection neurons rescued timing performance as well as medial frontal activity in a rodent model of schizophrenia-related frontal dysfunction. These data provide insight into how the cerebellum influences medial frontal networks and the role of the cerebellum in cognitive processing.
INTRODUCTION
Frontal brain regions are dysfunctional in schizophrenia1,2 and contribute to debilitating and difficult to treat negative symptoms including impaired cognitive processes such as working memory, attention and planning.2,3 Few therapies specifically target frontal dysfunction or reliably improve cognitive dysfunction in schizophrenia. One unexplored possibility is to target cerebellar circuits.4,5 The cerebellum contributes to cognitive function,6–8 and functional neuroimaging studies reveal lateral cerebellar activations in concurrence with frontal activation during cognitive tasks requiring working memory and executive function.9 Output from cerebellar hemispheres is relayed through lateral deep cerebellar nuclei10 and these projections to medial frontal cortex (MFC) can be compromised in schizophrenia.11 A recent proof of principle study indicated that cerebellar stimulation can attenuate some negative symptoms of schizophrenia.12 A randomized double-blind study further replicated these findings,13 yet the neural mechanism underlying the efficacy of cerebellar stimulation is unknown. Electrical stimulation of lateral cerebellar nuclei (LCN; analogous to the dentate nuclei in humans) in animal models indicates that cerebellar modulation of dopamine release in frontal networks may be one possible mechanism.14 However, it is unclear how cerebellar circuits interact with frontal networks and influence cognitive processing.
To investigate this issue, we studied cerebellar-frontal interactions in rodent models and in patients with schizophrenia during an elementary cognitive task: interval timing. This task requires working memory for temporal rules, attention to the passage of time and planning when to move. In general, these processes are disrupted in patients with schizophrenia, and timing is specifically and reliably disrupted in patients as well as first-degree relatives, in addition to several animal models of schizophrenia.15–17 Thus, interval timing is particularly well suited for studying cognition in schizophrenia because it is disrupted in patients with schizophrenia and in rodent models and involves both cerebellar networks and the MFC.16,18–20
This prefrontal region is specifically dysfunctional in schizophrenia, has anatomical and functional homologies with rodent MFC1,21 and involves common patterns of neuronal activity in humans and rodents during timing tasks.22,23 Recent work from our group has shown that single MFC neurons are involved in temporal processing during interval timing.20,22,24 The MFC also exerts top-down control over neural activity in other brain areas to promote goal-oriented behavior.25,26 For instance, we have found that the MFC can promote neuronal activity related to waiting or to adaptive control in downstream brain areas such as motor cortex.23,27 These data imply that disruptions in MFC might have far-reaching behavioral consequences. We have also found that disrupting MFC D1-type dopamine receptors, which have been implicated in schizophrenia,28,29 specifically impairs temporal processing by MFC neuronal ensembles. Because the cerebellum can be involved in temporal processing, and because cerebellar projections may influence frontal networks,10 we explored how LCN stimulation influenced MFC networks during interval timing. We tested the specific hypothesis that stimulation of LCN projections to the thalamus can compensate for impaired MFC temporal processing during interval timing.
Here, we report that patients with schizophrenia also had attenuated MFC delta rhythms. We recorded from MFC and LCN neuronal ensembles during interval timing in rodents and found that MFC and LCN neurons had spectral interactions at delta frequencies. Finally, we found that delta-frequency optogenetic stimulation of LCN projections in the thalamus can compensate for MFC dysfunction in rodent models. These results illustrate how cerebellar projections can refine timing-related activity in medial frontal brain networks, which might help us understand cerebellar contributions to cognitive processing.
MATERIALS AND METHODS
Humans
The electroencephalography (EEG) arm of this study included nine patients with schizophrenia recruited from the Iowa Longitudinal Database and nine sex-, education- and age-matched controls recruited from the University of Iowa Department of Neurology’s Cognitive Neuroscience Registry for Normative Data (Table 1 for patient data). Written informed consent was obtained from every subject and all research protocols were approved by the University of Iowa Human Subjects Review Board.
Table 1.
Patient demographics for patients with schizophrenia and controls
| Human EEG demographics | Controls s.e. | Schizophrenia s.e. | Stat | D.f. | P-value |
|---|---|---|---|---|---|
| Age (years) | 22–65 (avg 43.43) ± 6.22 | 34–58 (avg 49.5) ± 2.90 | t = 1.28 | 8 | 0.235 |
| Age at diagnosis (years) | 15–37 (avg 25.56) ± 2.29 | ||||
| Ed (years) | 14.89 ± 0.59 | 13.22 ± 4.41 | t = − 1.66 | 8 | 0.134 |
| Sex | M7, F2 | M7, F2 | X2 = 0.00 | 1 | 1.000 |
| Medication status | 7 Atypical, 1 typical, 1 both | ||||
| Verbal fluency | 49.22 ± 4.64 | 29.44 ± 5.25 | t = − 3.10 | 8 | 0.001 |
| MOCA score | 28.00 ± 0.55 | 24.4 ± 1.09 | t = − 3.71 | 8 | 0.006 |
| Digit span | 21.11 ± 1.15 | 14.67 ± 1.21 | t = − 3.62 | 8 | 0.007 |
Abbreviations: D.f., degrees of freedom; Ed, education; EEG, electroencephalography; F, female; M, male; MOCA, Montreal Cognitive Assessment.
Human interval timing task
Interval timing was investigated in humans with and without schizophrenia (see Supplementary Methods and Figure 1a).22 The interval timing task consisted of four blocks of 40 trials (160 trials in total). Trials were presented in pseudorandom order. All trials began when a numerical cue stimulus appeared on the center of the screen indicating the temporal interval the participants were instructed to estimate (3 or 12–3 s trials were excluded in this study). Participants made responses by pressing the space bar on a keyboard using their dominant hand when they estimated the temporal interval had elapsed. Participants received feedback about their response time at the end of each trial. There was a 3–6 s interval between response and feedback. After feedback, participants moved to the next trial by pressing the space bar. The task was self-paced and the participants were asked not to count in their head during the task. Participants performed four practice trials before the real task. The interval timing task consisted of 160 trials with either a 3 or 12 s interval; only data from the 12 s interval was included in this manuscript.
Figure 1.
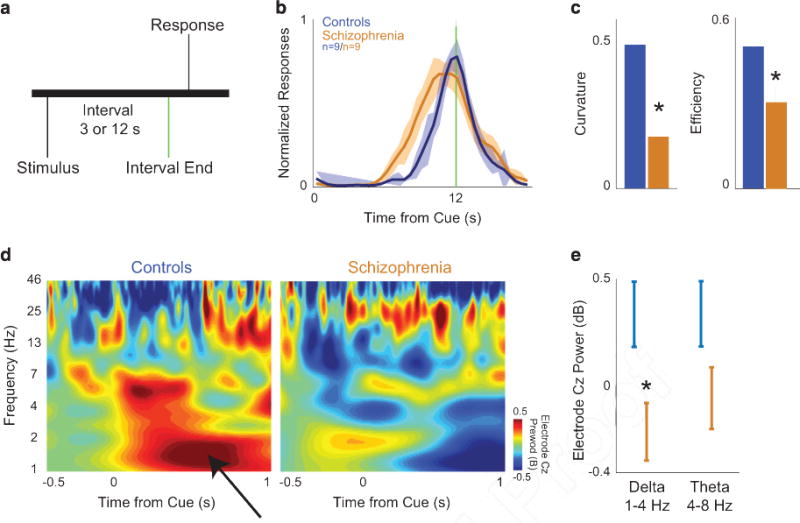
Medial frontal delta activity is attenuated in patients with schizophrenia and rodent models during interval timing. (a) We study elementary cognitive processing using an interval timing task, in which participants must estimate an interval of time by making a motor response. (b) Patients with schizophrenia (orange, n = 9) have broader estimates of the 12 s interval than demographically matched controls (blue, n = 9). (c) Patients with schizophrenia have flatter time-response histograms (curvature) and are less efficient during interval timing. (d) electroencephalography (EEG) activity from mid-frontal electrode Cz indicated that controls had prominent bursts of delta activity triggered by the instructional cue (black arrow). (e) Patients with schizophrenia had attenuated delta activity (1–4 Hz). Other bands, such as theta activity (5–8 Hz), were not significantly changed. All data are presented as mean ± s.e.m. Asterisks indicate significance at P<0.05. Also see Supplementary Figures S1 and S2.
For both human and rodent interval timing, we quantified behavioral performance using metrics that we and others have used extensively.4,22,30,31 Briefly, we measured the efficiency as the number of responses at 12 s divided by the total number of responses; this number is closer to 1 if most responses are at 12 s. Second, we measured the curvature of time-response histograms by calculating the deviation of the cumulative sum from a straight line; curvature has been used for over 50 years to quantify interval timing behavior.30 Curvature indices are higher with well-timed, more ‘curved’ time-response histograms. See Supplementary Methods and our prior work for further details.22
Neurophysioogical recording and time-frequency analysis
Twenty-one-lead 10/20 EEG recording and analysis was performed identically to prior work22,23 and are described at length in the Supplementary Methods. Bilateral mastoid re-referencing was used. Detailed methods for time-frequency measures for human and rodent data have been published in our previous work and in the Supplementary Methods.4,22–24 We used the KPSS (Kwiatkowski, Phillips, Schmidt and Shin) test to confirm that all EEG signals were stationary before subsequent analyses. Granger causality between rodent MFC and LCN was performed using the MGVC toolbox.32
Rodents
A total of 27 male Long–Evans rats (aged 2 months; 200–225 g) were included in this study. There were four groups in this study (1) simultaneous left MFC and right LCN recordings in 4 rodents, (2) pharmacological right LCN inactivation in 11 rodents, (3) optogenetic stimulation of the right LCN with left MFC dysfunction in 9 rodents and (4) left MFC recordings with LCN-VL (ventrolateral nucleus) stimulation in three rodents. Animals were trained in an interval timing task highly similar to the human version according operant techniques described in previous work and in the Supplementary Methods.20,22 The task had 3 and 12 s intervals, with a uniformly varying 6–12 s intertrial interval; early responses occurring before interval end were not reinforced. Surgical procedures, neurophysiological recordings and focal drug infusions were performed according to procedures described previously.20,24,33 The coordinates for left medial frontal implants were AP: +3.2; ML: ± 1.2; DV: − 3.5 at 12 °C in the lateral plane; for left VL: AP: − 2.3; ML: ± 1.8; DV: − 5.4; for right LCN AP: − 10.8; ML: ± 3.6; DV: − 6.2. LCN-VL projections were optogenetically stimulated during interval timing tasks using 473 nm light delivered at 10 mW power on 50% of trials. See the Supplementary Methods for further details.20,22 Animals were transcardially perfused at the completion of experiments.
RESULTS
MFC delta activity and interval timing in humans and rodents We studied the dynamics of LCN-MFC activity using an interval timing task. During this task, participants estimate an interval of several seconds as instructed by a cue (Figure 1a). We quantified interval timing performance in two ways. First, we calculated the efficiency of responses by calculating the fraction of responses between 11 and 12 s divided by the overall number of responses. As efficiency is closer to 1, the fraction of responses at 12 s is greater, indicating that participants guide their actions in time more accurately.24 Second, we calculated the ‘curvature’ of time-response histograms by measuring the deviation from the cumulative distribution of a straight line.4,22,30 This metric is 0 with a flat time-response curve during the interval and is closer to 1 when more responses are at 12 s and time-response histograms are more curved. Patients with schizophrenia were impaired on the interval timing task and estimated the end of the interval earlier relative to demographically matched controls (Table 1 and Figure 1b).34,35 By these metrics patients with schizophrenia had impaired interval timing (Figure 1c; efficiency P<0.01, power = 0.99; curvature P<0.01, power = 0.98; data from nine patients and nine controls; statistics in Table 2; raw data in Supplementary Figure S1).30 Our group has shown that bursts of MFC delta (1–4 Hz) and theta (5–8 Hz) activity that are triggered by the instructional cue during interval timing tasks can be measured by scalp EEG from electrode Cz (black arrow; Figure 1d).22,23 In patients with schizophrenia, MFC delta activity at Cz was attenuated (Figure 1e; P<0.05, power = 0.96; Supplementary Figures S2 and S3).
Table 2.
Statistical data
| Significant statistics | Stat | D.f. | P-value | Figure | Power |
|---|---|---|---|---|---|
| Controls vs schizophrenia: efficiency | t = 2.4 | 8 | 0.0110 | 1C | 0.99 |
| Controls vs schizophrenia: curvature | t = 3.3 | 8 | 0.0084 | 1C | 0.82 |
| Controls vs schizophrenia: Cz delta | t = 2.3 | 16 | 0.0382 | 1E | 0.94 |
| %Δ LCN muscimol vs saline: efficiency | t = − 3.3 | 10 | 0.0078 | 2B | 0.99 |
| %Δ LCN muscimol vs saline: curvature | t = 1.9 | 10 | 0.0880 | 2B | 0.8 |
| Correlation with response times early in trial | t = 2.3 | 28 | 0.0300 | 2H | 0.99 |
| LCN-MFC functional interactions vs shuffle | χ2 = 343.0 | 2316 | 0.0000 | 3D | 0.99 |
| LCN-MFC JPSTH interactions vs shuffle | χ2 = 6.9 | 2316 | 0.0090 | 3D | 0.99 |
| Differential directional connectivity (Granger) delta | t = 4.1 | 283 | 6.54−5 | 3E | 1.0 |
| Differential directional connectivity (Granger) beta | t = 7.9 | 291 | 4.64−14 | 3E | 1.0 |
| %Δ MFC Sch vs saline: efficiency | t = − 4.3 | 10 | 0.0017 | 4B | 0.99 |
| %Δ MFC Sch+LCN Stim at 2 Hz: efficiency | t = 2.4 | 8 | 0.0444 | 4B | 0.99 |
| %Δ MFC Sch vs saline: curvature | t = 2.5 | 10 | 0.0325 | 4B | 0.99 |
| %Δ MFC Sch vs MFC Sch+Stim at 2 Hz: curvature | t = 2.7 | 8 | 0.0279 | 4B | 0.99 |
| MFC Sch+Stim at 2 Hz: ramping neurons | χ2 = 4.2 | 23 | 0.0400 | 4C | 0.99 |
| MFC Sch: delta activity | t = 3.1 | 8 | 0.0200 | 4E | 0.93 |
Abbreviations: LCN, lateral cerebellar nuclei; JPSTH, joint-peristimulus time histogram; MFC, medial frontal cortex; Sch, ; Stim, stimulated.
Interactions between LCN and medial frontal neurons
The cerebellum has been shown to be involved in subsecond timing36 and in higher cognitive processes such as working memory.7 To study cerebellar contributions to timing over an interval of several seconds, we focally inactivated LCN using muscimol. We trained 11 rodents to perform an interval timing task using lever presses motivated by liquid rewards (Figure 1a and Supplementary Video S1). After animals were well trained, we unilaterally inactivated the right LCN via focal infusions of muscimol, a GABAA agonist (Figure 2a).20 Rodents with LCN inactivated tended to have impaired interval timing performance compared with control sessions with saline focally infused into the LCN (Figure 2b; efficiency: P<0.01; curvature trended P<0.09; data from 11 rodents). This result indicates that the LCN is involved in efficient suprasecond as well as subsecond interval timing.
Figure 2.
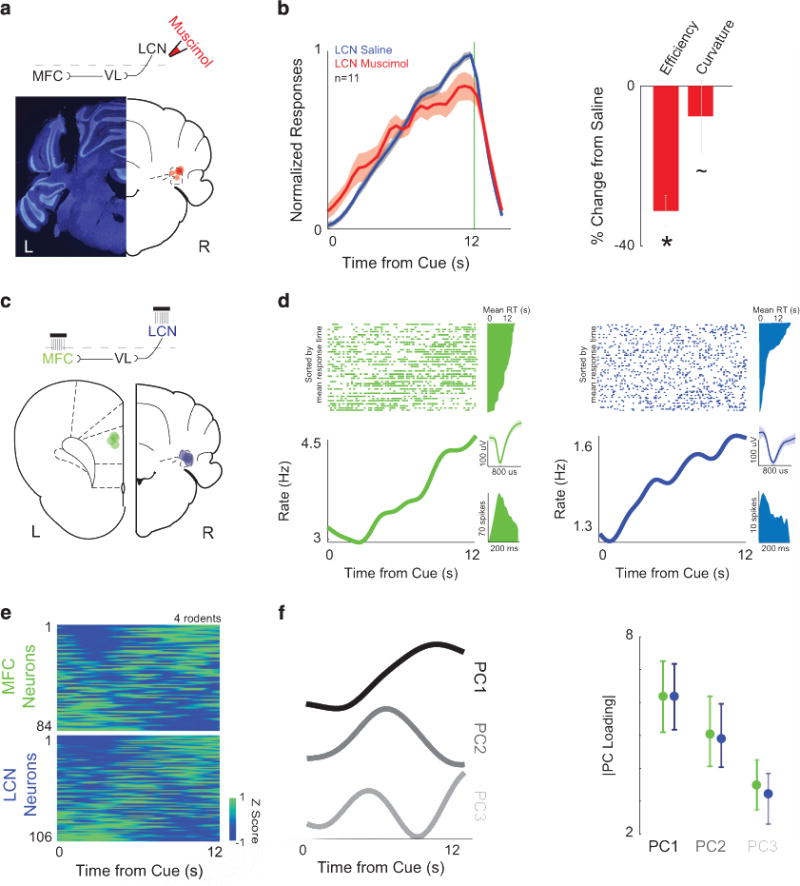
Lateral cerebellar nuclei (LCN) and medial frontal cortex (MFC) neurons are involved in temporal processing. (a) Cannula locations for focal infusions into LCN are illustrated with red dots on the right, while an individual example is shown on the left (note this image is mirrored as all cerebellar manipulations were on the right). (b) Inactivating LCN using muscimol (red) significantly impaired interval timing efficiency compared with control sessions (while curvature trended significant), with saline infused into the LCN (blue; n=11). (c) Simultaneous neural recordings from the left MFC (green, L—locations for each animal indicated by dots on schematic) and the right LCN (blue, R) in four rats during interval timing revealed (d) robust time-related ramping—that is, neural activity that consistently increased/decreased activity over the interval. Perievent rasters are sorted with respect to mean response time, with trials with a short mean response time on the bottom and trials with a longer mean response time on top. To the right of the rasters are the mean response times that correspond to each trial. Single neurons were isolated based on waveform (inset) and interspike intervals (green—MFC; blue—LCN). (e) Heatmaps of perievent time histograms revealed that time-related ramping was a ubiquitous feature of MFC and LCN ensembles (84 MFCs and 106 LCN neurons recorded simultaneously in four animals). Color scale is at bottom right. Neurons are sorted based on PC1 from (f) principal component analysis, a data-driven technique that identified time-related ramping as the first principal component in both MFCs and LCNs. There were no consistent differences between MFC and LCN neuronal ensembles for PCs 1–3; see Supplementary Figure S4 for scree plot of variance). All data from 84 MFC neurons and 106 LCN neurons in four rodents. Asterisk indicates significance at P<0.05 via t-test, displayed as mean ±s.e.m.
To identify how LCN neurons might influence MFC networks, we recorded neural activity simultaneously in the left MFC and right LCN while animals performed the interval timing task (note that LCN projections decussate in the midbrain).37,38 We recorded 84 MFC and 106 LCN neurons simultaneously in four rodents (Figure 2c). Work by our group and others have demonstrated that time-related ramping activity is a prominent feature of MFC neuronal activity.20,39–41 Our recordings indicate that LCN neuronal ensembles also had prominent ramping activity (Figures 2d and e) identified by data-driven techniques such as principal component analysis (ramping is PC1; Figure 2f and Supplementary Figure S4). MFC and LCN ensembles had similar projections for PCs 1–3 (Figure 2f). Finally, regression identified a similar fraction of neurons with significant linear and quadratic fits in the LCN and MFC (Supplementary Figure S5). The average slope for MFC neurons was 0.15 ± 0.05 spikes per s2, which was similar to the slope for the LCN (0.13 ± 0.03 spikes per s2).
These data indicate that despite the fact that LCN and MFC neuronal ensembles are not directly connected,10,42,43 they had similar patterns of activity during interval timing that could not be easily differentiated. When neuronal activity was aligned to responses, activity in MFC and LCN could correlate with when responses occurred in the interval (12% of MFC neurons and 10% of LCN neurons; data from ± 0.5 s around lever press); however, as we were focused on cue-triggered delta activity and cue-triggered neural activity (Figure 1), we did not further analyze-response-related activity. Taken together, these data indicate that LCN neurons are involved in temporal processing on a scale of several seconds.
Because we recorded in both LCN and MFC simultaneously, we were able to look at interactions between neuronal ensembles in each area. Consistent with prior anatomical evidence,10,42,43 we could not find consistent evidence of fast relationships between LCN and MFC via techniques such as cross-correlation. However, on a trial-by-trial basis LCN and MFC average firing rates had strong correlations (example: Figure 3a; analysis restricted to 12 s trials only). Surprisingly, across 2316 MFC/LCN pairs in four animals, ~ 25% had functionally significant interactions (compared with trial-shuffled data; 116 pairs in shuffled data; X2 = 343, P<0.05). To examine the temporal evolution of these interactions, we analyzed joint-peristimulus time histograms (JPSTHs),44 which examine the evolution of firing rate correlations over time (Figure 3b). We found more LCN-MFC JPSTH interactions at relatively large bins (1 s bins: 293/2316, or 13%) compared with smaller bins (0.1 s bins: 207 of 2316; X2 = 6.9, P<0.009; both more than in shuffled data). The average pattern of JPSTH interactions increased later in the interval (Figure 3c). In support of this temporal profile, we found stronger trial-by-trial correlations for 4–12 s than for 0–4 s (analysis identical to Figure 3a, except with 4 s bins; 265 interactions from 0 to 4 s vs 332 from 4 to 8 s and 342 from 8 to 12 s; X2 = 13.0 P<0.001). These paired recordings in both areas indicate that despite having no evidence of coherence, MFC and LCN neurons had significant time-varying functional relationships during interval timing, with more interactions later in the interval.
Figure 3.
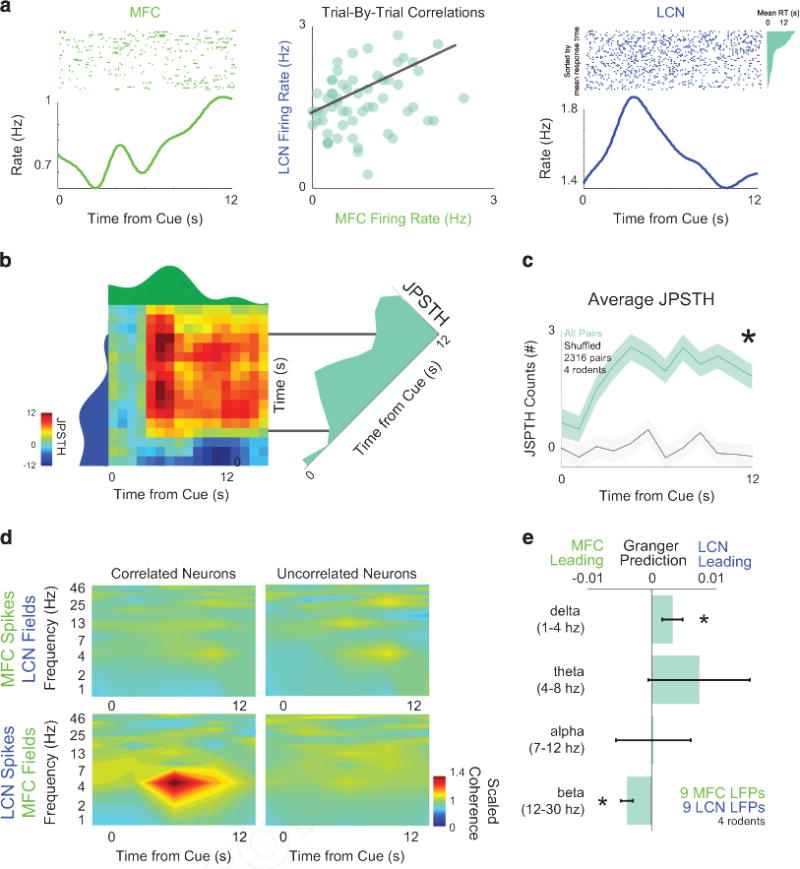
Interactions between lateral cerebellar nuclei (LCN) and medial frontal cortex (MFC). (a) Single MFC neurons (green) could have firing rate correlations with LCN neurons (blue) over trials; r = − 0.38; P<0.001. Rasters are sorted by mean response time and response times shown in teal for both simultaneously recorded neurons, as in Figure 2d. (b) To examine the evolution of these correlations during the interval, we turned to joint-peristimulus time histogram (JPSTH), in which the dynamics of correlations can be analyzed. For this representative pair of neurons, JPSTH revealed increased correlations later in the interval. (c) This pattern was consistent across all possible combinations of LCN-MFC neurons (blue-green). Data from 2316 pairs from 84 MFC and 106 LCN neurons in four animals. (d) Cross-area spike-field coherence revealed phase-locked activity at delta frequencies only for MFC LFPs with LCN neurons with significant trial-by-trial correlations. No other patterns of cross-regional spike-field coherence for LCN fields or for uncorrelated neurons were observed. (e) Granger causality (teal) indicates that LCN activity consistently led delta MFC activity in the delta band between 1 and 4 Hz (in nine LFPs from each area in three animals) in comparison with time-shuffled data (gray); notably, MFC led LCN activity in the beta band between 12 and 30 Hz. All data from 2316 MFC-LCN pairs in four rodents. Asterisk indicates significance at P<0.05 via analysis of variance (ANOVA). See Supplementary Figures S6 and S7.
To examine the spectral features of LCN-MFC interactions, we examined coherence among single neurons and local field potentials.45 Consistent with lack of fast interactions between MFC and LCN, we observed no spike-spike coherence between MFC and LCN neurons. However, for LCN neurons that had significant trial-by-trial correlations with MFC neurons, we found delta band cross-area spike-field coherence between LCN neurons and MFC field potentials (Figure 3d). This cross-area spike-field coherence was not present for correlated MFC neurons and LCN fields nor was it found for neurons without correlations. To examine the spectral features of this causality in detail, we used Granger causality (Supplementary Figure S7). Analyses of differential directional connectivity revealed LCN fields had a significant causal relationship to MFC in the delta frequency (1–4 Hz; P<0.000006). Conversely, MFC LFPs had a significant causal relationship to LCN LFPs in the beta range (12–30 Hz; Figure 3e; P<10−13). This reciprocal connectivity is supported by work by Kelly and Strick.46 Prior work from our group and others has suggested that activity in the delta-range couples neurons across frontal cortex in service of cognitive control.23,47 Cross-area LCN-MFC coherence could provide insight into how cerebellar circuits might influence cortical processing and leads to the hypothesis that LCN delta-range activity can modulate MFC neurons.
Cerebellar projections and medial frontal activity
Our results thus far suggest that LCN projections might have the potential to influence MFC activity. Because the MFC is dysfunctional in schizophrenia,1 we were interested to see if manipulations of cerebellar projections can compensate for MFC dysfunction. To model aspects of schizophrenia-related MFC dysfunction, we locally blocked MFC D1DRs using SCH23390.1,2,28,29 Patients with schizo phrenia have abnormalities in frontal D1DRs, and blocking these receptors has been used to model aspects of prefrontal dysfunction in schizophrenia.2,28,29 Although there are many models of schizophrenia, abnormal frontal D1 signaling reliably produces deficits in interval timing behavior, disrupts temporal processing by single MFC neurons and attenuates MFC delta activity (Supplementary Figure S8).20,24,38,48
In animals with MFC D1DR dysfunction, we optogenetically stimulated LCN projections to the thalamus. We stimulated synaptic terminals of LCN neurons in the VL expressing channelrhodopsin2 (Figure 4a, Supplementary Figures S8 and S9). Strikingly, optogenetic stimulation of LCN-VL projections at 2 Hz, but not 4, 10 or 20 Hz, rescued behavioral deficits induced by MFC D1DR blockade (efficiency P<0.04; curvature P<0.03 in 9 rodents; power = 0.998; Figure 4b, Supplementary Videos S2 and S3). In line with prior work on LCN, there was no clear effect of optogenetic LCN-VL terminal stimulation on lever pressing, rewards or open-field activity (Supplementary Figure S10).49 There was no effect in sessions without MFC D1 blockade or in separate control animals with AAV-mCherry (Supplementary Figures S11 and S12). These data intimate that increasing activity of the LCN can compensate for D1DR-dependent MFC deficits in the temporal control of action.
Figure 4.
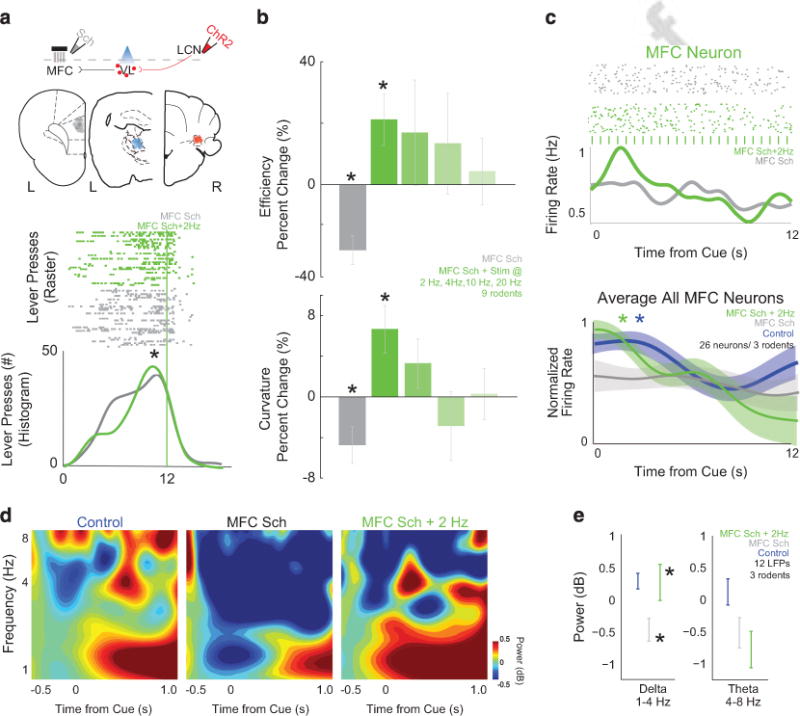
Stimulation of cerebellar projections can compensate for frontal dysfunction. We modeled aspects of frontal dysfunction in schizophrenia by pharmacologically disrupting medial frontal cortex (MFC) D1 dopamine receptors with SCH23390. (a) Histological analyses revealed electrode infusion cannula ensemble placement in the MFC (gray), optical cannula in the ventrolateral thalamus (VL, blue) and channelrhodopsin2 (ChR2) infusion/cannula placement in the lateral cerebellar nuclei (LCN) for individual animals as represented by each dot. Stimulating LCN-VL projections at 2 Hz could rescue behavioral deficits caused by MFC SCH23390 (lower plot in a; MFC SCH23390: gray— LCN-VL stimulation: green; ticks are individual lever presses from the entire experiment). (b) Group data from eight rodents showed impaired behavior with MFC SCH23390 and behavioral rescue with 2 Hz stimulation of LCN-VL projections. (c) During interval timing, MFC neurons have time-related ramping, which is decreased with MFC SCH23390 (gray). For this neuron, with LCN-VL 2 Hz stimulation, ramping increased (green); each blue tick is a 473 nm laser pulse. With LCN-VL 2 Hz stimulation, ramping activity increased as measured by average activity or by linear regression; MFC-saline without stimulation is labeled in blue. Data from 26 MFC neurons in three rodents; asterisk indicates significance at P<0.05 via χ2 test. (d) Similar to humans, rodents have a burst of delta/theta activity as shown by time-frequency analyses, which is decreased in animals with MFC D1DR blockade (gray—MFC SCH23390; n = 12 LFPs in three rodents). (e) With LCN-VL 2 Hz stimulation (green), delta MFC activity significantly improved. Asterisks indicate significance at P<0.05 via paired t-test displayed; all data are displayed as mean ± s.e.m.
If LCN stimulation improves behavior, then it should rectify temporal processing in the MFC that is disrupted with MFC D1DR blockade.20 To test this idea, we recorded MFC neuronal ensembles after focally infusing SCH23390 into the MFC and optogenetically stimulating LCN projections in the thalamus (Figure 4c). In 26 MFC neurons from three animals, 2 Hz stimulation of LCN-VL terminals increased ramping activity in individual neurons by increasing the firing rate for the first 3 s of the interval (Figure 4c; P<0.05). More MFC neurons were fit by linear regression on stimulation trials compared with non-stimulation trials (26% vs 4%; P<0.04). Both patients with schizophrenia and rodent models of MFC dysfunction have attenuated delta activity (Figures 1d, e and 4d).20,33 In these animals, 2 Hz LCN-VL stimulation increased MFC delta activity, restoring delta activity to MFC-saline levels (P<0.04; Figures 4d and e). Two hertz LCN-VL also trended to increase the number of MFC neurons with cue-triggered delta spike-field coherence, which our previous work has shown depends on MFC D1DRs (2 of 17 MFC neurons had significant cue-triggered delta spike-field coherence in MFC D1 blockade sessions, vs 7 MFC neurons with 2 Hz LCN-VL stimulation; X2 = 3.8; P = 0.05). These data indicate that 2 Hz stimulation of LCN-VL projections could rescue both behavioral deficits and MFC neuronal dysfunction caused by D1DR blockade, lending support to the idea that the cerebellar circuits can compensate for dysfunctional MFC activity.
DISCUSSION
We tested if cerebellar stimulation could normalize dysfunctional frontal networks and report three main findings: (1) patients with schizophrenia had attenuated MFC delta activity during interval timing and compromised projections from the cerebellum to the MFC, (2) MFC and LCN neuronal ensembles had shared temporal processing and extensive interactions, particularly at delta frequencies and (3) specifically stimulating LCN-VL projections could recover both behavioral performance and neuronal activity associated with elementary cognitive operations. Our results show that cerebellar and MFC networks interact at delta frequencies, providing evidence that cerebellar circuits can contribute to MFC cognitive processing, particularly when the MFC is not functioning optimally. This idea could be helpful in developing and optimizing treatments that target cerebellar circuits to improve MFC function in human diseases such as schizophrenia.4
The cerebellum is consistently active in cognitive tasks;7,18 however, patients with cerebellar lesions do not consistently have cognitive deficits.50 Cerebellar processing may be crucial in particular contexts, such as learning or compensating for cortical dysfunction.4 Thus, cerebellar circuits may be particularly well suited to therapeutic applications that seek to normalize cortical function. Our findings suggest a previously unappreciated role for the cerebellum in temporal processing on the scale of seconds, and indicate that cerebellar output nuclei have similar patterns of cognitive processing with the MFC. To our knowledge, our study is the first to probe neuronal activity in both the MFC and LCN during a cognitive task, and provides insight into cerebellar-frontal interactions during cognitive processing. Our working model is that LCN neurons provide temporal information to MFC neurons in the form of a ramping signal, and that this spiking activity arrives in-phase with 1–4 Hz delta MFC rhythms that engage temporal processing in the frontal cortex.20,24 We observe that with MFC D1 blockade these MFC delta rhythms are attenuated; however, we find that stimulating LCN synaptic terminals at delta frequencies increases MFC delta power and temporal processing, possibly by boosting corticothalamic spiking in-phase with MFC delta rhythms. Future work will elucidate precisely how LCN-VL input influences MFC spiking activity, as it could occur via a direct corticothalamic projection or an indirect projection to the VTA, striatum or other brain network.42 Extending this work to other tasks involving working memory, attention, planning and reasoning will further help define the role of the cerebellum in cognition.
Low-frequency stimulation of cerebellar networks has been partially effective at relieving some symptoms in treatment-resistant schizophrenia patients.12,13 In addition, cerebellar vermal transcranial magnetic stimulation can produce downstream changes in neuronal activity in the frontal cortex.51 Our data suggest that the cerebellar contribution to MFC activity is frequency-specific and that delta-range stimulation may be uniquely effective in modulating cognitive processing. Delta-range activity in MFC is coherent with single neuron activity; we found that LCN neurons were phase-locked with low-frequency MFC rhythms, and LCN field potentials causally predict MFC delta activity. More broadly, delta-range activity is found across diverse brain networks and appears to represent the need for cognitive control.47,52,53 Stimulating LCN projections in the thalamus might boost these cognitive control signals and counteract MFC dysfunction induced by disrupting cortical dopamine signaling.
Here we study cerebellar-frontal interactions in a highly limited model of aspects of frontal dysfunction in rodent models based on disrupting MFC D1DRs. This model has some clinical relevance and consistently affects interval timing as well as MFC neurons, and thus affords a unique opportunity to map this circuit in detail.20,24,28,29,48 There are many other animal models with relevance for schizophrenia, but mapping cerebellar-frontal interactions in these models will require extensive behavioral and neurophysiological characterization of MFC and LCN neurons before determining how best to intervene in these models. However, these efforts will likely generate a detailed understanding of the scope of cerebellar-frontal interactions, which could inform future translational efforts targeting the cerebellum for patients with schizophrenia.4
Supplementary Material
Acknowledgments
We thank members of the Narayanan Lab, Erik Carlson, MD, PhD, John Freeman, PhD and Vince Magnotta, PhD for scientific discussion and Alane Tranel and Tom Wassink, MD, PhD for assistance with patient recruitment. KLP has received generous funding to complete this research from the Brain and Behavior Foundation Young Investigator NARSAD Award, The Nellie Ball Research Trust and NIMH K01 MH106824. NSN has received funding from an NIND R01 NS089470.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
KLP and NN designed research, analyzed data and wrote the manuscript. KLP performed rodent research. NCA provided the human schizophrenia population, KLP and K-HC performed human studies, RMK conducted Granger Causality analyses, KLP, YK, AJN and VAM-E conducted histological analyses, and all authors provided feedback on manuscript.
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
References
- 1.Andreasen NC, O’Leary DS, Flaum M, Nopoulos P, Watkins GL, Boles Ponto LL, et al. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naïve patients. Lancet. 1997;349:1730–1734. doi: 10.1016/s0140-6736(96)08258-x. [DOI] [PubMed] [Google Scholar]
- 2.Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 4.Parker KL, Narayanan NS, Andreasen NC. The therapeutic potential of the cerebellum in schizophrenia. Front Syst Neurosci. 2014;8:163. doi: 10.3389/fnsys.2014.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker KL. Timing tasks synchronize cerebellar and frontal ramping activity and theta oscillations: implications for cerebellar stimulation in diseases of impaired cognition. Front Psychiatry. 2016;6:190. doi: 10.3389/fpsyt.2015.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80:807–815. doi: 10.1016/j.neuron.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 7.E K-H, Chen S-HA, Ho M-HR, Desmond JE. A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum Brain Mapp. 2012:35593–615. doi: 10.1002/hbm.22194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H, et al. Consensus Paper: the cerebellum’s role in movement and cognition. Cerebellum. 2013;13:151–177. doi: 10.1007/s12311-013-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoodley CJ. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 2011;11:352–365. doi: 10.1007/s12311-011-0260-7. [DOI] [PubMed] [Google Scholar]
- 10.Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H, Fan G, Xu K, Wang F. Changes in cerebellar functional connectivity and anatomical connectivity in schizophrenia: a combined resting-state functional MRI and diffusion tensor imaging study. J Magn Reson Imag. 2011;34:1430–1438. doi: 10.1002/jmri.22784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demirtas-Tatlidede A, Freitas C, Cromer JR, Safar L, Ongur D, Stone WS, et al. Safety and proof of principle study of cerebellar vermal theta burst stimulation in refractory schizophrenia. Schizophr Res. 2010;124:91–100. doi: 10.1016/j.schres.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garg S, Sinha VK, Tikka SK, Mishra P, Goyal N. The efficacy of cerebellar vermal deep high frequency (theta range) repetitive transcranial magnetic stimulation (rTMS) in schizophrenia: a randomized rater blind-sham controlled study. Psychiatry Res. 2016;243:413–420. doi: 10.1016/j.psychres.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Mittleman G, Goldowitz D, Heck DH, Blaha CD. Cerebellar modulation of frontal cortex dopamine efflux in mice: Relevance to autism and schizophrenia. Synapse. 2008;62:544–550. doi: 10.1002/syn.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clausen J. An evaluation of experimental methods of time judgment. J Exp Psychol. 1950;40:756–761. doi: 10.1037/h0056354. [DOI] [PubMed] [Google Scholar]
- 16.Ward RD, Kellendonk C, Kandel ER, Balsam PD. Timing as a window on cognition in schizophrenia. Neuropharmacology. 2012;62:1175–1181. doi: 10.1016/j.neuropharm.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penney TB, Meck WH, Roberts SA, Gibbon J, Erlenmeyer-Kimling L. Interval-timing deficits in individuals at high risk for schizophrenia. Brain Cogn. 2005;58:109–118. doi: 10.1016/j.bandc.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Ivry RB, Keele SW, Diener HC. Dissociation of the lateral and medial cerebellum in movement timing and movement execution. Exp Brain Res. 1988;73:167–180. doi: 10.1007/BF00279670. [DOI] [PubMed] [Google Scholar]
- 19.Merchant H, Harrington DL, Meck WH. Neural basis of the perception and estimation of time. Annu Rev Neurosci. 2013;36:313–336. doi: 10.1146/annurev-neuro-062012-170349. [DOI] [PubMed] [Google Scholar]
- 20.Parker KL, Chen K-H, Kingyon JR, Cavanagh JF, Narayanan NS. D1-dependent 4 Hz oscillations and ramping activity in rodent medial frontal cortex during interval timing. J Neurosci. 2014;34:16774–16783. doi: 10.1523/JNEUROSCI.2772-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laubach M. Neural Basis of Motivational and Cognitive Control. MIT Press; Cambridge, MA: 2011. A comparative perspective on executive and motivational control by the medial prefrontal cortex. [Google Scholar]
- 22.Parker KL, Chen K-H, Kingyon JR, Cavanagh JF, Naryanan NS. Medial frontal ~ 4 Hz activity in humans and rodents is attenuated in PD patients and in rodents with cortical dopamine depletion. J Neurophysiol. 2015;114:1310–1320. doi: 10.1152/jn.00412.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narayanan NS, Cavanagh JF, Frank MJ, Laubach M. Common medial frontal mechanisms of adaptive control in humans and rodents. Nat Neurosci. 2013;16:1888–1897. doi: 10.1038/nn.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker KL, Ruggiero RN, Narayanan NS. Infusion of D1 dopamine receptor agonist into medial frontal cortex disrupts neural correlates of interval timing. Front Behav Neurosci. 2015;9:294. doi: 10.3389/fnbeh.2015.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller BT, D’Esposito M. Searching for ‘the top’ in top-down control. Neuron. 2005;48:535–538. doi: 10.1016/j.neuron.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 27.Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–931. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–636. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- 30.Fry W, Kelleher RT, Cook L. A mathematical index of performance on fixed-interval schedules of reinforcement. J Exp Anal Behav. 1960;3:193–199. doi: 10.1901/jeab.1960.3-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narayanan NS, Land BB, Solder JE, Deisseroth K, DiLeone RJ. Prefrontal D1 dopamine signaling is required for temporal control. Proc Natl Acad Sci USA. 2012;109:20726–20731. doi: 10.1073/pnas.1211258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnett L, Seth AK. The MVGC multivariate Granger causality toolbox: a new approach to Granger-causal inference. J Neurosci Methods. 2014;223:50–68. doi: 10.1016/j.jneumeth.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 33.Parker KL, Chen K-H, Kingyon JR, Cavanagh JF, Naryanan NS. Medial frontal ~ 4 Hz activity in humans and rodents is attenuated in PD patients and in rodents with cortical dopamine depletion. J Neurophysiol. 2015;114:1310–1320. doi: 10.1152/jn.00412.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward RD, Kellendonk C, Kandel ER, Balsam PD. Timing as a window on cognition in schizophrenia. Neuropharmacology. 2011;62:1175–1181. doi: 10.1016/j.neuropharm.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elvevåg B, McCormack T, Gilbert A, Brown GDA, Weinberger DR, Goldberg TE. Duration judgements in patients with schizophrenia. Psychol Med. 2003;33:1249–1261. doi: 10.1017/s0033291703008122. [DOI] [PubMed] [Google Scholar]
- 36.Ivry RB, Spencer RM. The neural representation of time. Curr Opin Neurobiol. 2004;14:225–232. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Kim J, Jung AH, Byun J, Jo S, Jung MW. Inactivation of medial prefrontal cortex impairs time interval discrimination in rats. Front Behav Neurosci. 2009;3:38. doi: 10.3389/neuro.08.038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narayanan NS, Land BB, Solder JE, Deisseroth K, Dileone RJ. Prefrontal D1 dopamine signaling is required for temporal control. Proc Natl Acad Sci USA. 2012;109:20726–20731. doi: 10.1073/pnas.1211258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Narayanan NS. Ramping activity is a cortical mechanism of temporal control of action. Curr Opin Behav Sci. 2016;8:226–230. doi: 10.1016/j.cobeha.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J, Ghim J-W, Lee JH, Jung MW. Neural correlates of interval timing in rodent prefrontal cortex. J Neurosci. 2013;33:13834–13847. doi: 10.1523/JNEUROSCI.1443-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu M, Zhang S, Dan Y, Poo M. Representation of interval timing by temporally scalable firing patterns in rat prefrontal cortex. Proc Natl Acad Sci USA. 2014;111:480–485. doi: 10.1073/pnas.1321314111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teune TM, van der Burg J, van der Moer J, Voogd J, Ruigrok TJ. Topography of cerebellar nuclear projections to the brain stem in the rat. Prog Brain Res. 2000;124:141–172. doi: 10.1016/S0079-6123(00)24014-4. [DOI] [PubMed] [Google Scholar]
- 43.Deniau JM, Kita H, Kitai ST. Patterns of termination of cerebellar and basal ganglia efferents in the rat thalamus. Strictly segregated and partly overlapping projections. Neurosci Lett. 1992;144:202–206. doi: 10.1016/0304-3940(92)90750-2. [DOI] [PubMed] [Google Scholar]
- 44.Narayanan NS. Dynamic Brain Imaging. Springer; Berlin, Germany: Laubach MMethods for studying functional interactions among neuronal populations; pp. 135–1652009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM. The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol. 1989;53:1–31. doi: 10.1016/0079-6107(89)90004-7. [DOI] [PubMed] [Google Scholar]
- 46.Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujisawa S, Buzsáki G. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron. 2011;72:153–165. doi: 10.1016/j.neuron.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parker KL, Alberico SL, Miller AD, Narayanan NS. Prefrontal D1 dopamine signaling is necessary for temporal expectation during reaction time performance. Neuroscience. 2013;255:246–254. doi: 10.1016/j.neuroscience.2013.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joyal CC, Strazielle C, Lalonde R. Effects of dentate nucleus lesions on spatial and postural sensorimotor learning in rats. Behav Brain Res. 2001;122:131–137. doi: 10.1016/s0166-4328(00)00390-9. [DOI] [PubMed] [Google Scholar]
- 50.Spencer RMC, Ivry RB. Comparison of patients with Parkinson’s disease or cerebellar lesions in the production of periodic movements involving event-based or emergent timing. Brain Cogn. 2005;58:84–93. doi: 10.1016/j.bandc.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 51.Schutter DJLG, van Honk J, d’Alfonso AAL, Peper JS, Panksepp J. High frequency repetitive transcranial magnetic over the medial cerebellum induces a shift in the prefrontal electroencephalography gamma spectrum: a pilot study in humans. Neurosci Lett. 2003;336:73–76. doi: 10.1016/s0304-3940(02)01077-7. [DOI] [PubMed] [Google Scholar]
- 52.Chen K-H, Okerstrom KL, Kingyon JR, Anderson SW, Cavanagh JF, Narayanan NS. Startle habituation and midfrontal theta activity in Parkinson’s disease. J Cogn Neurosci. 2016:1–11. doi: 10.1162/jocn_a_01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cavanagh JF. Cortical delta activity reflects reward prediction error and related behavioral adjustments, but at different times. Neuroimage. 2015;110:205–216. doi: 10.1016/j.neuroimage.2015.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


