Abstract
Previous work has found that serum G-CSF was acutely elevated in mice 24 h but not one week after controlled cortical impact (CCI). The purpose of this study was to investigate whether blood G-CSF correlates with the elevated brain cytokines in mice after CCI and also if it correlates with traumatic brain injury (TBI) in humans. Here, we found in mice undergoing CCI, a procedure that induces direct injury to the brain, that serum G-CSF correlated directly or indirectly with several brain cytokines, indicating it is a useful marker for the neuroinflammation of TBI. A pilot study in humans (phase I, n = 19) confirmed that plasma G-CSF is acutely elevated on day 1 (p < 0.001) of TBI and has returned to baseline by one week. In a second human sample (phase II) (n = 80), we found plasma G-CSF peaks about 12 h after arriving in the emergency department (41.6 +/− 5.4 pg/ml). Aging was weakly associated (p < 0.05) with a less robust elevation in serum G-CSF, but there was no difference with gender. ISS, a measure of total severity of injury, correlated with the degree of elevation in serum G-CSF (r = .419; p < 0.05), but severity of head injury (via AIS) did not. The latter may have been because of the statistically narrow range of head injuries among our cases and the high number of cases diagnosed with closed head injury (a non-codable diagnosis). In conclusion, plasma G-CSF may be a useful biomarker of TBI, correlating with neuroinflammation in the animal model and in the human studies with time since injury and total severity of injury. As such, it may be useful in determining whether TBI has occurred within the last 24 h.
Keywords: G-CSF, Traumatic brain injury, Biomarker, Neuroinflammation, Blood–brain barrier
1. Introduction
Traumatic brain injury (TBI) has emerged as a common feature of both civilian and military lifestyles. In the form of blast injuries, it is common in the deployed military and, in the form of falls, automobile accidents and sports-related injuries, it is common in the civilian population (Sayer, 2012). Biomarkers are sought which might be useful as diagnostic indicators of TBI injury or as prognostic indicators of its outcome. To be useful, a TBI biomarker can serve any of several functions, such as an indicator of acute or chronic injury, a predictor of clinical outcomes, or a responder to an effective treatment. Because of the neuroinflammation which is prominent in TBI, cytokines are among the potential biomarkers (Morganti-Kossman et al., 1997). Cytokines are thought to play both deleterious and curative roles in TBI (Clausen et al., 2009; Ley et al., 2011; Sun et al., 2010, 2009; Ziebell and Morganti-Kossmann, 2010). Therefore, cytokine elevations in TBI could convey information relevant to severity of injury or predictive of outcomes.
Cytokine levels in brain or cerebrospinal fluid (CSF) would be most likely to reflect TBI events. However, blood is much easier to inventory than is the CSF. That cytokines in blood might reflect neuroimmune events in the CNS is suggested by the fact that cytokines can cross the blood–brain barrier (Banks, 2015; Chen and Reichlin, 1998; Quan and Banks, 2007; Romero et al., 1996). Our work with controlled cortical impact (CCI) in mice is consistent with this idea. With that model, we found that several brain cytokines, including granulocyte colony-stimulating factor (G-CSF), were elevated after CCI. However, only G-CSF was found to be elevated in serum. This suggested to us that blood G-CSF might be useful as a biomarker in TBI.
G-CSF is a 177 amino acid glycoprotein that was originally described as a hematopoietic growth factor and is currently used to promote the production of neutrophils in the face of chemotherapy-induced neutropenia (Bendall and Bradstock, 2014). It also mobilizes stem cells and several studies indicate that it has neuroprotective properties after its peripheral administration (Frank et al., 2012; Ghorbani et al., 2013; Hong et al., 2012). For example, G-CSF is neuroprotective against carbon monoxide toxicity and ischemia. Effectiveness after intravenous administration is likely a result of the ability of G-CSF to cross the BBB (Zhao et al., 2007). That peripheral G-CSF enters the brain to exert its neuroprotective effects is further suggested by the finding that when given by an intranasal method that fosters entry into the brain, G-CSF is protective against ischemic injury in rats (Sun et al., 2014). G-CSF has previously been suggested as a biomarker for other CNS diseases, with blood levels correlating with the severity of stroke (Yu et al., 2012) and being diagnostic but not prognostic in gliomas (Yildiz et al., 2011). High serum levels of growth factors in general, including G-CSF, are associated with a better functional outcome and a reduced lesion volume in intracerebral hemorrhage (Sobrino et al., 2009). In a rodent model of TBI, it was noted to have some short-term therapeutic benefits when administered intravenously (Acosta et al., 2014).
The elevation in serum G-CSF in the mouse model led to the current evaluations in which we investigated whether serum G-CSF correlates with the elevated brain cytokines in mice after CCI and whether plasma G-CSF correlates in humans who have suffered TBI with age, gender, time from injury, and measures of injury severity.
2. Materials and methods
2.1. Mouse studies
CCI model
All mouse studies were performed under protocols approved by the animal use committee at the Veterans Affairs Puget Sound Health Care System. A stereotactic impactor (Leica Microsystems, Inc) was used to induce CCI in 8 week old C57BL/6 male mice (Harlan Sprague Dawley). Anesthesia was induced with isoflurane and maintained by administration with a nose cone. The head was shaved, the skin overlying the skull infiltrated with lidocaine/bupivicaine mixture, and the mouse mounted in a stereotaxic apparatus that is part of a Lighthall stroke-contained pneumatic compression device. Using sterile technique, the skull was exposed through a midline incision and skin and muscle retracted. A 4 mm craniotomy was made lateral to the sagittal suture and centered between the lambda and the bregma. The bone was removed and the dura cut to expose the cortex. The cortex was compressed by using the impactor device to deliver a 3 mm diameter flat tip weight at a velocity of 5.82 m/s to a depth of 1.2 mm for a duration of 47 ms at a driving pressure of 73 psi. The injured area was covered with dura, the skin placed back into position and cemented, and the mice placed in an incubator at 37 °C until they regained consciousness (return of righting reflex and mobility). Sham mice went through the entire procedure except for delivery of impact.
Serum and brain cytokine measurements in mice
At 24 h and 7 days after CCI or sham surgery, mice were anesthetized with urethane, the carotid artery exposed, severed, and blood collected from it, and the hemibrain ipsilateral to CCI/sham surgery was obtained. The arterial blood was centrifuged at 4500g for 10 min at 4 C and cytokine levels measured on the resulting serum. The hemibrain ipsilateral to CCI/sham surgery was homogenized in 1 ml of extraction buffer (0.01 M phosphate buffered saline (PBS; 2.7 mM potassium chloride, 0.137 M sodium chloride, pH 7.4) 2.7 mM, 1 mM EDTA, 1 mM PMSF, Protease Inhibitor cocktail) using a mini beadbeater set at 4800 RPM for 30 s to produce the PBS homogenate. A volume of 0.625 ml of the PBS homogenate was added to 0.125 ml of extraction buffer containing 0.6% Triton X-100 and centrifuged at 20,000g for 10 min at 4 C. Levels of brain cytokines were measured on the resulting supernatant. The multiplex kit for murine cytokines from BioRad was used to measure 23 cytokines: IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 (p40), IL-12(p70), IL-13, IL-17, eotaxin (CCL11), G-CSF, GM-CSF, IFN-γ, KC (CXCL1), MCP-1 (CCL2), MIP-1α (CCL3), MIP-1β (CCL4), RANTES (CCL5), and TNF-α. Brain levels are reported relative to the protein content of the brain sample.
2.2. Human studies
Study design
Both Phase I and Phase II were observational prospective cohort studies.
Recruitment of human subjects
For both the Phase I and II samples discussed below, a trained member of the research team approached participants or their legally authorized representative to obtain informed consent within 24 h of injury. Once informed consent was obtained, a member of the research team extracted injury-related data from the medical record. Institutional Review Board approval was obtained for these studies.
Phase I study sample characteristics and procedures
Participants with blunt TBI were recruited from the population of eligible patients seeking medical treatment at a level I trauma center emergency department (ED; n = 10) from 2009 to 2010. Non-injured adult controls were recruited from the greater Seattle area (n = 9) over the same period. Participants with TBI met the following inclusion criteria: (1) Arrival in the emergency department within 24 h of injury, (2) primary diagnosis of TBI by physician, nurse practitioner or physician assistant, (3) initial Glasgow Coma Score (GCS) at presentation to ED of 9–15 (indicating mild-moderate brain injury). The following exclusion criteria were applied to the TBI sample: (1) cervical spinal cord injury, (2) lower extremity fracture, (3) prior history of TBI, stroke or dementia, (4) hospitalization within the past 6 months and (5) autoimmune disorder. To be included in the study as a control, participants must be (1) independent in activities of daily living, (2) not have prior history of TBI, stroke or dementia and (3) no hospitalization in past 6 months. Blood was drawn in CPT processing tubes (Becton-Dickenson, Franklin Lakes, NJ) at enrollment within 24 h of injury, 1 week and 1 month post-injury. The 1 week and 1 month samples were both drawn before 10AM. Samples were processed within 2 h of draw for plasma and PMBCs. Plasma samples were aliquoted and then immediately frozen and stored at −70 °C until processing. In non-injured older adult controls, blood was drawn at baseline and again at 1 month post-enrollment and processed as described above.
Phase II study sample characteristics and procedures
This sample was secondary data analysis of an ongoing, larger prospective cohort study of the immune response to TBI. The study enrolled both persons with mild TBI (n = 41) and age-gender matched controls (n = 39) from a large level I trauma center. The current sample was taken from the first 80 subjects enrolled in 2012–13. TBI subjects must be 21 years of age or older and have a clinical diagnosis of mild TBI using CDC criteria (confusion or disorientation; amnesia near the time of injury and GCS of 15 by 24 h; a loss of consciousness up to 30 min; neurological or neuropsychological problems, and/or an initial score of 13 or higher on the GCS 21) from a blunt mechanism of injury within the past 24 h. Potential subjects were excluded if they were pregnant, had cervical spine injury, prior head injury or stroke in past year, history of dementia or if they had a diagnosis of autoimmune disorder or were using a therapeutic agent that would affect the immune response (e.g., immune suppressive agents, steroids). Controls were also excluded if they had a similar history of TBI, stroke, dementia or were on similar agents. Further, control subjects must have been able to independently perform activities of daily life at time of enrollment. All subjects consented to participation prior to any study procedures. Blood was drawn from subjects within 24 h of injury (or at enrollment for controls) in EDTA-anticoagulated blood tubes (Becton Dickinson, Franklin Lakes, NJ) and processed for plasma within 2 h of collection. Aliquots of samples were stored at −70 °C until evaluation.
Injury related data
Glasgow Coma Scale (GCS) the GCS is a measure of impaired consciousness following TBI or other disorders. For the purposes of this study, the investigators used the first reported GCS in the ED.
Abbreviated Injury Scale-head (AIS-head) is a systematic method for rating the severity of individual injuries to the cranium and brain rated from 1 (minor) to 6 (maximum/nonsurvivable) (2008). This was extracted from the medical record by trained investigators and verified with the trauma registry.
Injury severity score (ISS) is a measure of the overall severity of multiple trauma. The ISS is the sum of the squares of the most severe injury in each of the three most severely injured body regions and is scored on a scale of 1 (least severe) to 75 (most severe). The six body regions on which the ISS is based are head/neck, face, chest, abdomen/pelvic contents, extremities/pelvic girdle, and external (Baker and O’Neill, 1976; Baker et al., 1974). Similar procedures to the AIS were followed.
Glasgow outcome scale-extended (GOS-E)
This is a brief descriptive outcome scale that assesses functional outcome after injury. It yields an overall score ranging from 1 = “dead” to 8 = “upper good recovery”.
Measurement of G-CSF in human plasma
G-CSF was measured using ELISA kits from R&D systems (Minneapolis, MN). At time of assay, samples were diluted one to one with the assay buffer provided by the kit. Values are reported in pg/ml.
Statistical methods
Means are reported with their standard errors. Following evaluation of normality, means were compared by t-test and multiple comparisons were made by one-way ANOVA followed by Newman-Keuls post-test. The receiver operating characteristic (ROC) curve with area under the curve analysis (AUC; c-statistic) were performed to assess the ability of G-CSF to predict TBI versus control group. The ROC curve was analyzed to identify thresholds for prediction. Values with p < 0.05 were taken as statistically significant.
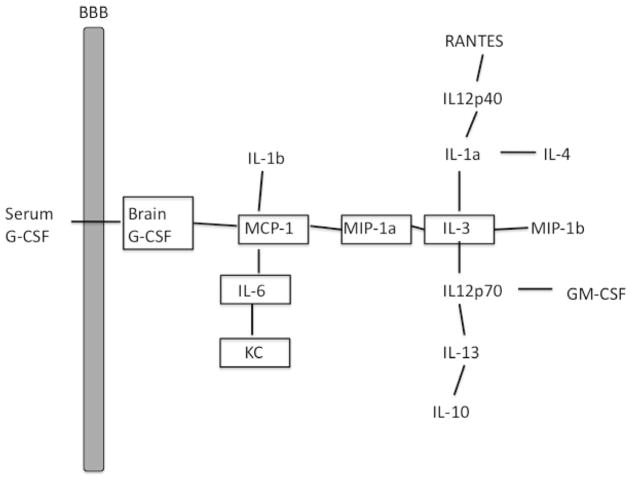
Path analysis based on methods as outlined by Baron and Kenny (1986) was used to further explore relations among cytokines. Such analysis allows the numerous relations among cytokines to be ranked in a hierarchical fashion with the statistically more robust correlations being assigned roles as direct relations. The remaining correlations are revealed to be indirect relations, being mediated through the more robust correlations. We performed this analysis in a series of steps. First, statistically significant (p < 0.001) Spearman correlation coefficients (r) between the serum G-CSF and brain cytokine/chemokine pairings were ranked in order from high to low. Second, starting with the pairs with the highest correlation coefficients, pairs in which the correlation coefficient was statistically significant were graphed (see Fig. 1) unless the cytokines were already connected indirectly through other pairings. Violation of this rule would create a chain passing through a cytokine more than once and was most easily noted visually in Fig. 1 in that it would produce a closed loop. The cytokines producing this indirect pathway are termed mediators. Third, to determine the extent to which the mediators accounted for the correlation between the pair producing the violation (i.e., closing the loop), the correlation coefficients of all the mediators were multiplied and subtracted from the correlation coefficient of the violating pair. If the residual (direct) correlation coefficient remained statistically significant, then it was deemed that the pair was directly connected and not just related through the mediating cytokines. This results in a graph that, based on the given data set, shows which cytokines are mostly closely related to one another and those which are indirectly related through other cytokines.
Fig. 1.
Path analysis of relation of serum G-CSF to brain cytokines in a mouse model of TBI. Results show that serum G-CSF was most directly correlated with brain G-CSF levels and so was related to several other brain cytokines.
3. Results
Animal study
Here, we found these levels of G-CSF in serum correlated with the simultaneously obtained G-CSF levels in brain and with five other brain cytokines (Table 1; n = 24). Path analysis showed that serum G-CSF was linked to brain cytokines most directly through brain G-CSF and less directly through an MCP-1/MIP-1 alpha/IL-3 pathway with nine other brain cytokines (Fig. 1).
Table 1.
Correlations of serum G-CSF levels with levels of brain cytokines in mouse.
| Cytokine | r; p | Cytokine | r; p value |
|---|---|---|---|
| G-CSF | 0.868; p < 0.001 | KC | 0.713; p < 0.001 |
| IL-3 | 0.438; p < 0.05 | MCP-1 | 0.715; p < 0.001 |
| IL-6 | 0.761; p < 0.001 | MIP-1alpha | 0.687; p < 0.001 |
r = correlation coefficient.
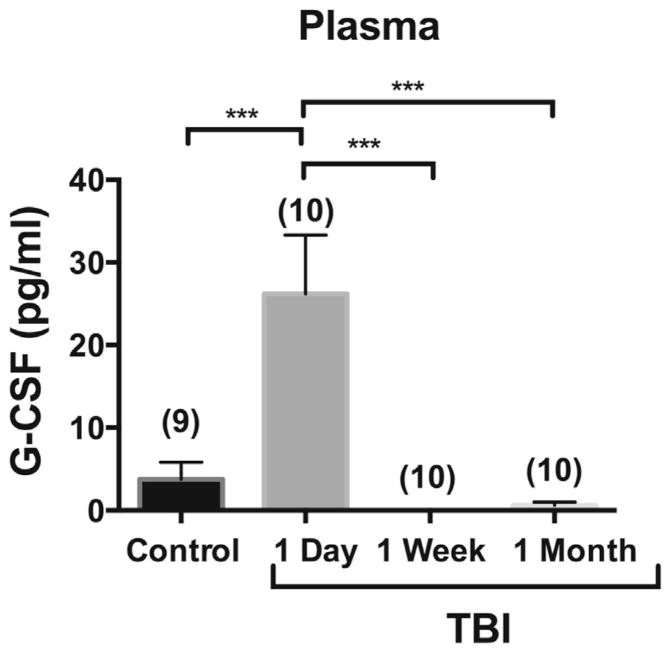
Human Phase I study
Demographics and injury characteristics of the Phase I and II studies are given in Table 2. In Phase I, plasma G-CSF was measured 1 day, one week, and one month after TBI (Fig. 2). There was a statistically significant elevation on the day of TBI. At the subsequent time points of one week and one month, plasma G-CSF levels did not differ from non-injured controls.
Table 2.
Demographic and injury characteristics of the Phase I (n = 19) and Phase II (n = 80) samples by TBI status. Data are presented as means/range unless otherwise noted*.
| Phase I sample* | Phase II sample** | |||
|---|---|---|---|---|
|
|
|
|||
| Controls (n = 9) | TBI (n = 10) | Controls (n = 39) | TBI (n = 41) | |
| Age | 74.1 (71–90) | 77.0 (65–87) | 42.8 (22–76) | 42.7 (21–81) |
| % Male | 33% | 40% | 69% | 68% |
| Mechanism of injury (%) | n/a | MVC 30 | n/a | MVC 41.5 |
| Fall 70 | Pedestrian 9.8 | |||
| Fall 21.9 | ||||
| Assault 12.2 | ||||
| Other 14.6 | ||||
| Initial ED GCS | n/a | 14.1 (10–15) | n/a | 14.9 (14–15) |
| Confirmed loss of consciousness (%) | n/a | Data not available | n/a | 54% |
| Possible loss/altered consciousness (%) | n/a | n/a | 19.5% | |
| Post-traumatic amnesia (%) | n/a | n/a | 48.8% | |
| % having head CT performed | n/a | 100% | n/a | 75.6% |
| % CTs positive for intracranial pathology | 90% | 29% | ||
| Injury severity score (ISS)** | n/a | 17.1 (95% CI 11.3; 25.3)) | n/a | 5.6 (95% CI 4.2; 7.0)) |
| GOS-E | n/a | n/a | ||
| 3 months | 6.1 (4–8) | 7.6 (5–8) | ||
| 6 months | 6.2 (4–8) | 7.7 (5–8) | ||
Phase I sample only enrolled older subjects.
Phase II sample restricted enrollment to AIS < 3 in systems other than head. n/a = not available
Fig. 2.
Phase I study: levels of plasma G-CSF in ten patients followed serially with blood taken immediately, one week, and one month after TBI and compared to nine control patients. Plasma G-CSF was elevated immediately after TBI, but not later. The n per group is shown in parentheses.
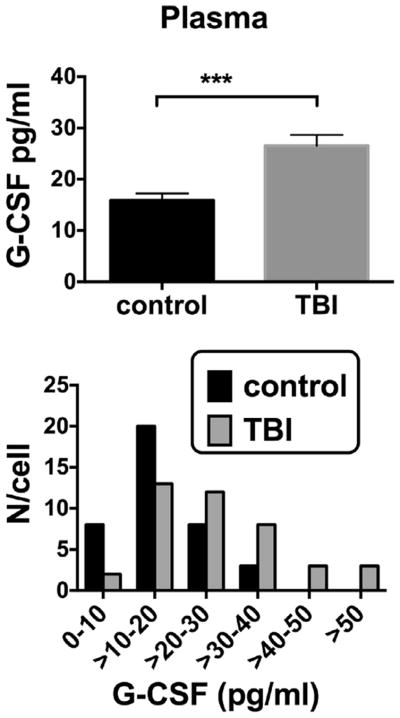
Human Phase II study
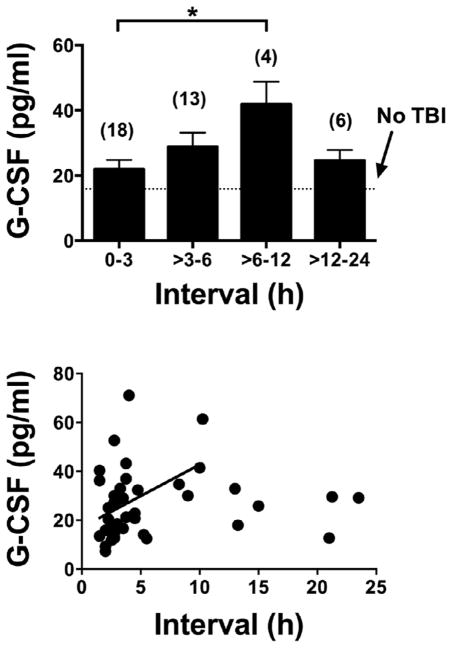
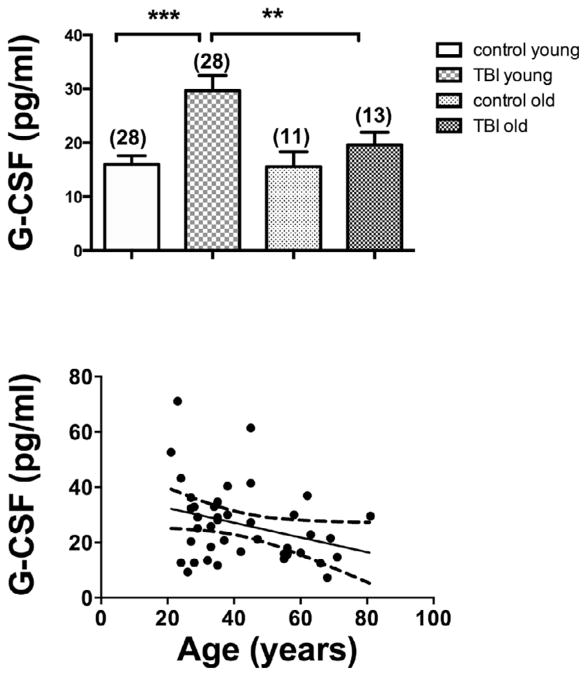
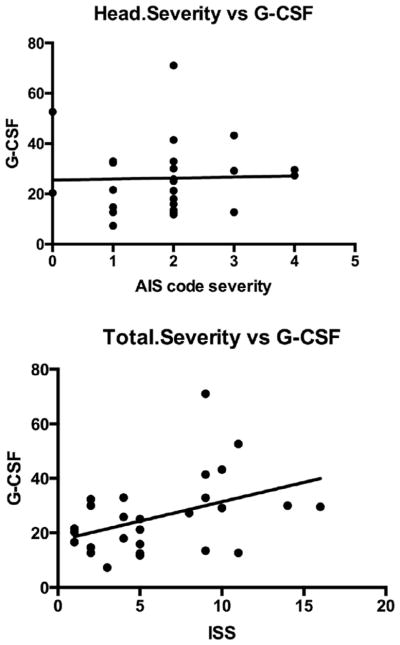
G-CSF was elevated in TBI patients who reported to an inner city emergency room in comparison to age-gender matched individuals who did not have TBI: Control 15.9 +/− 1.4 pg/ml, n = 39 vs TBI 26.5 +/− 2.2 pg/ml, n = 41; t(78) = 4.10; p < 0.001 (Fig. 3, upper panel). The lower panel of Fig. 3 shows the distribution pattern for G-CSF levels in plasma to be shifted to the right in TBI. Fig. 4 upper panel shows that plasma G-CSF levels peaked between 6 and 12 h after reporting to the emergency room. There was a linear relation between this interval and G-CSF levels for those results that included data with an interval of 12 h or less (Fig. 4, lower panel; r = 0.400, n = 35, p < 0.05). Subsequent sub analysis showed a significant elevation in those younger than age 55, but a non-significant arithmetic increase in those aged 55 years or more (Fig. 5, upper panel). Regression between age and plasma G-CSF levels for those with TBI showed a trend towards correlation (r = 0.308, n = 41, p = 0.0502; Fig. 4b), but not for controls or all cases. Plasma G-CSF levels did not show an effect of gender either when cases were considered all together or when segregated into TBI and non-TBI cases (data not shown). There was no correlation between plasma G-CSF levels and head injury severity by AIS coding (Fig. 6 upper panel), but there was a correlation between serum G-CSF levels and injury severity score (ISS) (Fig. 6, lower panel, r = 0.419, p < 0.05). A total of 36 TBI patients had GOS-E scores at 3 months and 35 TBI patients had GOS-E at 6 months following TBI. In neither case did GOS-E scores correlate with plasma G-CSF levels (data not shown).
Fig. 3.
Phase II study: upper panel: plasma G-CSF was elevated in a cohort of 39 control and 41 TBI patients. Lower panel: histogram for plasma G-CSF levels in controls and TBI patients. These Phase II study patients were the cohort further evaluated in Figs. 4–6.
Fig. 4.
Interval between arriving in the emergency room and blood drawing vs plasma G-CSF levels. Upper panel: levels of G-CSF peaked in blood samples drawn 6–12 h after arriving in the emergency room. Lower panel: a statistically significant correlation existed between plasma G-CSF levels and interval for the first 12 h.
Fig. 5.
Age and plasma G-CSF levels. Upper panel: younger patients, but not older patients showed an increase in G-CSF after TBI. Young and older controls showed no differences. Lower panel: a trend (p = 0.054) between age and plasma G-CSF occurred among those who had suffered TBI.
Fig. 6.
Severity of injury and plasmaG-CSF levels. Upper panel: no correlation existed between the measure of severity of head injury and G-CSF levels. Lower panel: a significant correlation existed between the measure of total injury severity and G-CSF levels.
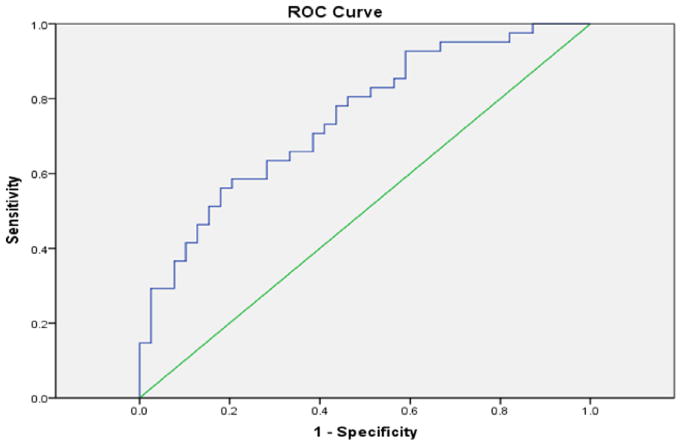
The ROC curve was used to assess the ability of G-CSF within 24 h of injury to identify TBI subjects (Fig. 7). When comparing levels in TBI versus non-injured controls, the AUC was 0.748 (95% CI .642–.854; p < 0.0005), indicating a reasonable marker for predictive purposes. At this time point, a level >15 pg/mL was found to have 80% sensitivity and 54% specificity to detect TBI.
Fig. 7.
Receiver operator characteristics (ROC) curve. Data used was from the validation study. The curve had an AUC of 0.748 and a predictive value of 15 pg/ml.
4. Discussion
These results extend previous findings in animal studies suggesting that serum G-CSF could be useful as a biomarker for TBI. Those previous studies found that G-CSF was the only cytokine of 23 measured whose serum levels were elevated after TBI in a murine model (Dohi et al., 2014). Here, we further evaluated that animal data by determining whether serum G-CSF levels correlated with brain cytokine levels in mouse. Using simple regression analysis, we found that serum G-CSF correlated directly with 6 of the brain cytokines, including with brain G-CSF, which showed the strongest statistical correlation. Path analysis showed that serum G-CSF directly or indirectly correlated with a total of 16 brain cytokines. Brain G-CSF and brain MCP-1 linked serum G-CSF with the other cytokines, including with IL-3 which acted as a major nidus with other cytokines. Overall, these results suggest that serum G-CSF can be used as a biomarker of the neuroinflammation of TBI, correlating directly and indirectly with many brain cytokines.
Several mechanisms could explain the linking of serum G-CSF with brain G-CSF and the other brain cytokines. G-CSF is produced both peripherally and centrally. G-CSF can cross the BBB, thus providing a mechanism by which brain and blood levels could be correlated (Zhao et al., 2007), and other mechanisms, including acting at circumventricular organs and through vagal afferent and efferent pathways, provide other ways through which peripheral and central sources of cytokines interact (Blatteis, 1992; Goehler et al., 1999; Quan and Banks, 2007). Other studies have found elevations in brain cytokines, including G-CSF, but could not detect a difference between arterial and jugular blood levels (Helmy et al., 2011). Such arterio-jugular gradients can indicate whether the brain is primarily taking up or secreting substances. However, it may be that the amount of G-CSF crossing in either the blood-to-brain or the brain-to-blood direction is too small to result in a measureable arterio-jugular difference. Therefore, the source of G-CSF could be either peripheral, CNS, or both.
The studies done here in humans showed similarities to our findings in mice. The mouse studies showed that serum G-CSF was elevated 24 h after CCI, but not at later time points (Dohi et al., 2014). Here, we found in the human plasma samples that G-CSF was elevated 1 day after TBI, but not 1 week or 1 month after TBI. This short time could be useful, especially in conditions where multiple injuries are likely to occur as during sports or in war zones. Future studies should include serial daily measures between day 0 and day 7 post-injury to better define the complete resolution of the G-CSF elevation. Further, G-CSF was found to have reasonable predictive ability for TBI in humans, particularly at thresholds above 15 pg/mL. Because as found in both the human and the murine data serum G-CSF returns to baseline soon after injury, it could be used repeatedly to detect subsequent injuries.
Further analysis was conducted in another set of patients whose blood was obtained within 24 h of a traumatic brain injury. In this group, plasma G-CSF levels were measured using an ultrasensitive kit and were elevated by about 60%. Further subset analysis showed no difference between men and women, but age analysis was ambiguous. A cut point of 55 years of age was used as this (a) is the age found to be the inflection point for when trauma mortality increases (MacKenzie et al., 2006) and (b) it is the recommended older age categorization per the guidelines for field triage (Sasser et al., 2012). In patients older than 55 that had suffered TBI, the arithmetic increase in plasma G-CSF did not reach statistical significance, suggesting that elevations may not be so robust in the aged (Sasser et al., 2012). However, there was no statistically significant correlation between G-CSF levels and age, although there was a strong trend. It may be that younger patients respond more robustly to a TBI event than older patients in terms of their G-CSF, or it may be that older patients report to the emergency room with less severe cases of TBI or that they have a less robust response to a given level of trauma.
These patients further defined the ephemeral nature to the elevation in plasma G-CSF following TBI. All had blood drawn within 24 h of injury. This analysis clearly showed a trend towards increasing levels for the first 12 h with a subsequent decline thereafter.
Finally, we found that severity of injury, as indicated by ISS score, but not severity of head injury, correlated with plasma G-CSF. However, the lack of correlation with head injury may be because the range was very narrow for the purposes of statistical analysis. Also, many individuals in the sample had a diagnosis of “closed head injury”, which is not a usable ICD-9-CM diagnosis for AIS purposes (receives a “9,” not codable). Alternatively, it may be the peripheral tissue injury, rather than the CNS injury, that dictates how much blood G-CSF is elevated. However, our animal studies, which involved only direct CNS injury and no trauma to the peripheral tissues, argues against peripheral tissue injury being the sole cause of elevations in serum G-CSF after TBI.
Here, we did not find a correlation between plasma G-CSF and outcomes at 3 and 6 months post TBI as measured by GOS-E. To the extent that G-CSF reflects severity of injury, it should also be linked to worse outcomes. However, peripheral administration of G-CSF has shown neuroprotective properties in carbon monoxide poisoning (Ghorbani et al., 2013), a Parkinson’s disease model (Frank et al., 2012), in focal cerebral ischemia (Hong et al., 2012), and in a TBI model (Acosta et al., 2014). Thus, a more robust response in G-CSF levels could be neuroprotective and so correlate with a better outcome. Contrary trends of higher levels with more severe injuries and higher levels also being neuroprotective could counterbalance each other and produce a net effect of no correlation. Although we sought to control potential sources of bias through inclusion/exclusion criteria, we did not evaluate all potential confounders such as co-morbid health conditions on G-CSF samples. This should be viewed as a study limitation. Additionally, the use of the GOS-E as an outcome variable for persons with mild TBI may not be optimal as we noted a very narrow range with most patients having values of 7 or 8. Future work should consider associations with other outcomes such as symptom burden, results of neuropsychological testing, and CT imaging. Additionally, future experiments should include comparison of CSF and plasma levels of G-CSF in humans given that it is not brain specific.
In conclusion, we found that blood G-CSF is acutely elevated after TBI in both mice and humans. The mouse studies showed that after direct injury to the brain, serum G-CSF correlates with and so is a biomarker for neuroinflammation, correlating directly with 6 cytokines and indirectly with 10 others. The human studies con-firm that G-CSF is elevated early after TBI, peaking between 6 and 12 h after arrival in the emergency room, may weakly correlate with age but not with gender, and relates to severity of injury. In sum, these findings suggest that G-CSF may be a useful marker in acute TBI, indicating degree of overall injury severity and correlating with neuroinflammation.
Acknowledgments
Supported by grants from the National Institutes of Health, National Center for Research Resources KL2RR025015 (HT), the National Institute of Neurologic Diseases and Stroke R01NS077913 (HT), the Department of Veterans Affairs (WAB), and the National Institute on Aging R01AG029839 (WAB) and R01AG046619 (WAB).
Footnotes
5. Author disclosures
No author has any financial disclosures to make.
References
- Acosta SA, Tajiri N, Shinozuka K, Ishikawa H, Sanberg PR, Sanchez-Ramos J, Song S, Kaneko Y, Borlongan CV. Combination therapy of human umbilical cord blood cells and granulocyte colony stimulating factor reduces histopathological and motor impairments in an experimental model of chronic traumatic brain injury. PLoS One. 2014;9:e90953. doi: 10.1371/journal.pone.0090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SP, O’Neill B. The injury severity score: an update. J Trauma. 1976;16:882–885. doi: 10.1097/00005373-197611000-00006. [DOI] [PubMed] [Google Scholar]
- Baker SP, O’Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- Banks WA. The blood–brain barrier in neuroimmunology: tales of separation and assimilation. Brain Behav Immun. 2015;44:1–8. doi: 10.1016/j.bbi.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bendall LJ, Bradstock KF. G-CSF: from granulocpoietic stimulant to bone marrow stem cell mobilizing agent. Cytokine Growth Factor Rev. 2014;25:355–367. doi: 10.1016/j.cytogfr.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Blatteis CM. Role of the OVLT in the febrile response to circulating pyrogens. In: Ermisch A, Landgraf R, Ruhle HJ, editors. Circumventricular Organs and Brain Fluid Environment. Elsevier; Amsterdam: 1992. pp. 409–412. [DOI] [PubMed] [Google Scholar]
- Chen G, Reichlin S. Clearance of [125 I]-tumor necrosis factor-alpha from the brain into the blood after intracerebroventricular injection into rats. Neuroimmunomodulation. 1998;5:261–269. doi: 10.1159/000026346. [DOI] [PubMed] [Google Scholar]
- Clausen F, Hanell A, Bjork M, Hillered L, Mir AK, Gram H, Marklund N. Neutralization of interleukin-1 beta modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury. Eur J Neurosci. 2009;30:385–396. doi: 10.1111/j.1460-9568.2009.06820.x. [DOI] [PubMed] [Google Scholar]
- Dohi K, Kraemer BC, Erickson MA, McMillian PJ, Kovac A, Flachbartova Z, Hansen KM, Shah GN, Sheibani N, Salameh T, Banks WA. Molecular hydrogen in drinking water protects against neurodegenerative changes induced by traumatic brain injury. PLoS One. 2014;24(29):e108034. doi: 10.1371/journal.pone.0108034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank T, Klinker F, Falkenburger BH, Laage R, Luhder F, Goricke B, Schneider A, Neurath H, Desel H, Liebetanz D, Bahr M, Weishaupt JH. Pegylated granulocyte colony-stimulating factor conveys long-term neuroprotection and improves functional outcome in a model of Parkinson’s disease. Brain. 2012;135:1914–1925. doi: 10.1093/brain/aws054. [DOI] [PubMed] [Google Scholar]
- Ghorbani M, Moallem S, Abnous K, Tabatabaee Yazdi SA, Movassaghi AR, Azizzadeh M, Mohamadpour AH. The effect of granulocyte colony-stimulating factor administration on carbon monoxide neurotoxicity in rats. Drug Chem Toxicol. 2013;36:102–108. doi: 10.3109/01480545.2012.737802. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Nguyen KT, Lee JE, Tilders FJ, Maier SF, Watkins LR. Interleukin-1 beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J Neurosci. 1999;19:2799–2806. doi: 10.1523/JNEUROSCI.19-07-02799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy A, Carpenter KL, Menon DK, Pickard JD, Hutchinson PJ. The cytokine responses to human tramatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J Cereb Blood Flow Metab. 2011;31:658–670. doi: 10.1038/jcbfm.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Deng C, Zhang J, Zhu J, Li Q. Neuroprotective effect of granulocyte colony-stimulating factor in a focal cerebral ischemia rat model with hyperlipidemia. J Huazhong Univ Sci Technol Med Sci. 2012;32:872–878. doi: 10.1007/s11596-012-1050-2. [DOI] [PubMed] [Google Scholar]
- Ley EJ, Clond MA, Singer MB, Shouhead D, Salim A. IL6 deficiency affects function after traumatic brain injury. J Surg Res. 2011;170:253–256. doi: 10.1016/j.jss.2011.03.006. [DOI] [PubMed] [Google Scholar]
- MacKenzie EJ, Rivara FP, Jurkovich GJ, Nathens AB, Frey KP, Egleston BL, Salkever DS, Scharfstein DO. A national evalualtion of the effect of trauma-center care on mortality. N Engl J Med. 2006;356:366–378. doi: 10.1056/NEJMsa052049. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossman MC, Lenzlinger PM, Hans V, Stahel P, Csuka E, Ammann E, Stocker R, Trentz O, Kossmann T. Production of cytokines following brain injury: beneficial and deleterious for the damaged tissue. Mol Psychiatry. 1997;2:133–136. doi: 10.1038/sj.mp.4000227. [DOI] [PubMed] [Google Scholar]
- Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Romero LI, Kakucska I, Lechan RM, Reichlin S. Interleukin-6 (IL-6) is secreted from the brain after intracerebroventricular injection of IL-1 β in rats. Am J Physiol. 1996;270:R518–R524. doi: 10.1152/ajpregu.1996.270.3.R518. [DOI] [PubMed] [Google Scholar]
- Sasser SM, Hunt RC, Faul M, Sugerman D, Pearson WS, Dulski T, Wald MM, Jurkovich GJ, Newgard CD, Lerner EB, Cooper A, Wang SC, Henry MC, Salomone JP, Galli RL. Centers for disease control and prevention guidelines for field triage of injured patients: recommendation of the national expert panel of field triage, 2011. MMWR Recomm Rep. 2012;61:1–20. [PubMed] [Google Scholar]
- Sayer NA. Traumatic brain injury and its neuropsychiatric sequelae in war veterans. Annu Rev Med. 2012;63:405–419. doi: 10.1146/annurev-med-061610-154046. [DOI] [PubMed] [Google Scholar]
- Sobrino T, Arias S, Rodriguez-Gonzalez R, Brea D, Silva Y, de la Ossa NP, Agulla J, Blanco M, Pumar JM, Serena J, Davalos A, Castillo J. High serum levels of growth factors are associated with good outcome in intracerebral hemorrhage. J Cereb Blood Flow Metab. 2009;29:1968–1974. doi: 10.1038/jcbfm.2009.182. [DOI] [PubMed] [Google Scholar]
- Sun D, Bullock MR, McGinn MJ, Zhou Z, Altememi N, Hagood S, Hamm R, Colello RJ. Basic fibroblast growth factor-enhanced neurogenesis contributes to cognitive recovery in rats following traumatic brain injury. Exp Neurol. 2009;216:56–65. doi: 10.1016/j.expneurol.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Bullock MR, Altememi N, Zhou Z, Hagood S, Rolfe A, McGinn MJ, Hamm R, Colello RJ. The effect of epidermal growth factor in the injured brain after trauma in rats. J Neurotrauma. 2010;27:923–938. doi: 10.1089/neu.2009.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B-L, He M-Q, Han X-Y, Sun J-Y, Yang M-F, Yuan H, Fan C-D, Zhang S, Mao L-L, Li D-W, Zhang Z-Y, Zheng C-B, Yang X-Y, Li YV, Stetler RA, Chen J, Zhang F. Intranasal delivery of granulocyte colony-stimulating factor enhances its neuroprotective effects against ischemic brain injury in rats. Mol Neurobiol. 2014 doi: 10.1007/s12035-014-8984-2. epub. [DOI] [PubMed] [Google Scholar]
- The Abbreviated Injury Scale: 2008 update, 2008. Association for the Advancement of Automotive Medicine; Des Plaines, IL: [Google Scholar]
- Yildiz R, Coskun U, Buyukberber S, Sancak B, Kaya AO, Gulbahar O, Benekli M. Serum granulocyte colony-stimulating factor levels in gliomas. J BUON. 2011;16:138–141. [PubMed] [Google Scholar]
- Yu SC, Kuo CL, Huang CS, Chang CS, Wu SL, Su SL, Liu CS. Endogenous granulocyte colony-stimulating factor: a biomarker in acute ischemic stroke. Biomarkers. 2012;17:319–324. doi: 10.3109/1354750X.2012.668712. [DOI] [PubMed] [Google Scholar]
- Zhao LR, Navalitloha Y, Singhal S, Mehta J, Piao CS, Guo WP, Kessler JA, Groothuis DR. Hematopoietic growth factors pass through the blood–brain barrier in intact rats. Exp Neurol. 2007;204:569–573. doi: 10.1016/j.expneurol.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebell JM, Morganti-Kossmann MC. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 2010;7:22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]