Abstract
Background
Incident heart failure (HF) is the most common non-fatal event in patients with atrial fibrillation (AF), although strategies for HF prevention are lacking.
Objectives
To identify modifiable risk factors and estimate the impact of risk factor modification on HF risk in women with new-onset AF.
Methods
We assessed 34 736 participants in the Women’s Health Study free of prevalent cardiovascular disease at baseline. Cox models with time-varying assessment of risk factors after AF diagnosis were used to identify significant modifiable risk factors for incident HF.
Results
Over a median follow-up of 20.6 years, 1495 women developed AF without prevalent HF. In multivariable models, new-onset AF was associated with an increased risk of HF (HR 9.03 [95% CI: 7.52-10.85]). Once women with AF developed HF, all-cause (HR 1.83 [1.37-2.45]) and cardiovascular mortality (HR 2.87 [1.70-4.85]) increased. In time-updated, multivariable models accounting for changes in risk factors after AF diagnosis, systolic blood pressure > 120 mmHg, body mass index ≥ 30 kg/m2, current tobacco use, and diabetes mellitus were each associated with incident HF. The combination of these 4 modifiable risk factors accounted for an estimated 62% [23-83] of the population attributable risk of HF. Compared to women with 3 or 4 risk factors, those who maintained or achieved optimal risk factor control had a progressive decreased risk of HF (HR for 2 risk factors: 0.60 [0.37-0.95], 1 risk factor: 0.40 [0.25-0.63], 0 risk factors: 0.14 [0.07-0.29]).
Conclusion
In women with new-onset AF, modifiable risk factors including obesity, hypertension, smoking, and diabetes accounted for the majority of the population risk of HF. Optimal levels of modifiable risk factors were associated with decreased HF risk. Prospective assessment of risk factor modification at the time of AF diagnosis may warrant future investigation.
Introduction
The onset of atrial fibrillation (AF) has been consistently associated with increased mortality in diverse populations, including those with low cardiovascular disease burden (1). Improvements in thromboembolic risk prediction, coupled with the proliferation of anticoagulation agents, have led to important declines in stroke-related mortality for patients with AF (2). Despite these major advances, improvement in overall survival for patients with AF has been modest, with age-adjusted 5-year mortality rates of nearly 40% in one contemporary cohort (2). There remains a significant need to identify additional determinants of mortality in this population.
To that end, recent studies have suggested a shifting epidemiology of cardiovascular risk after new-onset AF (3). In particular, HF now represents the most common incident cardiovascular event in patients with AF, occurring at a rate nearly twice that of stroke (4). AF and HF frequently co-exist and the combination confers a greater mortality risk than either alone (5). However, in contrast to stroke where established preventive approaches exist, there are few, if any, preventive strategies for reducing the incidence of HF in patients with AF.
We therefore utilized the Women’s Health Study (WHS) (6) - a large, longitudinal cohort of women without prevalent cardiovascular disease at baseline – to examine the population risk, prognostic implications, and risk factors for incident HF in women with new-onset AF. We hypothesized that modifiable risk factors might account for a significant proportion of population and individual HF risk in AF, and thus, might provide important targets for prevention.
Methods
Study Cohort
The study was comprised of 39 876 female healthcare professionals in the United States enrolled in the WHS, an ongoing observational follow-up study that began in 1993 as a 2×2 randomized controlled trial of Vitamin E and low-dose aspirin for the primary prevention of cardiovascular disease (CVD) and cancer (6). Women were aged 45 years or older and free of cardiovascular disease and cancer at study entry. After the end of randomized treatment on March 31, 2004, participants were invited to participate in an observational follow-up study including serial questionnaires about cardiovascular risk factors and updated health outcomes. All participants provided written, informed consent and the study was approved by the institutional review board of Brigham and Women’s Hospital.
Risk Factor Ascertainment
Participants self-reported cardiovascular risk factors and interval health events at baseline and on annual questionnaires. Covariates of interest included baseline demography (age, race/ethnicity, height, weight) as well as time-updated assessment of both clinical risk factors (diabetes mellitus, systolic blood pressure, anti-hypertensive medication use, hyperlipidemia, lipid-lowering medication use, use of hormone replacement therapy) and lifestyle habits (smoking status [never, former, current], physical activity [metabolic equivalents per week], alcohol consumption [number of drinks per day]).
Ascertainment of AF and Cardiovascular Endpoints
Details regarding AF ascertainment have been previously described (1). Briefly, at study entry, 48 months, and annually thereafter, women were asked to report diagnoses of incident AF. Medical records pertaining to the AF diagnosis, ECGs and rhythm strips were reviewed by an end-point committee of cardiologists. Confirmation of AF required the presence of electrocardiographic evidence or a medical report clearly indicating a history of AF.
Ascertainment of cardiovascular endpoints (HF, stroke, MI) and death in WHS has been previously described (1). Women reported new physician diagnoses of cardiovascular endpoints via annual follow-up questionnaires. Similar to AF, women were first asked to report HF on the 48 month questionnaire. Information on MI and stroke was collected from the beginning of the study. Deaths were usually reported by family members or postal authorities or ascertained through the National Death Index. All events were adjudicated according to predefined criteria in a blinded fashion by an endpoint committee of physicians. HF was confirmed if either Framingham Heart Study (7) or Cardiovascular Health Study (8) criteria were met. Incident HF was further categorized by left ventricular ejection fraction (LVEF) within 3 months of HF diagnosis (9). HF subtypes were classified as HF with preserved ejection fraction (HFpEF) if LVEF was ≥ 50% or HF with reduced ejection fraction (HFrEF) if LVEF was < 50%. Deaths were confirmed to be from cardiovascular causes on the basis of autopsy reports, death certificates, medical records and information obtained from family members.
Population for Analysis
For the present analysis, women with a history of AF at study entry (N=876) or the presence of a cardiovascular event prior to randomization (stroke, myocardial infarction [MI], heart failure; N=59) were excluded. We also excluded women who were either lost to follow-up during the initial trial (N=1246) or opted out of observational follow-up (N=2959) at the end of the trial in 2004 because incident AF and subsequent cardiovascular events could not be reliably confirmed. The final study cohort included 34 736 women.
Statistical Analysis
First, we quantified the relative and absolute risk of incident HF associated with the development of incident AF in the entire study population. Person-years of follow-up were defined from the date of return of the baseline questionnaire until the first occurrence of HF, death, loss to follow-up or December 31, 2014. Cox proportional hazard models were used to calculate hazard ratios (HR) and 95% confidence intervals (CI). Incident AF was entered into the model as time-varying covariates. Models were initially adjusted for age and randomization assignment (vitamin E, ASA) with subsequent multivariable, time-varying adjustment for HF risk factors (legend, Table 2). In secondary analyses, we assessed the association between AF and incident HF subtypes (HFpEF, HFrEF). In addition, as the association between AF and HF may differ by the proximity of their temporal relationship,(5,10) we performed sensitivity analyses which censored HF events < 30 days after AF diagnosis (N=61).
Table 2.
Risk Factors for Incident HF after AF Diagnosis
| Multivariable-Adjusted Hazard Ratio [95% CI]* | p-value | |
|---|---|---|
|
| ||
| Diabetes mellitus | 1.57 [1.07-2.32] | 0.0001 |
|
| ||
| Smoking status | ||
| Never smoker | Reference | – |
| Prior smoker | 0.99 [0.73-1.34] | 0.94 |
| Current smoker | 2.11 [1.11-4.03] | 0.02 |
|
| ||
| Body Mass Index, kg/m2 | ||
| <25 | Reference | – |
| 25-29 | 0.97 [0.66-1.43] | 0.89 |
| ≥30 | 1.62 (1.10-2.40] | 0.02 |
| P, trend =0.011 | ||
|
| ||
| SBP, mmHg | ||
| < 120 | Reference | – |
| 120-139 | 2.12 [1.33-3.36] | 0.002 |
| 140-159 | 1.76 [1.02-3.02] | 0.041 |
| ≥ 160 | 2.50 [1.10-5.71] | 0.029 |
| P, trend =0.044 | ||
|
| ||
| Physical activity, ≥ 7.5 METS/week | 0.95 [0.70-1.28] | 0.71 |
|
| ||
| EtOH ≥ 2 drinks/day | 1.37 [0.75-2.50] | 0.30 |
|
| ||
| Hyperlipidemia | 1.06 (0.73-1.54) | 0.77 |
|
| ||
| Age, per year | 1.06 (1.04-1.08] | <0.0001 |
|
| ||
| Race/Ethnicity | ||
| White | Reference | – |
| Black | 1.23 [0.30-5.02] | 0.77 |
| Hispanic | † | 1.0 |
| Other | 0.72 [0.10-5.26] | 0.72 |
|
| ||
| History of MI at AF diagnosis | 0.44 [0.15-1.27] | 0.13 |
|
| ||
| Medication Use | ||
| Vitamin E | 0.79 [0.59-1.07] | 0.12 |
| Aspirin | 1.23 [0.92-1.66] | 0.17 |
| HRT | 0.73 [0.53-1.0] | 0.05 |
| Statin | 0.72 [0.70-1.28] | 0.06 |
|
| ||
| Anti-hypertensive medication use | 1.26 [0.89-1.76] | 0.19 |
|
| ||
| Chronic Kidney Disease | 4.02 [2.31-7.0] | <0.0001 |
|
| ||
| Incident Coronary Heart Disease | 2.44 [1.62-3.67] | <0.0001 |
Multivariable adjustment was for age, race, randomization assignment (ASA, Vitamin E), and history of myocardial infarction at AF diagnosis as well as time-updated assessment of medication use (statin, anti-hypertensive, hormone replacement therapy), incident coronary heart disease (MI and/or revascularization), chronic kidney disease, and modifiable risk factors shown (diabetes mellitus, physical activity, alcohol consumption, smoking, body mass index, SBP, hyperlipidemia).
Hazard ratios were not calculable secondary to small sample size. AF, atrial fibrillation; HF, heart failure; CI, confidence interval; METS, metabolic equivalents; EtOH, alcohol; SBP, systolic blood pressure; HRT, hormone replacement therapy; MI, myocardial infarction.
To quantify the association between modifiable risk factors and the development of incident HF in those with new onset-AF, we utilized Cox models incorporating time-varying HF risk factors limited to the subpopulation of women with new-onset AF and without prevalent HF (N=1495). In these models, each woman contributed person-years of follow-up from the date of AF diagnosis to the first occurrence of HF, death, loss to follow-up or December 31, 2014. Baseline covariates in the AF subpopulation were updated to reflect the assessment most proximate to the date of AF diagnosis, followed by subsequent time-varying adjustment of HF risk factors after AF diagnosis (see legend, Table 4). To estimate the joint risk reduction associated with risk factor modification, we constructed a risk factor score comprised of significant modifiable risk factors (1 point each for: SBP > 120 mmHg, BMI ≥ 30 kg/m2, current smoking, diabetes). Using a similar method for time-varying assessment as outlined above for the individual risk factors, we then compared multivariable-adjusted hazard ratios for participants with the least favorable risk factor profile (3 or 4 points) to those with progressively favorable profiles (2, 1, and 0 points). Patients with 3 or 4 points were combined to maintain similarly sized risk factor strata given the rare prevalence of women with all 4 risk factors (i.e. 4 points).
Table 4.
Impact of Incident HF on Cardiovascular Mortality, Stroke, and Myocardial Infarction in New-Onset AF
| No HF | HF | p-value | |
|---|---|---|---|
|
| |||
| All-Cause Mortality | |||
| No. of events | 241 | 69 | – |
| Incidence rate [95% CI]* | 25.2 [21.1-29.4] | 42.3 [28.9-55.7] | |
| Hazard Ratio [95% CI] | |||
| Age-adjusted | 1 [Reference] | 2.11 [1.60-2.77] | <0.0001 |
| Multivariable-adjusted† | 1 [Reference] | 1.83 [1.37-2.45] | <0.0001 |
|
| |||
| CV Mortality | |||
| No. of events | 59 | 26 | – |
| Incidence rate [95% CI] | 6.3 [4.3-8.3] | 16.0 [7.6-24.4] | |
| Hazard Ratio [95% CI] | |||
| Age-adjusted | 1 [Reference] | 3.47 [2.15-5.60] | <0.0001 |
| Multivariable-adjusted | 1 [Reference] | 2.87 [1.70-4.85] | <0.0001 |
|
| |||
| Stroke | |||
| No. of events | 81 | 15 | – |
| Incidence rate [95% CI] | 8.8 [6.3-11.3] | 9.9 [3.4-16.5] | |
| Hazard Ratio [95% CI] | |||
| Age-adjusted | 1 [Reference] | 1.11 [0.53-2.32] | 0.78 |
| Multivariable-adjusted | 1 [Reference] | 0.89 [0.42-1.90] | 0.76 |
|
| |||
| Myocardial Infarction | |||
| No. of events | 36 | 15 | – |
| Incidence rate [95% CI] | 3.4 [1.8-5.1] | 10.0 [3.2-16.8] | |
| Hazard Ratio [95% CI] | |||
| Age-adjusted | 1 [Reference] | 4.96 [2.38-10.33] | <0.0001 |
| Multivariable-adjusted | 1 [Reference] | 4.90 [2.29-10.50] | <0.0001 |
Incidence rates are age-adjusted and reported per 1000 person-years.
Multivariable adjustment was for demography (age, white race, education), randomization assignment (aspirin, Vitamin E), and anticoagulation use at the time of AF diagnosis with time-varying adjustment for body mass index, coronary revascularization, diabetes mellitus, hypertension, hyperlipidemia, statin use, alcohol consumption, exercise, smoking status, and hormone replacement therapy. Mortality (all-cause, cardiovascular) models included additional time-varying adjustment for stroke and MI after AF diagnosis. HF, heart failure; No., number; AF, atrial fibrillation; CI, confidence interval; CV, cardiovascular; MI, myocardial infarction.
We then assessed the absolute and relative risk of stroke, MI, and mortality associated with the development of HF in this AF sub-population through the use of age-adjusted cumulative incidence curves and proportional hazard models with incident HF as a time-varying covariate and cardiovascular endpoints (stroke, MI) and mortality (all-cause, cardiovascular) as outcomes. Person-years of follow-up were derived from the date of AF diagnosis to the first occurrence of death, stroke, MI, loss to follow-up, or December 31, 2014 as appropriate. Models included time-varying adjustment for established risk factors for cardiovascular morbidity and mortality (see legend, Table 5). Mortality models (all-cause, cardiovascular) included additional time-varying adjustment for stroke and MI after AF diagnosis. To explore whether prognostic implications differed by timing of HF after AF, we repeated the mortality analyses excluding participants with HF < 30 days after AF diagnosis (N=61).
For all the above outcomes, we calculated the population attributable fraction (PAF) and 95% confidence interval, which reflects the proportion of the outcome that would not have occurred if the risk factor were not present (assuming a causal relationship). To calculate the PAF, we estimated the relative risk from multivariable pooled logistic regression models, which allowed for updated assessment of risk factor prevalence and direct inclusion of age in the model. In this approach each 2-year interval was treated as an independent follow-up study and observations over all intervals were pooled, as previously described (11). Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc, Cary, North Carolina). A 2-tailed P<0.05 was considered to indicate statistical significance.
Results
Study Cohort
Baseline characteristics of the study cohort are shown in Table 1. In 34 736 women free of prevalent cardiovascular disease at baseline, 1534 women developed new-onset AF (4.4% of the study cohort) and 687 developed HF (2.0% of the study cohort) over a median follow-up of 20.6 (interquartile range [IQR], 19.6-21.1) years. Among 1534 women with new-onset AF, there were 226 HF events, the majority of which occurred after AF diagnosis (N=187; 82.7%). Excluding women with HF prior to AF (N=39), the baseline characteristics of the 1495 women with new-onset AF and without prevalent HF, updated to the time of AF diagnosis, are also shown in Table 1. Of women with new-onset AF and with available echocardiography at the time of AF diagnosis (N=1064), only a minority demonstrated structural heart disease as reflected by the presence of LV hypertrophy (N=419 [39%]), mitral regurgitation (N=157 [15%]), or left atrial enlargement (N=493 [46%]).
Table 1.
Baseline characteristics of total cohort at enrollment and new-onset AF without HF at AF diagnosis
| Total Cohort at Enrollment (N=34,736) | New-Onset AF without HF at AF diagnosis (N=1495) | |
|---|---|---|
|
| ||
| Age, years | 55±7 | 69±8 |
|
| ||
| Race/ethnicity | ||
| White | 32,759 (94) | 1454 (97) |
| Black | 720 (2) | 12 (1) |
| Hispanic | 348 (1) | 4 (<1) |
| Other | 909 (3) | 25 (2) |
|
| ||
| BMI, kg/m2 | 26±5 | 28.1±6.1 |
|
| ||
| Diabetes mellitus, n (%) | 938 (3) | 172 (12) |
|
| ||
| Hypertension, n (%) | 9176 (26) | 1140 (76) |
| Systolic Blood Pressure, mmHg | ||
| <120 | 15324 (45) | 314 (21) |
| 120-139 | 14604 (43) | 826 (55) |
| 140-159 | 3970 (11) | 311 (21) |
| ≥160 | 406 (1) | 43(3) |
|
| ||
| Hyperlipidemia, n (%) | 10505 (30) | 954 (64) |
|
| ||
| History of coronary revascularization, n (%) | 0 (0) | 67 (5) |
|
| ||
| History of MI, n (%) | 0 (0) | 24 (2) |
|
| ||
| History of stroke, n (%) | 0 (0) | 36 (2) |
|
| ||
| Chronic Kidney Disease, n (%) | 7 (<1) | 12 (1) |
|
| ||
| Smoking status, n (%) | ||
| Never | 17920 (52) | 720 (48) |
| Past | 12484 (36) | 696 (47) |
| Current | 4306 (12) | 79 (5) |
|
| ||
| ≥ 2 drinks EtOH/day, n (%) | 1360 (4) | 91 (6) |
|
| ||
| Physical activity (≥ 7.5 METS/week) | 18923 (55) | 856 (57) |
|
| ||
| Anticoagulation use, n (%) | – | 663 (44) |
|
| ||
| CHADSVASc score* | ||
| 1 | 159 (11) | |
| 2 | 392 (26) | |
| 3 | – | 523 (35) |
| 4 | 349 (23) | |
| 5 | 55 (4) | |
| 6 | 16 (1) | |
| 7-9 | 1 (<1) | |
Continuous variables expressed mean±STDEV.
CHADSVASc score reflects thrombo-embolic risk: hypertension (1 point), age (65-74 years: 1 point; ≥ 75 years: 2 points), diabetes mellitus (1 point), history of stroke, TIA or thromboembolism (2 points), vascular disease (1 point), female sex (1 point). AF, atrial fibrillation; BMI, body mass index; EtOH, alcohol; METS, metabolic equivalents; ASA, aspirin.
AF as Risk Factor for Incident HF
Following the diagnosis of AF, the age-adjusted cumulative incidence rate of HF was 17.4 cases/1000 person-years [95% CI: 13.4-21.4]) over a median follow-up of 6.8 years [IQR: 3.7-10.3]. The incidence of HF after new-onset AF was greater than age-adjusted incidences of stroke (9.0 cases/1000 person-years [95% CI: 6.6-11.3]) and MI (4.4 cases/1000 person-years [95% CI: 2.6-6.1]). In multivariable-adjusted analyses, AF was associated with 9-fold increased risk of incident HF (HR 9.03 [95% CI: 7.52-10.84], p<0.0001), with similar risk estimates for incident HFpEF (HR 9.63 [95% CI: 7.67-12.86], p<0.0001) and HFrEF (HR 10.02 [95% CI: 7.38-13.61], p<0.001). After censoring HF events occurring within 30 days of AF diagnosis (N=61), the association between AF and incident HF was attenuated, but remained significant in multivariable-adjusted models (HR 4.99 [95% CI: 4.05-6.13], p<0.0001) (Supplementary Table 1). Assuming a causal association between AF and incident HF and after accounting for population-level risk factors for HF, the population attributable risk of new-onset AF for incident HF was 26% (95% CI: 15-37).
Risk Factors for Incident HF after New-Onset AF
We next examined risk factors associated with incident HF in women with new-onset AF (Table 2). In multivariable and time-updated models accounting for changes in risk factors after AF diagnosis, 4 directly modifiable risk factors (diabetes mellitus, current smoking, obesity, and elevated systolic blood pressure (SBP)) were each significantly associated with the development of incident HF. Additional risk factors associated with HF in women with new-onset AF included age, chronic kidney disease, and incident myocardial infarction. Borderline significant inverse associations with HF risk were also observed for statin and hormone replacement therapy use.
At the time of AF diagnosis, 85% of women (1264 of 1495) had at least one directly modifiable risk factor associated with HF risk (diabetes, smoking, BMI ≥ 30 kg/m2, or SBP > 120 mmHg; Table 3). Increasing BMI was associated with a significant increased risk of incident HF (multivariable-adjusted HR: 1.15 per 5 kg/m2 [95% CI: 1.01-1.28]; p, continuous=0.04), with the most significant risk confined to those with BMI ≥ 30 kg/m2 (HR 1.62 [95% CI: 1.10-2.40], p=0.02) when compared to normal weight (BMI <25 kg/m2) women. In addition, increasing systolic blood pressure (SBP) was associated with an increased risk of incident HF (HR 1.16 [95% CI: 1.05-1.28] per 10 mmHg increase, p=0.003). When assessed categorically, the relative risk of incident HF was elevated even at mild elevations in systolic blood pressure (SBP; HR 2.12 [95% CI: 1.33-3.36], p=0.002 for SBP 120-139 vs. < 120 mmHg) and persisted for higher values of SBP (p, trend across SBP categories = 0.044).
Table 3.
Modifiable Risk Factors and HF Risk in New Onset AF
| # of Modifiable Risk Factors at AF Baseline* | Subgroup N | Incident HF Events | Person-Years Follow-up | Age-adjusted Incidence Rate† (95% CI) | Multivariable-Adjusted Hazard Ratio‡ | p-value |
|---|---|---|---|---|---|---|
|
| ||||||
| 0 | 231 | 9 | 1896.5 | 6.3 (2.2-10.4) | 0.14 [0.07-0.29] | <0.0001 |
| 1 | 758 | 88 | 5679.4 | 16.2 (12.1-20.4) | 0.40 [0.25-0.63] | <0.0001 |
| 2 | 381 | 65 | 2743.7 | 25.8 (17.3-34.2) | 0.60 [0.37-0.95] | 0.029 |
| 3-4 | 125 | 25 | 779.3 | 36.0 (17.4-54.6) | Reference | – |
Modifiable risk factors as assessed at the time of AF diagnosis include: diabetes mellitus, current smoking, BMI ≥ 30 kg/m2, and SBP > 120 mmHg.
Incidence rates are age-adjusted, reported per 1000 person-years, and reflect risk factor profiles at the time of AF diagnosis.
Multivariable models reflect time-updated assessment of risk factor profiles (i.e. allows for changes in risk factor profiles after AF diagnosis). Specifically, adjustment was for age, race, randomization assignment (ASA, Vitamin E), and history of myocardial infarction at AF diagnosis as well as time-updated assessment of medication use (statin, anti-hypertensive, hormone replacement therapy), myocardial infarction, coronary revascularization, chronic kidney disease, and modifiable risk factors. BMI, body mass index; SBP, systolic blood pressure; CI, confidence interval.
To assess the joint impact of these modifiable risk factors on incident HF, we utilized multivariable-adjusted models with time-updated assessment of risk factors after AF diagnosis (Table 3; Supplemental Table 2). Compared to women with the least favorable risk factor profile (3-4 risk factors), women who maintained or achieved optimal risk factor levels were at significantly decreased risk of HF, with a graded reduction in risk across progressively more optimal risk factors levels (Table 3; Figure 1). For example, women with optimal levels of all 4 modifiable risk factors (SBP < 120 mmHg, BMI < 30 kg/m2, absent smoking, absent diabetes) were at 86% lower risk of incident HF (HR 0.14 [95% CI: 0.07-0.29]) compared to women with the least favorable risk factor profile (3-4 risk factors). Assuming a causal relationship between modifiable risk factors and HF, an estimated 62% (95% CI: 23-83) of the population attributable risk of incident HF after new-onset AF was attributable to these 4 directly modifiable risk factors, with the greatest contributing risk factor being SBP (Supplemental Table 3).
Figure 1. Heart Failure Risk in Atrial Fibrillation – Impact of Risk Factor Modification.
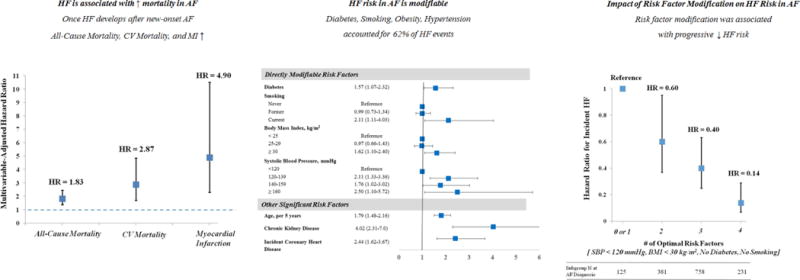
This study estimated the influence of risk factor modification on HF risk in women with new-onset AF. In women with new-onset AF, the onset of HF was associated with significant increases in mortality (all-cause, cardiovascular) and morbidity (myocardial infarction) (Left Panel). Risk factors for incident HF in women with new-onset AF included directly modifiable factors (obesity, hypertension, smoking, diabetes) as well as other significant risk factors (age, chronic kidney disease, incident coronary heart disease [myocardial infarction, revascularization]). All 4 directly modifiable risk factors accounted for 62% of the population attributable fraction of HF in the cohort (Middle Panel). Compared to women with the least favorable risk factor profile (3-4 modifiable risk factors), women who maintained or achieved more favorable risk factor profiles after new-onset AF were at progressively lower risk of incident HF (Right Panel).
Impact of Incident HF on Mortality and Cardiovascular Events after AF
Among the 1495 women with new-onset AF without prevalent HF, 310 deaths, 96 strokes, and 51 MIs occurred over a median follow-up of 7.4 [IQR: 4.4-11.0] years. Age- and multivariable-adjusted estimates for the association between incident HF and mortality (all-cause, cardiovascular), stroke, and MI in women with new-onset AF are shown in Table 4. In multivariable-adjusted models inclusive of time-updated accounting for stroke and myocardial infarction, incident HF was associated with a 2-fold increase in all-cause mortality (HR 1.83 [95% CI: 1.37-2.45], p<0.0001), with similar mortality risk associated with incident HFpEF and HFrEF (Supplemental Table 4). Incident HF was also associated with an increased risk of cardiovascular mortality (HR 2.87 [95% CI: 1.70-4.85], p<0.0001) and MI (HR 4.90 [95% CI: 2.29-10.50], p<0.0001) in multivariable-adjusted models, but was not associated with an increased risk of stroke. In our cohort, nearly every woman with subsequent HF (181 of 187; 97%) had a CHADSVASC2 score of ≥2 at the time of AF diagnosis. After taking into account population-level risk factors for mortality and morbidity, we estimated that 9.9% (95% CI: 2.2-18.3) of all deaths and 18.2% (95% CI: 1-35) of cardiovascular deaths in women with new-onset AF could be attributed to incident HF.
Discussion
In women with new-onset AF, HF was the most common non-fatal event and its onset was associated with increased all-cause and cardiovascular mortality. Directly modifiable risk factors – obesity, diabetes mellitus, elevated systolic blood pressure, and current tobacco smoking – were jointly associated with 62% of the population risk of incident HF. In time-updated models accounting for changes in risk factors after AF diagnosis, women who maintained or achieved an optimal risk factor profile were at significantly reduced risk of HF. Taken together, these findings highlight the potential population and individual level impact of risk factor modification on HF risk in new-onset AF.
Previous studies of incident HF in those with AF (12–14) have focused primarily on risk prediction and, as such, have incorporated both modifiable and non-modifiable risk factors assessed at AF baseline. The clinical impact of these studies is limited, however, by the absence of evidence for HF preventive therapies in AF. By incorporating time-updated assessments of directly modifiable risk factors after AF diagnosis, our study is the first to estimate the association between optimal levels of risk factors and HF risk in patients with AF. We identified a consistent decrement in HF risk associated with progressively optimal risk factor profiles, with a striking 86% lower HF risk among women with optimal levels of modifiable risk factors (obesity, hypertension, smoking, diabetes). These factors have also been associated with incident AF (15–17) and thus, our study suggests that risk factor improvement even after the diagnosis of AF may decrease HF risk and improve outcomes. While hypothesis-generating, our findings more firmly establish a necessary evidence base to support future investment in intervention trials aimed at risk factor modification in AF patients and further support the inclusion of HF as endpoint in such prospective assessments.
With respect to individual risk factors, we found that blood pressure elevations accounted for a large proportion of the population attributable risk of incident HF and observed individual increases in HF risk associated with even modestly elevated systolic blood pressures (i.e. 120-139 mmHg). These findings are consistent with previous reports of SBP and HF risk in the general population (18) which found increased HF risk at modest elevation in SBP with a generally fixed hazard across increasing SBP categories. Our findings may add to the emerging literature supporting the salutary cardiovascular effects of lower SBP targets for antihypertensive therapy (19). We would note that the lack of a clear gradation of HF risk in categories of SBP ≥ 140 mmHg may also be due to the relatively low numbers of women with persistent elevations in SBP above this level in this health professional cohort. Further studies in hypertensive AF populations would be needed to fully evaluate this question. Other directly modifiable risk factors associated with HF risk included BMI ≥ 30 kg/m2, a finding consistent with recent studies highlighting the pathophysiological relationship between obesity and HF (20). Finally, to the extent that incident MI likely mediates some of the association between our modifiable risk factors and HF, our adjusted estimates regarding the impact of risk factor modification on HF risk are conservative.
We additionally estimate the potential clinical impact of HF prevention on other adverse outcomes in our cohort. Consistent with recent estimates from contemporary cohorts of mixed gender (3,4,10), we find that HF was the most common non-fatal cardiovascular event after AF, reinforcing the predominant impact of HF on morbidity after AF even among women, who are known to be at elevated risk for stroke. Once HF developed in women with AF, we found that cardiovascular morbidity and mortality were increased. While previous reports have identified similar relative risk elevations for cardiovascular and total mortality (5), we add to this previous literature by quantifying the potential impact of HF prevention on mortality in AF. Assuming a causal association between incident HF and mortality, we estimated that prevention of HF in women with new-onset AF could potentially lead to an estimated 10% reduction in total and 18% reduction in cardiovascular mortality. Similar to a recent report in patients with establish AF (14), incident HF was not associated with subsequent stroke risk in women with new-onset AF. This finding, which is seemingly contradictory to older reports in the literature (21), may reflect the successful real-world institution of anticoagulation either prior to or at the time of HF diagnosis. Finally, we observed a bidirectional association between HF and incident MI, which to our knowledge, has not been previously reported. Concordant with this finding, statin use was associated a numerically lower, albeit statistically insignificant, risk of HF. This finding is based upon small numbers, and if confirmed in other cohorts, could provide further justification for aggressive risk factor modification (including lipids) in AF patients.
Our study has several strengths including prospective assessment of risk factors, large sample size, adjudication of endpoints, and incorporation of time-updated risk factors of incident HF risk. Nonetheless, the findings of this study should be interpreted in the context of its design and limitations. First, the study population was comprised of healthy, middle-aged and predominantly white female health professionals. The population attributable fraction estimates in this study – which are population-specific – may not generalize to men or non-white ethnicities. Second, risk factor modification was not randomized at the time of AF diagnosis, and the associations identified including PAF estimates cannot be directly taken as causal. Third, modifiable risk factors including BMI and SBP were self-reported, although non-differential misclassification would have biased our findings towards the null. The prognostic value of self-reported BP and BMI in WHS (15) has been previously reported. Fourth, the methodology of AF ascertainment may have underestimated asymptomatic cases of AF. In addition, the diagnosis of AF may have led to increased medical surveillance resulting in ascertainment bias for incident HF and thus magnifying AF-HF risk estimates. Fifth, information regarding some HF risk factors (e.g. sleep apnea, coronary disease without myocardial infarction) were not available and could not be assessed. In addition, echocardiographic evaluation was not systematically performed in this cohort and therefore the contribution of potential cardiac structural risk factors (e.g. valvular heart disease) to HF risk could not be assessed. Sixth, although adjustment for multiple comparisons was not performed, the primary findings of the study would remain significant even with Bonferroni correction. Finally, as pharmacotherapy for AF was assessed two years after AF diagnosis, we were unable to assess the impact of pharmacotherapy strategies (e.g. anti-arrhythmic use, β-blockade) strategies on HF risk.
Conclusion
In women free of prevalent cardiovascular disease at baseline, new-onset AF was associated with an increased risk of HF which, in turn, was associated with increased mortality and morbidity. Our data provide support for the concept that targeting modifiable risk factors including obesity, smoking, elevated systolic blood pressure, and diabetes mellitus in patients with new-onset AF has the potential to significantly reduce the individual risk and population burden of HF.
Supplementary Material
Perspectives.
Competency in Patient Care and Procedural Decision Making
Cardiovascular risk in AF is changing in the contemporary era of anticoagulation. In a large cohort of women with new-onset atrial fibrillation (AF), heart failure (HF) was the most common incident cardiovascular event, occurring at a rate nearly twice that of stroke. The onset of HF was associated with a more than 2-fold increase in mortality and 4-fold increase risk of myocardial infarction.
Competency in Patient Care and Procedural Decision Making
In women with new-onset AF, directly modifiable risk factors – obesity, smoking, elevated systolic blood pressure, and diabetes mellitus – accounted for 62% of the population burden of HF. Compared to women with the least favorable risk factor profile (3-4 modifiable risk factors), women who maintained or achieved optimal levels of risk factors after AF diagnosis (BMI < 30 kg/m2, SBP < 120 mmHg, non-smoker, absent diabetes) had an 86% lower risk of incident HF.
Translational Outlook
Future studies are needed to examine the impact of risk factor modification at the time of AF diagnosis on incident HF risk. Prevention of HF may represent an important endpoint of AF intervention trials in the future and a relevant target for improving survival in AF.
Acknowledgments
Funding Sources: Dr. Chatterjee was supported by NHLBI T-32 HL-007575. The WHS was supported by grants HL-043851, HL-080467, and HL-099355 from the NHLBI and grant CA-047988 from the National Cancer Institute.
Footnotes
Disclosures: None
References
- 1.Conen D, Chae CU, Glynn RJ, et al. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA. 2011;305:2080–7. doi: 10.1001/jama.2011.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet (London, England) 2015;386:154–62. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piccini JP, Hammill BG, Sinner MF, et al. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J. 2014;35:250–6. doi: 10.1093/eurheartj/eht483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ (Clinical research ed) 2016;354:i4482. doi: 10.1136/bmj.i4482. [DOI] [PubMed] [Google Scholar]
- 5.Santhanakrishnan R, Wang N, Larson MG, et al. Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved Versus Reduced Ejection Fraction. Circulation. 2016;133:484–92. doi: 10.1161/CIRCULATIONAHA.115.018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 7.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. The New England journal of medicine. 1971;285:1441–6. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Annals of epidemiology. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 9.Chae CU, Albert CM, Moorthy MV, Lee IM, Buring JE. Vitamin E supplementation and the risk of heart failure in women. Circ Heart Fail. 2012;5:176–82. doi: 10.1161/CIRCHEARTFAILURE.111.963793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vermond RA, Geelhoed B, Verweij N, et al. Incidence of Atrial Fibrillation and Relationship With Cardiovascular Events, Heart Failure, and Mortality: A Community-Based Study From the Netherlands. J Am Coll Cardiol. 2015;66:1000–7. doi: 10.1016/j.jacc.2015.06.1314. [DOI] [PubMed] [Google Scholar]
- 11.Chiuve SE, Fung TT, Rexrode KM, et al. Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. Jama. 2011;306:62–9. doi: 10.1001/jama.2011.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potpara TS, Polovina MM, Licina MM, Marinkovic JM, Lip GY. Predictors and prognostic implications of incident heart failure following the first diagnosis of atrial fibrillation in patients with structurally normal hearts: the Belgrade Atrial Fibrillation Study. Eur J Heart Fail. 2013;15:415–24. doi: 10.1093/eurjhf/hft004. [DOI] [PubMed] [Google Scholar]
- 13.Schnabel RB, Rienstra M, Sullivan LM, et al. Risk assessment for incident heart failure in individuals with atrial fibrillation. Eur J Heart Fail. 2013;15:843–9. doi: 10.1093/eurjhf/hft041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandey A, Kim S, Moore C, et al. Predictors and Prognostic Implications of Incident Heart Failure in Patients With Prevalent Atrial Fibrillation. JACC Heart failure. 2017;5:44–52. doi: 10.1016/j.jchf.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Conen D, Tedrow UB, Koplan BA, Glynn RJ, Buring JE, Albert CM. Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation. 2009;119:2146–52. doi: 10.1161/CIRCULATIONAHA.108.830042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoen T, Pradhan AD, Albert CM, Conen D. Type 2 diabetes mellitus and risk of incident atrial fibrillation in women. J Am Coll Cardiol. 2012;60:1421–8. doi: 10.1016/j.jacc.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tedrow UB, Conen D, Ridker PM, et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women’s health study) J Am Coll Cardiol. 2010;55:2319–27. doi: 10.1016/j.jacc.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Britton KA, Gaziano JM, Djousse L. Normal systolic blood pressure and risk of heart failure in US male physicians. Eur J Heart Fail. 2009;11:1129–34. doi: 10.1093/eurjhf/hfp141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko MJ, Jo AJ, Park CM, Kim HJ, Kim YJ, Park DW. Level of Blood Pressure Control and Cardiovascular Events: SPRINT Criteria Versus the 2014 Hypertension Recommendations. J Am Coll Cardiol. 2016;67:2821–31. doi: 10.1016/j.jacc.2016.03.572. [DOI] [PubMed] [Google Scholar]
- 20.Kitzman DW, Shah SJ. The HFpEF Obesity Phenotype: The Elephant in the Room. J Am Coll Cardiol. 2016;68:200–3. doi: 10.1016/j.jacc.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Chugh Y, Faillace RT. The C of CHADS: Historical perspective and clinical applications for anticoagulation in patients with non valvular atrial fibrillation and congestive heart failure. International journal of cardiology. 2016;224:431–436. doi: 10.1016/j.ijcard.2016.09.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


