Abstract
Objective
To differentiate developmental encephalopathies by creating a novel quantitative phenotyping tool.
Study design
We created the Developmental Encephalopathy Inventory (DEI) to differentiate disorders with complex multisystem neurodevelopmental symptoms. We then used the DEI to study the phenotype features of 20 subjects with FOXG1 disorder and 11 subjects with MECP2 disorder.
Results
The DEI identified core domains of fine motor and expressive language that were severely impaired in both disorders. Individuals with FOXG1 disorder were overall more severely impaired. Subjects with FOXG1 disorder were less able to walk, had worse fine motor skills, more disability in receptive language and reciprocity, and had more disordered sleep than did subjects with MECP2 disorder (P < .05). Covariance, cluster, and principal component analysis confirmed a relationship between impaired awareness, reciprocity, and language in both disorders. In addition, abnormal ambulation was a first principal component for FOXG1 but not for MECP2 disorder, suggesting that impaired ambulation is a strong differentiating factor clinically between the 2 disorders.
Conclusions
We have developed a novel quantitative developmental assessment tool for developmental encephalopathies and propose this tool as a method to identify and illustrate core common and differential domains of disability in these complex disorders. These findings demonstrate clear phenotype differences between FOXG1 and MECP2 disorders.
A class of disorders has been recognized in which features of intellectual disability and autism coexist with dysfunction of multiple other neurodevelopment domains, including the autonomic nervous system, breathing rhythm, specific types of epilepsy, movement disorders, and other findings. We use the term “developmental encephalopathy” to capture the full spectrum of phenotype in these conditions. MECP2 disorder is a classic example of a developmental encephalopathy. First described in 1966,1 causative loss-of-function mutations in MECP2 were found in 1999.2 Individuals with MECP2-related disorder often have normal development until 6–18 months of age, after which they begin to lose purposeful hand movements, motor, and language skills.3,4 Other characteristic signs include postnatal microcephaly, hand stereotypies, breathing irregularities, and autonomic disturbances.5 Although the disorder has been termed a “syndromic form of autism,”6 these additional features illustrate that the phenotype extends beyond that typically seen in autism spectrum disorders.
In 1985, a female patient was described with congenital hypotonia, loss of eye contact at 16 months, and emergence of hand stereotypies at 18 months.7 Others also noted individuals with a clinical diagnosis of “Rett syndrome” had congenital hypotonia,8 and soon criteria for both a “congenital Rett variant” and an “early seizure variant” were proposed.9,10 The “early seizure variant” was found to be caused by mutations in the gene CDKL5.11 The “congenital Rett variant” was found to be due to chromosome 14q12 deletions and loss-of-function mutations in FOXG1.12,13
Individuals with FOXG1 disorder present with global developmental delay during infancy, postnatal microcephaly, and malformations of the corpus callosum.14,15 Other symptoms reported include severe gastrointestinal dysfunction, hyperkinesis, and sleep abnormalities.15 Epilepsy is common, with multiple seizure types developing after 1 year of age that are refractory to antiseizure medications.16 The autonomic, respiratory, and cardiac complications seen in MECP2 disorder17–19 are not described in individuals with FOXG1 disorder. Finally, most individuals with FOXG1 disorder have chorea-dystonia,20 movement patterns distinct from MECP2 disorder; however, patients with FOXG1 mutations continue to be reported in the literature as if they have MECP2 disorder.21–23
Despite these differences in genetic cause, developmental phenotypes, and brain malformations, distinguishing between MECP2 and FOXG1 disorders is a complex task, and clinical recognition is made difficult by the existence of other similar disorders. Except for genetic diagnosis and clinical experience, there are no established tools or methods to differentiate among these disorders, impeding clinical diagnosis, phenotyping, and natural history studies. To address this need, our research team created the Developmental Encephalopathy Inventory (DEI) to assess and distinguish between disorders like FOXG1 and MECP2. Our broader intent was to establish tools and methods to study developmental encephalopathies rigorously, to improve clinical knowledge about diagnosis and prognosis, and to improve counseling for affected families.
Methods
All subjects underwent informed consented through the Genetic Studies of Developmental Brain Disorders protocol approved by the University of Rochester Medical Center Research Subjects Review Board. For consistency of phenotyping of MECP2 disorder, we included only individuals who, on review of their medical records, were identified by their treating child neurologist, geneticist, or developmental pediatrician as meeting classical criteria for the syndrome.5 All subjects completed chromosomal microarray, FOXG1, and/or MECP2 gene sequencing as part of routine clinical care. Subjects with 14q12 copy number variants identified by chromosomal microarray had the de novo status of these variants confirmed by fluorescence in situ hybridization of parental samples according to routine clinical practice. Subjects with FOXG1 or MECP2 sequence abnormalities had the inheritance confirmed by standard Sanger methods.
The DEI was created to quantify the physical, neurologic, and neurobehavioral phenotypes of individuals with overlapping features of autism spectrum disorders and intellectual disability. Such persons may be too severely impaired to be assessed with standard autism diagnostic tools such as the Autism Diagnostic Inventory (Revised) or the Autism Diagnostic Observation Scale.24 In addition, because many of the developmental encephalopathies are rare, our intent was to develop a measure that could be administered remotely by parent proxy (eg, telephone, video conference) and would not require direct examination by an expert clinician. Creation of the DEI was an iterative process. We first compiled the phenotypes of 10 disorders described in published reports agreed on by the authors as meeting criteria for developmental encephalopathies (Table I; available at www.jpeds.com).
Table I.
Ten example disorders meeting the criteria for developmental encephalopathies
|
|
We next applied definitions to the neurobehavioral phenotypes by using 3 publicly available ontologies: the Human Phenotype Ontology (http://www.human-phenotypeontology.org/index.php/hpo_home.html), the Medical Subject Headings vocabulary (http://www.ncbi.nlm.nih.gov/mesh/), and the Unified Medical Language System (http://www.nlm.nih.gov/research/umls/). For phenotypes not defined by these ontologies, we used definitions from the primary literature and child neuropsychological assessment tools (The Bayley Scales of Infant and Toddler Development-III: Observation Checklist,25 the Vineland Adaptive Behavior Scales 2nd edition,26 and the Scales of Independent Behavior – Revised27).
A tree data structure was then assigned to the items grouped within neurobehavioral domains. This resulted in grouping of phenotypes into 14 parent domains of neurobehavioral function (Table II). Table III (available at www.jpeds.com) lists all domains and their respective items. Because several of the domains included items that do not emerge until after 2–3 years of age (eg, expressive language), we limited the DEI assessment to individuals older than 3 years of age. The initial version of the DEI incorporated domains to assess epilepsy and movement disorders; however, definitions within those domains required direct reviews of electroencephalography reports (for epilepsy) and either video or direct observation (for movement disorders) by a trained clinician. Therefore, the current form of the DEI focused on the 12 remaining domains. Data were collected by structured telephone interview with the parents of subjects. DEI scores for each item within each domain ranged from 0 to 3. Lower scores indicated better function, with a score of ≥2 indicative of frequent impairment or worse, with interference with daily function. A complete copy of this pilot version DEI as administered is available as Table III.
Table II.
Fourteen domains of neurobehavioral function included in the DEI
|
For this study, movements and seizures were assessed separately.
Table III.
The DEI
| Number | Item description | Variable name | Score type |
|---|---|---|---|
| A. | Metadata | ||
| 1 | Subject ID | $id | |
| 2 | Age, y | $age_year | |
| 3 | Age, mo | $age_month | |
| 4 | Interviewer | $interviewer | |
| 5 | Timestamp | $ts | |
| B. | Ambulation | ||
| 1 | Can crawl or roll to get around room | $crawls | 2 |
| 2 | Can walk independently for at least 6 feet without holding onto anything or another person (ie, wall or furniture or another person) | $walks | 2 |
| 3 | Can run independently for at least 6 feet without needing to hold onto anything | $runs | 2 |
| 4 | Trips and/or falls | $falls | 1 |
| 5 | Comments | $ambulation_comments | |
| C. | Autonomic nervous system | ||
| 1 | Tachycardia | $tachycardia | 1 |
| 2 | Hypertension | $hypertension | 1 |
| 3 | Edema of extremities | $edema | 1 |
| 4 | Bradycardia | $bradycardia | 1 |
| 5 | Hypotension | $hypotension | 1 |
| 6 | Constipation | $constipation | 1 |
| 7 | Aspiration | $aspiration | 1 |
| 8 | Absent sphincter control | $absent_sphincter | 1 |
| 9 | Gastroesophageal reflux | $gerd | 1 |
| 10 | Vomiting | $vomiting | 1 |
| 11 | Hyperthermia | $hyperthermia | 1 |
| 12 | Hypothermia | $hypothermia | 1 |
| 13 | Comments | $ans_comments | 1 |
| D. | Awareness of self and environment | ||
| 1 | Turns toward any sound or noise | $turns_sound | 2 |
| 2 | Turns head toward parent/caregiver when hearing parent/caregiver's voice | $turns_head_parent | 2 |
| 3 | Responds to his/her own name when spoken | $responds_name | 2 |
| 4 | Cries/fusses when hungry or wet | $cries_hungry_wet | 2 |
| 5 | Comments | $awareness_comments | |
| E. | Breathing | ||
| 1 | Intermittent overbreathing/hyperventilation | $overbreathing | 1 |
| 2 | Breathing dysrhythmia/mixed hypeventilation/hypoventilation | $breathing_dysrhythmia | 1 |
| 3 | Hypoventilation | $hypoventilation | 1 |
| 4 | Comments | $breathing_comments | |
| F. | Fine motor | ||
| 1 | Moves small objects (ie, a toy block or Lego) from one hand to the other | $moves_obj_hands | 2 |
| 2 | Swallows food without choking (ie, cooked vegetables, chopped meats) | $swallows_no_choking | 2 |
| 3 | Drinks from a cup or glass (may spill), holding cup to mouth, can be a sippy cup | $drinks_cup | 2 |
| 4 | Able to use a utensil or tool appropriately (ie, spoon, toy hammer, toothbrush, hairbrush) | $uses_utensil | 2 |
| 5 | Takes off clothing that opens in the front (ie, coat) by undoing a zipper or buttons | $removes_clothing | 2 |
| 6 | Picks up small object with thumb and fingers (ie, toy block or Lego) | $pincer_grasp | 2 |
| 7 | Comments | $fm_comments | |
| G. | Food behavior | ||
| 1 | Able to feed self if food is placed in front of child | $feeds_self | 2 |
| 2 | Eats nonfood items (pica) | $pica | 1 |
| 3 | Anorexia/refusal to eat/not interested in eating. For example, turns head away when food is offered, does not eat food placed in front of him/her if able to feed self, spits food out, does not cry/fuss at times when you would expect child to be hungry | $anorexia | 1 |
| 4 | Hyperphagia/overeating/obsessed with food. (ie, does not ever seem to get full, complains of being hungry or fusses as if hungry even if has recently eaten what should be a sufficient amount, sneaks extra food. | $hyperphagia | 1 |
| 5 | Comments | $feeding_comments | |
| H. | Language (expressive) | ||
| 1 | Makes sounds or gestures when wants activity to stop or to keep going (ie, crying, grunting, reaching, pushing item away) | $makes_sounds_stop_go | 2 |
| 2 | Makes nonword baby sounds (babbling) | $babbles | 2 |
| 3 | Says “da-da,” “ma-ma,” or another consistent or recognizable name for parent/caregiver (even if a nickname) that is clearly directed at a specific person, and is not general babbling OR uses sign language that means the same | $names_caregiver | 2 |
| 4 | Repeats or tries to repeat common words immediately after hearing them (does not have to be fully intelligible) OR uses sign language that means the same | $repeats_words | 2 |
| 5 | Names at least 3 common objects that s/he is familiar with (ie, cat, toy) OR uses sign language that means the same | $names_3_obj | 2 |
| 6 | Makes one-word requests such as “up,” “give,” “no,” or “mine” OR uses sign language that means the same | $one_word_request | 2 |
| 7 | States own first name spontaneously or when asked directly “What's your name?” or when pointed to and asked “Who's this?” Does not have to be pronounced clearly OR uses sign language that means the same | $says_name | 2 |
| 8 | Says at least 50 recognizable words that can be understood by someone who knows the child and is familiar with his/her speech patterns OR uses sign language that means the same | $says_50_words | 2 |
| 9 | Comments | $lang_expr_comments | |
| I. | Language (receptive) | ||
| 1 | Responds to spoken request (ie, “Give me the toy please” or “Where is your nose?”) | $responds_request | 2 |
| 2 | Demonstrates understanding of the word “no,” or a word or gesture with the same meaning (ie, stops current activity, if only briefly) | $understands_no | 2 |
| 3 | Directs attention to storyteller or reader | $attention_story | 3 |
| 4 | Points to 3 body parts when asked | $points_3_bodyparts | 2 |
| 5 | Knows difference between 2 toys | $knows_2_toys | 2 |
| 6 | Responds differently to 2 different requests (ie, “Do you want to play?” vs “Do you want to go to bed?” | $knows_2_requests | 2 |
| 7 | Comments | $lang_recept_comments | |
| J. | Mood | ||
| 1 | Exhibits shyness and/or social anxiety about situations and people | $shy | 1 |
| 2 | Is easily irritated (negative response to a mild stimulus) | $easily_irritated | 1 |
| 3 | Expresses fear or worries or anxieties that are unreasonable | $anxiety | 1 |
| 4 | Is hyperactive, ie, always “on the go,” as if driven by a motor, difficulty staying still in situations that call for it. Note that activities are not purposeless. | $hyperactive | 1 |
| 5 | Laughs/smiles inappropriately (ie, when gets hurt or witnesses someone else getting hurt or when someone cries or appears sad or angry) | $laughs_inapprop | 1 |
| 6 | Obsesses about things, situations, or people. Gets thoughts or ideas stuck in his/her head and cannot stop thinking about them (ie, constantly thinks about or talks about certain toys or activities). | $obsesses | 1 |
| 7 | Exhibits self-injurious behaviors (ie, deliberately attempts to harm self by biting, kicking, scratching, or other risk taking behavior that is intended to result in bodily harm). | $self_injurious | 1 |
| 8 | Slow to settle down/difficulty calming or transitioning after a (positive or negative) stimulation | $slow_to_settle | 1 |
| 9 | Comments | $mood_comments | |
| K. | Reciprocity | ||
| 1 | Sustains gaze when parent makes eye contact with child | $sustains_gaze | 2 |
| 2 | Imitates or tries to imitate parent/caregiver facial expressions (ie, smiling, open mouth, smacking lips, blinking eyes) | $imitates_expressions | 2 |
| 3 | Follows pointed finger to an object | $follows_pointed_finger | 2 |
| 4 | Waves goodbye when another person waves, or when another person says “goodbye” to child | $waves | 2 |
| 5 | Shows interest in children by sharing toys, seeking eye contact, sustaining eye contact, attempting to communicate verbally or physically (making sounds to get other child's attention, touching other child, scooting, crawling, walking over to be closer to other child), watches what other child is doing | $interest_in_children | 2 |
| 6 | Makes or tries to make social contact with people by smiling, laughing, offering toys or other items, touching person, moving to be near a person, saying or attempting to say person's name or get their attention | $makes_social_contact | 2 |
| 7 | Smiles when smiled at | $smiles_response | 2 |
| 8 | Child engages in parallel play (engages in similar activity as another child nearby, but does not interact directly with that child) | $parallel_play | 2 |
| 9 | Child engages in behavior that is intended to elicit response from others (ie, to provoke laughter, or “testing the limits”) | $behavior_response | 2 |
| 10 | Comments | $reciprocity_comments | |
| L. | Sensory | ||
| 1 | Blunted response to pain/pain insensitivity (ie, does not appear to notice painful stimuli) | $pain_insensitivity | 1 |
| 2 | Hypersensitive to pain/cries in pain easily (ie, cries or complains of pain with stimulus that should not be painful) | $pain_hypersensitivity | 1 |
| 3 | Cortical visual impairment | $cvi | 1 |
| 4 | Sensorineural hearing loss | $snhl | 1 |
| 5 | Comments | $sensory_comments | |
| M. | Sleep | ||
| 1 | Hypersomnolent/difficult to keep awake during day | $hypersomnolent | 1 |
| 2 | Abnormal sleep onset | $abnl_sleep_onset | 1 |
| 3 | Abnormal awakening after sleep onset | $awakens_after_sleep_onset 1 | |
| 4 | Hyposomnolent (does not appear to need same amount of sleep as other his/her age) | $hyposomnolent | 1 |
| 5 | Comments | $sleep_comments |
Score type 1: 0 = does not occur; 1 = rarely occurs and does not interfere with routine activities; 2 = frequently (ie, weekly) interferes with routine activities; 3 = constantly (ie, daily) interferes with routine activities.
Score type 2: 0 = always able to (100% independently); 1 = usually able to (75% of the time, needs prompting); 2 = sometimes able to (can attempt, but result is not good); 3 = never able to (too hard, too difficult, not safe, not appropriate).
Score type 3: 0 = >1 minute; 1 = 1 minute; 2 = 30 seconds; 3 = not at all.
Statistical Analyses
All statistical tests were performed with R version 3.1.2 (http://cran.r-project.org/). Differences between mean DEI scores were evaluated with the Mann-Whitney U test for non-parametric data. Covariance was evaluated with Spearman rho. P values <.05 were interpreted as significant. Hierarchical cluster analysis was performed with the R package pvclust (http://www.sigmath.es.osaka-u.ac.jp/shimo-lab/prog/pvclust/). Principle component analysis was performed with the R package FactoMineR version 1.29 (http://cran.r-project.org/web/packages/FactoMineR/index.html). A stand-alone R script dei.R that performs the statistical analyses reported here is available for free at https://github.com/Paciorkowski-Lab/DEI.
Circos plots were used to illustrate differential and overlapping DEI domains scoring ≥2 (frequently impaired or worse) and were created with circos-0.56 (http://circos.ca/).28 Code to create Circos visualizations is available at https://github.com/Paciorkowski-Lab/DEI/tree/master/circos_files.
Results
A total of 20 individuals with FOXG1 disorder (18 intragenic loss-of-function de novo mutations and 2 deletions of 14q12) and 11 individuals with clinically typical MECP2 disorder (2 missense and 9 loss-of-function de novo mutations) participated in the study. Table IV (available at www.jpeds.com) summarizes subject demographics and genotype data.
Table IV.
Subjects with FOXG1 and MECP2 disorders included in this study
| Subjects | Diagnostic genetics | Sex | Age at time of study, y |
|---|---|---|---|
| DB12-001 | FOXG1 c.651C>G/p.Y217X | M | 4.5 |
| DB12-002 | FOXG1 p.Gln86X | F | 4.9 |
| DB12-004 | 1.1 Mb del 14q12 (FOXG1) | F | 3.6 |
| DB12-005 | FOXG1 c.430G>T, p.E144X | M | 9.5 |
| DB12-006 | FOXG1 c.460dupG/p.Glu154GlyfsX301 | M | 6.3 |
| DB12-009 | FOXG1 c.460dupG/p.Glu154GlyfsX301 | F | 16.25 |
| DB12-015 | FOXG1 c.133_469del377insACCCACCGCCCC | M | 6.9 |
| DB12-016 | FOXG1 c.577G>A/p.Ala193Thr | M | 13.75 |
| DB12-017a1 | FOXG1 c.515_517del63/p.Gly172_Met192del | F | 9.6 |
| DB12-017a2 | FOXG1 c.515_517del63/p.Gly172_Met192del | F | 6.8 |
| DB13-006 | FOXG1 c.506delG | M | 3.9 |
| DB13-007 | FOXG1 c.586C>T/p.Gln196X | M | 3.6 |
| DB13-012 | FOXG1 c.755G>T/p.Gly252Val | F | 6.5 |
| DB13-019 | 4.1 Mb del 14q12 (FOXG1) | M | 10 |
| DB13-028 | FOXG1 c.735delC | M | 5.6 |
| DB13-029a1 | FOXG1 c.460dupG/p.Glu154GlyfsX301 | F | 25.8 |
| DB13-029a2 | FOXG1 c.460dupG/p.Glu154GlyfsX301 | M | 22 |
| DB13-041 | FOXG1 c.222_223dupGC/p.Pro75ArgfsX118 | M | 8 |
| DB13-052a1 | FOXG1 c.460dupG/p.Glu154GlyfsX301 | F | 5 |
| DB13-052a2 | FOXG1 c.460dupG/p.Glu154GlyfsX301 | F | 5 |
| DB13-042 | MECP2 c.808C>T/p.R270X | F | 31 |
| DB13-044 | MECP2 p.R294X | F | 8.6 |
| DB13-050 | MECP2 c.1163_1188del26/p.P388fs | F | 8.5 |
| DB13-058 | MECP2 p.R255X | F | 28 |
| DB13-059 | MECP2 c.916C>T, p.R306C | F | 4.25 |
| DB13-060 | MECP2 p.R255X | F | 20 |
| DB14-011 | MECP2 p.R255X | F | 15 |
| DB14-012 | MECP2 p.R255X | F | 26 |
| DB14-023 | MECP2 del exons 3-4 | F | 5.9 |
| DB14-025 | MECP2 p.R133C | F | 6 |
| DB14-027 | MECP2 del exon 4 | F | 9 |
F, female; M, male.
Figure 1, A (available at www.jpeds.com) shows the age distribution for subjects with FOXG1 disorder (mean age 8.9 years; median 6.7 years). Figure 1, B shows the distribution of ages for subjects with MECP2 disorder (mean age 14.75 years; median 9.0 years). Although most subjects in both groups were between 5 and 10 years of age, there were more subjects with FOXG1 disorder younger than 5 years of age and more subjects with MECP2 disorder older than 25 years of age.
Figure 1.
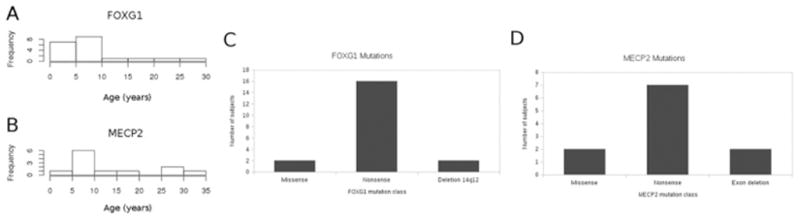
Histogram illustrating distribution of ages at the time of study of subjects with FOXG1 and MECP2. The mean age of subjects with FOXG1 was 8.9 years, and the median age was 6.7 years. The mean age of subjects with MECP2 disorder was 14.75 years, and the median age was 9.0 years. There were slightly more subjects with MECP2 disorder in the 25- to 35-year category.
Mutation Distribution
Most of the mutations in both FOXG1 and MECP2 were nonsense mutations that introduced premature stop codons and therefore were expected to cause loss of function of the encoded protein. Several individuals with FOXG1 had deletions of 14q12, or missense intragenic mutations, and several individuals with MECP2 had deletions of 1 or more exons, or missense mutations. The mutational distribution for each disorder is illustrated in Figure 1, C and D.
DEI Describes Areas of Severe Disability in Both FOXG1 and MECP2 Disorders
Table V presents the mean domain scores, within each parent-rated domain of function, for FOXG1 and MECP2 disorders, respectively, and between-group comparisons. Two DEI domains had a mean severity of ≥2 (frequently impaired or worse) in both FOXG1 and MECP2 disorders. These were the domains of fine motor and expressive language, areas classically described as core to the clinical phenotype of both disorders. Collectively, Tables VI–XVII (available at www.jpeds.com) present the mean scores for FOXG1 disorder and MECP2 disorder groups for all items across all domains, and group comparisons. Within the fine motor domain, subjects with FOXG1 or MECP2 disorder were rated as having severe difficulties with moving objects with hands, using utensils, and removing clothing (Table X).
Table V.
Mean DEI scores from parent domains for subjects with FOXG1 and MECP2
| DEI domains | FOXG1 mean | MECP2 mean | Mann-Whitney U P value |
|---|---|---|---|
| Ambulation | 2.4 | 1.4 | .03 |
| Autonomic nervous system | 0.8 | 0.9 | .72 |
| Awareness | 0.9 | 0.6 | .35 |
| Breathing | 0.1 | 1.4 | 6.7e-05 |
| Fine motor | 2.5 | 2.1 | .08 |
| Food behavior | 1.3 | 1.0 | .3 |
| Language (expressive) | 2.5 | 2.4 | .17 |
| Language (receptive) | 1.8 | 1.1 | .03 |
| Mood | 0.8 | 0.8 | .85 |
| Reciprocity | 1.9 | 1.3 | .017 |
| Sensory | 0.8 | 0.6 | .33 |
| Sleep | 1.3 | 0.7 | .02 |
Table VI.
Mean DEI scores for ambulation items
| Ambulation items | FOXG1 | MECP2 | Mann-Whitney U P value |
|---|---|---|---|
| Crawls | 1.2 | 0.8 | .4 |
| Walks | 3.0 | 1.1 | 7.5e-05* |
| Runs | 2.95 | 2.1 | .005* |
Bold values indicate statistical significance.
P ≤ .05.
Table XVII.
Mean DEI scores for sleep items
| Sleep items | FOXG1 | MECP2 | Mann-Whitney U P value |
|---|---|---|---|
| Hypersomnolent | 0.5 | 0.9 | .2 |
| Abnormal sleep onset | 1.4 | 0.7 | .1 |
| Awakens after sleep onset | 2.2 | 0.9 | .002* |
| Hyposomnolent | 1.3 | 0.3 | .02* |
Bold values indicate statistical significance.
P ≤ .05.
Table X.
Mean DEI scores for fine motor items
| Fine motor items | FOXG1 | MECP2 | Mann-Whitney U P value |
|---|---|---|---|
| Moves object with hands | 2.2 | 2.2 | 1 |
| Swallows without choking | 1.6 | 1.2 | .3 |
| Drinks from cup | 2.9 | 1.6 | .003* |
| Uses utensil | 2.8 | 2.6 | .5 |
| Removes clothing | 2.9 | 2.8 | 1 |
| Pincer grasp | 2.7 | 2.0 | .06 |
Bold values indicate statistical significance.
P ≤ .05.
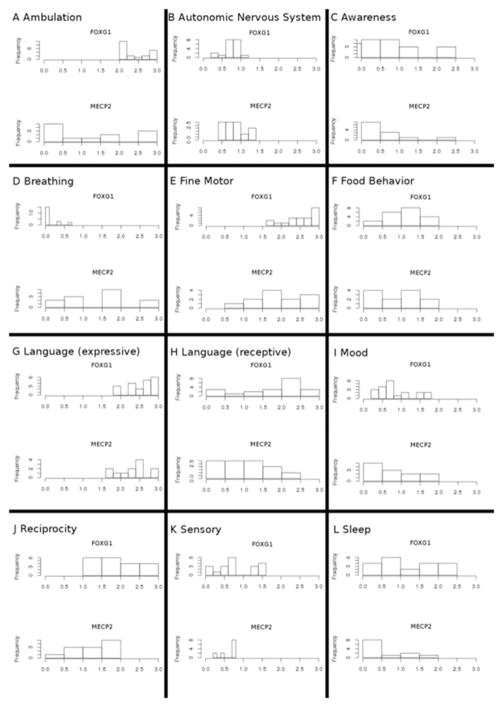
Within the expressive language domain, individuals in both groups were rated as having severe difficulties naming their caregiver, repeating words, saying a one-word request, saying their name, and exhibiting a vocabulary of at least 50 words that were intelligible to someone who knew the individual well (Table XII). Table XVIII (available at www.jpeds.com) presents the mean scores and group comparisons for items within other domains, on which subjects from both groups were rated as having severe difficulty (severity score >2): ability to run (ambulation), absent toilet training/sphincter control (autonomic nervous system), ability to point to 3 body parts when asked (receptive language), several reciprocity domains, and marked pain insensitivity (sensory). Combined with the severe disabilities seen in the fine motor and expressive language domain, these clinical features describe both FOXG1 and MECP2 disorder well. The distributions of DEI scores for all parent domains are illustrated in Figure 2 (available at www.jpeds.com).
Table XII.
Mean DEI scores for expressive language items
| Expressive language items | FOXG1 | MECP2 | Mann-Whitney U P value |
|---|---|---|---|
| Makes sounds stop/go | 1.2 | 0.7 | .4 |
| Babbles | 1.8 | 1.7 | .7 |
| Names caregiver | 2.8 | 2.1 | .03* |
| Repeats words | 3.0 | 2.8 | .09 |
| Names 3 objects | 3.0 | 3.0 | NA |
| One word request | 2.9 | 2.7 | .4 |
| Says name | 3.0 | 2.9 | .2 |
| Says 50 words | 3.0 | 3.0 | NA |
Bold values indicate statistical significance.
P ≤ .05.
Table XVIII.
Additional items where both individuals with FOXG1 and MECP2 disorder had severe disability (mean DEI score ≥2.0)
| DEI domain: items | FOXG1 mean | MECP2 mean |
|---|---|---|
| Ambulation: runs | 2.95 | 2.0 |
| Autonomic nervous system: absent sphincter control | 2.3 | 2.8 |
| Receptive language: points to 3 body parts when asked | 2.5 | 2.4 |
| Reciprocity: imitates expressions | 2.5 | 2.3 |
| Reciprocity: waves | 2.5 | 2.8 |
| Reciprocity: parallel play | 2.9 | 2.4 |
| Sensory: pain insensitivity | 2.3 | 2.5 |
Figure 2.
Distributions of Developmental Encephalopathy Inventory (DEI) scores for subjects with FOXG1 and MECP2 disorders. Twelve neurobehavioral domains were assayed in this study. For both disorders, G, expressive language, was consistently severely affected in all subjects.
DEI Differentiates Features of FOXG1 Disorder and MECP2 Disorder
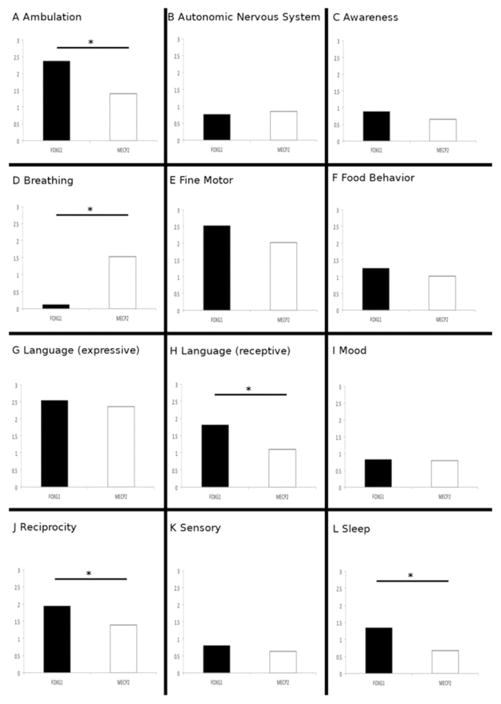
Despite these clinical similarities between the 2 disorders, the DEI identified phenotypic features that differentiate FOXG1 and MECP2 disorder. Significant group differences on the DEI were found in the domains of ambulation, breathing, receptive language, reciprocity, and sleep (Table V).
With the exception of breathing, FOXG1 disorder was more severe (Figure 3). There was no significant association between age and any of the domain scores within the FOXG1 group; however, there were a greater number of younger subjects in our FOXG1 sample. In the MECP2 group, there was a signifi-cant positive correlation between age and disability score in the domain of awareness, and interestingly a negative correlation between age and mood dysfunction (Table XIX; available at www.jpeds.com). The distributions of DEI scores for all parent domains in relation to age are shown in Figure 4 (available at www.jpeds.com).
Figure 3.
Comparison of mean DEI scores for subjects with FOXG1 and MECP2 in the 12 neurobehavioral domains assayed in this study. Significant differences were found in A, ambulation; H, receptive language; J, reciprocity; and L, sleep with FOXG1 subjects more severe in all realms. The only domain where subjects with MECP2 were significantly more severe was that of D, breathing. *P < .05.
Table XIX.
Covariance with age for FOXG1 and MECP2
| Domains | Spearman rho/P value |
|---|---|
| FOXG1 | |
| Ambulation | 0.261/P = .267 |
| Autonomic nervous system | −0.162/P = .496 |
| Awareness | −0.014/P = .954 |
| Breathing | −0.125/P = .601 |
| Fine motor | −0.132/P = .578 |
| Food behavior | 0.120/P = .613 |
| Language (expressive) | 0.354/P = .125 |
| Language (receptive) | 0.014/P = .955 |
| Mood | 0.066/P = .782 |
| Reciprocity | 0.079/P = .739 |
| Sensory | −0.347/P = .134 |
| Sleep | −0.219/P = .353 |
| MECP2 | |
| Ambulation | 0.082/P = .801 |
| Autonomic nervous system | 0.304/P = .336 |
| Awareness | 0.656/P = .021* |
| Breathing | −0.479/P = .115 |
| Fine motor | 0.308/P = .331 |
| Food behavior | 0.089/P = .784 |
| Language (expressive) | 0.426/P = .167 |
| Language (receptive) | 0.009/P = .978 |
| Mood | −0.771/P = .003* |
| Reciprocity | 0.522/P = .082 |
| Sensory | 0.385/P = .216 |
| Sleep | 0.101/P = .756 |
Bold values indicate statistical significance.
P ≤ .05.
Figure 4.
Covariance of DEI scores in 12 neurobehavioral domains with age at the time of study. For all domains, subjects with FOXG1 disorder showed no significant covariance with age, suggesting that maximal disability did not increase over the lifespan. Among subjects with MECP2 disorder, there was significant positive covariance between age and awareness of self and environment and significant negative covariance between age and mood disorders.
When covariance analysis was performed between domains, we found differing between-group patterns. For example, in the FOXG1 group there was a significant covariance of disability in awareness with both expressive and receptive language, as well as reciprocity and sleep with expressive language. These covariances among subjects with FOXG1 disorder are illustrated in Figure 5 (available at www.jpeds.com). A significant covariance relationship between awareness and reciprocity also was seen among subjects with MECP2 disorder. Interestingly, subjects with MECP2 disorder also demonstrated significant covariance in the domains of awareness, reciprocity, and sensory dysfunction, suggesting a relationship between pain insensitivity, awareness of self and the environment, and reciprocal behavioral interactions. Finally, we observed significant negative covariance among autonomic nervous system dysfunction and receptive language, breathing and receptive language, and expressive language and mood disorder among subjects with MECP2 (Figure 6; available at www.jpeds.com). Significant covariant domains are listed in Table XX (available at www.jpeds.com).
Figure 5.
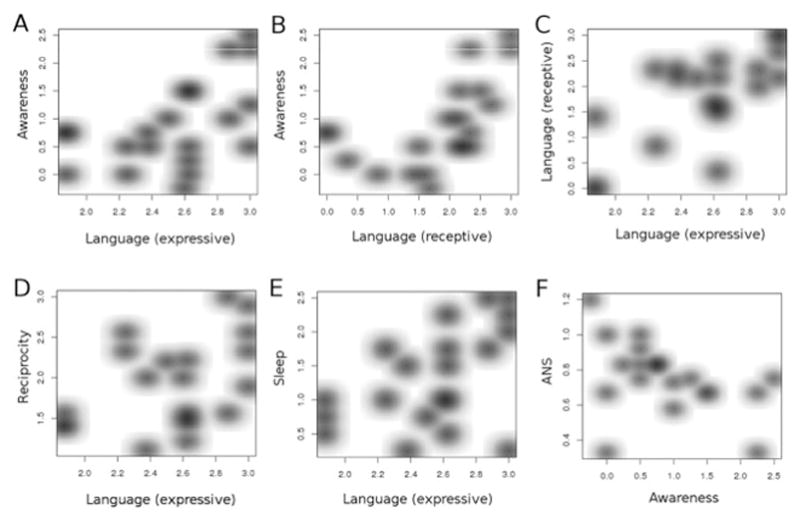
Covariance between DEI domains among subjects with FOXG1 disorder. There were significant relationships between A, awareness and expressive language, B, awareness and receptive language, C, receptive and expressive language, D, reciprocity and expressive language, and E, sleep and expressive language. F, Significant negative variance was found between autonomic nervous system disorders and awareness. ANS, autonomic nervous system.
Figure 6.
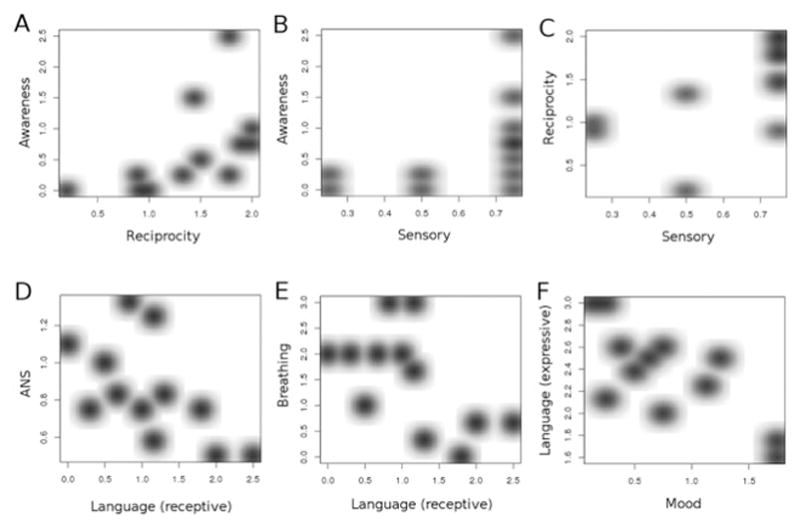
Covariance between DEI domains among subjects with MECP2 disorder. There were significant relationships between A, awareness and reciprocity; B, awareness and sensory; and C, reciprocity and sensory. D, Significant negative variance was found between ANS and receptive language; E, breathing and receptive language; and F, expressive language and mood.
Table XX.
Significant covariance analyses for subjects with FOXG1 and MECP2
| Covariant domains | Spearman rho/P value |
|---|---|
| FOXG1 | |
| Significant positive covariant domains | |
| Awareness and Language (expressive) | Rho = 0.513 P = .0205 |
| Awareness and Language (receptive) | Rho = 0.661 P = .00149 |
| Language (receptive) and Language (expressive) | Rho = 0.6289 P = .00297 |
| Reciprocity and Language (receptive) | Rho = 0.5987 P = .00528 |
| Sleep and Language (expressive) | Rho = 0.4896 P = .0284 |
| Significant negative covariant domains | |
| Awareness and ANS | Rho = −0.4929 P = .027 |
| MECP2 | |
| Significant positive covariant domains | |
| Reciprocity and Awareness | Rho = 0.722 P = .008 |
| Sensory and Awareness | Rho = 0.612 P = .035 |
| Sensory and Reciprocity | Rho = 0.7049 P = .0104 |
| Significant negative covariant domains | |
| Language (receptive) and ANS | Rho = −0.587 P = .044 |
| Language (receptive) and Breathing | Rho = −0.603 P = .038 |
| Mood and Language (expressive) | Rho = −0.6749 P = .016 |
ANS, autonomic nervous system.
Cluster Analysis and Principal Component Analysis Confirm Differentially Affected Domains in FOXG1 and MECP2 Disorders
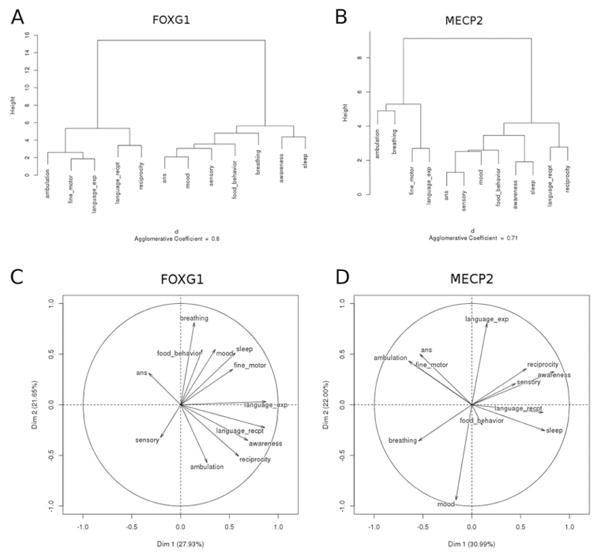
In both FOXG1 and MECP2 disorder we found 2 clusters of the most severely impaired domains. For FOXG1, ambulation, fine motor, and expressive language clustered together, as did receptive language and reciprocity (Figure 7, A). The clustering of less affected domains in FOXG1 disorder included autonomic nervous system, mood, sensory, food behavior, and breathing. All of these were consistent with the mean DEI scores for these domains. Similarly, for the MECP2 group, ambulation and breathing clustered together in increased severity, as did fine motor and expressive language, again consistent with the mean DEI scores for these domains (Figure 7, B). The cluster of less affected domains included autonomic nervous system, sensory, mood, food behavior, awareness, and sleep.
Figure 7.
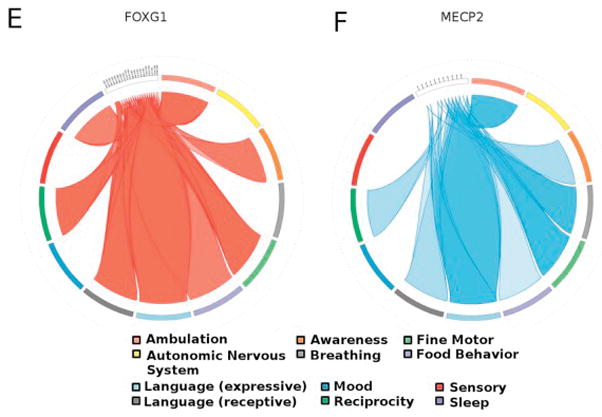
Dendrograms showing clusters of significant impairment among subjects with FOXG1 and MECP2 disorders. A, Subjects with FOXG1 shared in common disordered ambulation, fine motor skills, and expressive language. A secondary cluster of abnormal receptive language and reciprocity was also found. B, Subjects with MECP2 similarly shared disordered fine motor skills and expressive language, as well as an additional cluster of impaired ambulation and breathing. Principal component analysis factormaps for subjects with FOXG1 and MECP2. C, For FOXG1 ambulation, reciprocity, awareness, and receptive language were most important for the first principal component. All were negatively correlated with DEI score for autonomic nervous system dysfunction. For the second principal component, the realms expressive language, breathing, and sleep are the most important, and these variables are not correlated to ambulation, reciprocity, awareness, and receptive language. D, For subjects with MECP2, sleep and receptive language were most important for the first principal component, and these were negatively correlated with ambulation, fine motor dysfunction, and autonomic nervous system dysfunction. For the second principal component the realms expressive language, reciprocity, and awareness were most important, and these were negatively correlated with breathing. Circos plot illustrating core dysfunctions shared and variant between E, FOXG1 and F, MECP2 disorders. Severe abnormalities in expressive language, ambulation, and fine motor function are seen in both disorders; however, sleep dysregulation and breathing dysfunction emerged as variant between the 2 conditions. DEI scores >2.0 (frequently impaired or worse) were plotted.
For FOXG1, principal component analysis identified ambulation, reciprocity, awareness, and receptive language as the domains that loaded most robustly on the first principal component. For the second principal component, the domains of expressive language, breathing, and sleep were the most important, and these variables were not correlated with ambulation, reciprocity, awareness, and receptive language (Figure 7, C). These patterns were consistent with those revealed in our covariance and cluster analyses. For subjects with MECP2 disorder, sleep and receptive language loaded most robustly on the first principal component. For the second principal component, the domains expressive language, reciprocity, and awareness were most important, and these were negatively correlated with breathing (Figure 7, D). These findings highlight some interesting relationships, namely that for subjects with FOXG1 abnormalities in awareness, reciprocity, and language cluster together prominently. For subjects with MECP2 disorder, these factors were not as strongly characteristic of the disorder, and there was an uncoupling of expressive from receptive language. This finding was consistent with the observed mean DEI scores for expressive and receptive language, wherein expressive language was impaired severely for subjects with both FOXG1 and MECP2 disorders, and receptive language was significantly more impaired for individuals with FOXG1.
When our statistical analyses of DEI data for these 2 disorders are taken in summary, different patterns of impairments emerge, illustrating that these conditions are each distinct developmental encephalopathies. Figure 7, E and F, illustrates these patterns. Although shared abnormalities were indeed found between FOXG1 and MECP2 disorders in the domains of fine motor impairment, abnormal expressive and receptive language, and reciprocity, other domains served to strongly differentiate these disorders. These were breathing (more severe for MECP2 disorder) and sleep (more severe for FOXG1 disorder).
Discussion
When initially described, the clinical features of individuals with developmental encephalopathies may overlap with intellectual disability, autism spectrum disorder, and other neurobehavioral characteristics such that one disorder is superficially difficult to distinguish from another. This has been the case with FOXG1 and MECP2 disorders such that individuals with FOXG1 disorder may receive inaccurate counseling about medical comorbidities and natural history. Confusion about natural history also may result in inaccurate identification of target symptoms in future treatment trials.
The DEI confirmed shared domains of fine motor and expressive language that were severely impaired in both FOXG1 and MECP2 disorders, consistent with the published literature. Other distinct items that were affected severely in both disorders included the ability to run, absent toilet training, ability to point to 3 body parts when asked, imitation of expressions, waving goodbye, parallel play, and marked pain insensitivity. These are all common manifestations of developmental disabilities seen across a spectrum of individuals with intellectual disability and autistic features.
The strength of the DEI was its ability to distinguish specific domains that were differentially affected between the 2 disorders. Individuals with FOXG1 disorder emerged as more impaired overall at any age. These subjects were less able to walk, had worse fine motor skills, more disability in receptive language and reciprocity, and had more disordered sleep. The one symptom in which individuals with MECP2 disorder showed greater impairment was in breathing rhythm abnormalities.
Covariance, cluster, and principal component analyses con-firmed common impairments and relationships among DEI domains that differed between FOXG1 and MECP2 disorders. We observed a relationship among impaired awareness, reciprocity, and language in both disorders with the additional observation in subjects with MECP2 disorder of a relationship between pain insensitivity, awareness of self and the environment, and reciprocal behavioral interactions. The vectors for receptive language, expressive language, awareness, and reciprocity did not emerge among the first principal components for MECP2-related disorder, as they did in FOXG1. In addition, abnormal ambulation was a first principal component for FOXG1 but was decidedly not for MECP2, allowing us to observe that impaired ambulation is a strong differentiator between the 2 disorders.
Our cohort with FOXG1 disorder was larger than our cohort with MECP2 disorder, and the mean age of subjects with FOXG1 disorder was younger than the MECP2 cohort. With few exceptions, we did not find significant covariance of DEI scores with age for most domains, so this age difference between the cohorts should not influence our results. Age-related covariance was found in the group with MECP2 disorder between age and worse impairment in the domain of awareness and a negative correlation between age and mood dysfunction. This finding suggests that a feature of MECP2 disorder includes increasing impairment of awareness of self and the environment with age but improvement in mood regulation over time. Although this hypothesis cannot be tested in this cross-sectional study, it is an example of questions that can be answered with the DEI for longitudinal natural history studies.
The relationship between developmental encephalopathies and autism spectrum disorder is of interest, because individuals with MECP2 mutations have been described as having one of the “genetic forms of autism,”6 and some individuals with missense mutations in FOXG1 or duplications of FOXG1 have been diagnosed with autism.29 The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, criteria for diagnosis of autism spectrum disorder30 map to domains within the DEI (Figure 8; available at www.jpeds.com). Deficits in social communication and social interaction map to the awareness of self and environment and language domains. Restricted, repetitive patterns of behavior, interests, or activities map to the movements and sensory function domains. In this way, autism spectrum disorders can be conceptualized within developmental encephalopathies, and we propose that the phenotyping of individuals with autism spectrum disorder diagnoses with the DEI may reveal further insights into subtypes of autism.
Figure 8.
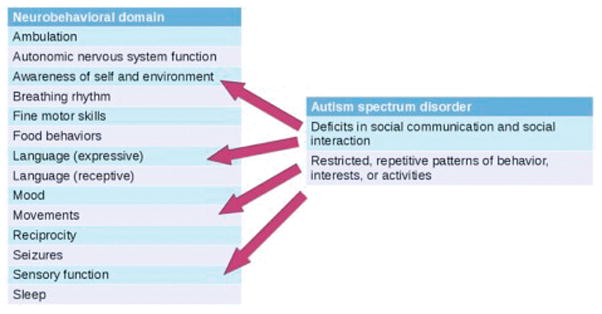
Diagnostic criteria for autism spectrum disorder (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition) map to domains within the DEI. Deficits in social communication and social interaction map to the domains of awareness of self and environment and language (primarily expressive). Restricted, repetitive patterns of behavior, interests, or activities map to the domains movements and sensory function.
The DEI was designed for ease of administration as a care-giver survey, and the information gathered is already part of routine medical and developmental history and review of systems during a clinical encounter. With experience with the tool, administering the DEI requires less than 30 minutes. Early working versions of the DEI included the domains of movements and seizures, considered key phenotypic elements of the developmental encephalopathies. It became clear, however, that proper assessment and classification of movement disorders required direct neurologist review, either in person or via video, of the movements. Similarly, meaningful inclusion of any seizure-related data required direct neurologist review of electroencephalography reports, neurologist notes, video of seizures, or all three. For this reason, the Movement and Seizure domains were removed from the caregiver survey version of the DEI. The data for these domains must be collected separately. We have published epilepsy-related data for FOXG1 disorder16 and a study of the associated movement disorder elsewhere.20 Ultimately, comprehensive phenotyping of the developmental encephalopathies may involve a multimethod, multi-informant approach that integrates information from caregiver surveys, review of records, and direct examination. With the study of larger cohorts using the DEI, it is our hope that this tool will prove useful as a predictive model, with its output guiding genetic testing.
We recognize several limitations of the present study. First, the DEI relies on proxy report by caregivers, rather than direct observation or examination. In addition to the potential for imprecise reporting of symptoms by caregivers without formal clinical training, this method also results in multiple raters (ie, as many raters are there are affected families). Another potential concern is that administration of the DEI might require specific training and therefore unlikely to be useful in a clinical setting; however, as noted previously, the majority of DEI item content is similar to questions raised in a general developmental evaluation, and thus unique previous experience with these or other rare disorders is not required. Our future work will consider approaches such as a “manual of procedures” and standardized training to ensure ongoing consistent use and to maintain consistency within individual investigators over time.
Small sample sizes also limit our ability to understand the full spectrum of disease severity for the conditions described here. To some degree, this is a common and unavoidable problem in studying rare disorders. Our ongoing longitudinal work, including reassessment of subjects over time as well as accrual of new subjects, will help to expand our understanding of the range of disease severity.
Longitudinal work also will illustrate the DEI’s responsiveness to changes in symptom expression over time, which will be particularly relevant as a tool for clinical trials. Finally, we note that the DEI is not yet a predictive tool that will a priori distinguish between FOXG1 and MECP2 disorders, or other related disorders. As larger sample sizes are identified and ascertained, we wish to evaluate whether the DEI can aid in phenotyping leading to diagnosis.
We have developed a novel quantitative assessment tool for developmental encephalopathies. The DEI was designed to capture the spectrum of abnormalities in affected individuals older than 3 years of age and facilitates robust statistical analysis of the resulting data. A modified version of the DEI is also under development for use in children younger than 3 years of age. It was our intention to pilot the DEI on FOXG1 and MECP2 disorders so that a tool will be available to assess the effectiveness of future treatment interventions. We also intended that the tool can be used in the description and differentiation of other developmental encephalopathies. We propose this tool as a method to identify and illustrate shared and differential domains of disability among complex neurodevelopmental disorders.
Table VII.
Mean DEI scores for autonomic nervous system items
| Autonomic nervous system items | FOXG1 | MECP2 | Mann-Whitney U P value |
|---|---|---|---|
| Tachycardia | 0 | 0.1 | .2 |
| Hypertension | 0 | 0 | NA |
| Edema | 0.2 | 0.4 | .8 |
| Bradycardia | 0 | 0 | NA |
| Hypotension | 0 | 0.1 | .2 |
| Constipation | 1.8 | 2.2 | .3 |
| Aspiration | 1.2 | 0.4 | .04* |
| Absent sphincter control | 2.3 | 2.8 | .3 |
| Gastroesophageal reflux | 1.3 | 1.9 | .2 |
| Vomiting | 0.9 | 0.5 | .3 |
| Hyperthermia | 0.95 | 0.8 | .8 |
| Hypothermia | 0.6 | 0.8 | .6 |
NA, not available.
Bold values indicate statistical significance.
P ≤ .05.
Table VIII.
Mean DEI scores for awareness items
| Awareness items | FOXG1 | MECP2 | Mann-Whitney U P value |
|---|---|---|---|
| Turns head to sound | 0.4 | 0.5 | .9 |
| Turns head to parent voice | 0.7 | 0.5 | .6 |
| Responds to name | 0.8 | 0.6 | .7 |
| Cries when hungry/wet | 1.8 | 1.0 | .06 |
Table IX.
Mean DEI scores for breathing items
| Breathing items | FOXG1 | MECP2 | Mann-Whitney U P value |
|---|---|---|---|
| Overbreathing | 0.2 | 1.5 | .003 |
| Breathing dysrhythmia | 0 | 1.1 | .001 |
| Hypoventilation | 0.2 | 1.5 | .001 |
Table XI.
Mean DEI scores for food behavior items
| Food behavior items | FOXG1 | MECP2 | Mann-Whitney U P value |
|---|---|---|---|
| Feeds self | 2.6 | 0.9 | .0002* |
| Pica | 1.0 | 1.3 | .6 |
| Anorexia | 1.2 | 0.4 | .03* |
| Hyperphagia | 0.2 | 1.5 | .003* |
Bold values indicate statistical significance.
P ≤ .05.
Table XIII.
Mean DEI scores for receptive language items
| Receptive language items | FOXG1 | MECP2 | Mann-Whitney U P value |
|---|---|---|---|
| Responds to request | 2.4 | 1.5 | .07 |
| Understands no | 2.0 | 0.3 | .0009* |
| Directs attention to story | 1.2 | 0.5 | .3 |
| Points to 3 body parts when asked | 2.5 | 2.4 | .5 |
| Knows difference between 2 toys | 0.7 | 0.9 | .6 |
| Knows difference between 2 requests | 2.2 | 1.3 | .08 |
Bold values indicate statistical significance.
P ≤ .05.
Table XIV.
Mean DEI scores for mood items
| Mood items | FOXG1 | MECP2 | Mann-Whitney U P value |
|---|---|---|---|
| Exhibits shyness | 0.3 | 0.3 | .8 |
| Easily irritated | 0.5 | 0.7 | .5 |
| Expresses fear/anxiety | 0.2 | 0.6 | .08 |
| Hyperactive | 1.4 | 1.2 | .8 |
| Inappropriate laughter | 1.8 | 1.5 | .5 |
| Obsesses | 0.7 | 0.5 | .6 |
| Self-injurious behavior | 0.9 | 1.4 | .2 |
| Slow to settle down | 1.0 | 0.3 | .05 |
Table XV.
Mean DEI scores for reciprocity items
| Reciprocity items | FOXG1 | MECP2 | Mann-Whitney U P value |
|---|---|---|---|
| Sustains gaze | 1.2 | 0.4 | .03* |
| Imitates expressions | 2.5 | 2.2 | .5 |
| Follows pointed finger | 2.5 | 1.7 | .01* |
| Waves | 2.5 | 2.8 | .2 |
| Interest in other children | 1.1 | 0.6 | .3 |
| Makes social contact | 1.6 | 0.8 | .1 |
| Smiles when smiled at | 1.3 | 0.09 | .02* |
| Parallel play | 2.9 | 2.4 | .06 |
| Elicits response | 2.2 | 1.2 | .1 |
Bold values indicate statistical significance.
P ≤ .05.
Table XVI.
Mean DEI scores for sensory items
| Sensory items | FOXG1 | MECP2 | Mann-Whitney U P value |
|---|---|---|---|
| Pain insensitivity | 2.3 | 2.5 | .8 |
| Pain hypersensitivity | 0 | 0 | NA |
| Cortical visual impairment | 1.1 | 0.0 | .02* |
| Sensorineural hearing loss | 0 | 0 | NA |
Bold values indicate statistical significance.
P ≤ .05.
Acknowledgments
Supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (R01NS058721 [to W.D.] and K08NS078054 [to A.P.]).
We acknowledge the FOXG1 Foundation, as well as Susan Hyman, MD (supported by the National Institute of Mental Health [R34 MH100254]), Alan Percy, MD (supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development [NICHD; U54 HD0612222], Neuron Pharmaceuticals, and the Civitan International Research Center), Jeffrey Neul, MD (supported by NICHD [U54 HD083092] and National Institute of Neurological Disorders and Stroke [NINDS; R21 NS089366]), and Timothy Benke, MD (supported by NINDS [P30 NS048154]), for referring subjects to this study.
Glossary
- DEI
Developmental Encephalopathy Inventory
Footnotes
The authors declare no conflicts of interest.
References
- 1.Rett A. On a unusual brain atrophy syndrome in hyperammonemia in childhood. Wien Med Wochenschr. 1966;116:723–6. [PubMed] [Google Scholar]
- 2.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–8. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 3.Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: report of 35 cases. Ann Neurol. 1983;14:471–9. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- 4.Percy AK. Rett syndrome: clinical correlates of the newly discovered gene. Brain Dev. 2001;23(Suppl 1):S202–5. doi: 10.1016/s0387-7604(01)00350-3. [DOI] [PubMed] [Google Scholar]
- 5.Neul JL, Kaufmann WE, Glaze DG, Christodoulou J, Clarke AJ, Bahi-Buisson N, et al. Rett syndrome: revised diagnostic criteria and nomenclature. Ann Neurol. 2010;68:944–50. doi: 10.1002/ana.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young DJ, Bebbington A, Anderson A, Ravine D, Ellaway C, Kulkarni A, et al. The diagnosis of autism in a female: could it be Rett syndrome? Eur J Pediatr. 2008;167:661–9. doi: 10.1007/s00431-007-0569-x. [DOI] [PubMed] [Google Scholar]
- 7.Rolando S. Rett syndrome: report of eight cases. Brain Dev. 1985;7:290–6. doi: 10.1016/s0387-7604(85)80030-9. [DOI] [PubMed] [Google Scholar]
- 8.Opitz JM, Lewin SO. Rett syndrome—a review and discussion of syndrome delineation and syndrome definition. Brain Dev. 1987;9:445–50. doi: 10.1016/s0387-7604(87)80061-x. [DOI] [PubMed] [Google Scholar]
- 9.Lin MY, Wang PJ, Lin LH, Shen YZ. The Rett and Rett-like syndromes: a broad concept. Brain Dev. 1991;13:228–31. doi: 10.1016/s0387-7604(12)80054-4. [DOI] [PubMed] [Google Scholar]
- 10.Hagberg BA, Skjeldal OH. Rett variants: a suggested model for inclusion criteria. Pediatr Neurol. 1994;11:5–11. doi: 10.1016/0887-8994(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 11.Weaving LS, Christodoulou J, Williamson SL, Friend KL, McKenzie OLD, Archer H, et al. Mutations of CDKL5 cause a severe neurodevelopmental disorder with infantile spasms and mental retardation. Am J Hum Genet. 2004;75:1079–93. doi: 10.1086/426462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ariani F, Hayek G, Rondinella D, Artuso R, Mencarelli MA, Spanhol-Rosseto A, et al. FOXG1 is responsible for the congenital variant of Rett syndrome. Am J Hum Genet. 2008;83:89–93. doi: 10.1016/j.ajhg.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papa FT, Mencarelli MA, Caselli R, Katzaki E, Sampieri K, Meloni I, et al. A 3 Mb deletion in 14q12 causes severe mental retardation, mild facial dysmorphisms and Rett-like features. Am J Med Genet A. 2008;146A:1994–8. doi: 10.1002/ajmg.a.32413. [DOI] [PubMed] [Google Scholar]
- 14.Bahi-Buisson N, Nectoux J, Girard B, Van Esch H, De Ravel T, Boddaert N, et al. Revisiting the phenotype associated with FOXG1 mutations: two novel cases of congenital Rett variant. Neurogenetics. 2010;11:241–9. doi: 10.1007/s10048-009-0220-2. [DOI] [PubMed] [Google Scholar]
- 15.Kortüm F, Das S, Flindt M, Morris-Rosendahl DJ, Stefanova I, Gold-stein A, et al. The core FOXG1 syndrome phenotype consists of postnatal microcephaly, severe mental retardation, absent language, dyskinesia, and corpus callosum hypogenesis. J Med Genet. 2011;48:396–406. doi: 10.1136/jmg.2010.087528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seltzer LE, Ma M, Ahmed S, Bertrand M, Dobyns WB, Wheless J, et al. Epilepsy and outcome in FOXG1-related disorders. Epilepsia. 2014;55:1292–300. doi: 10.1111/epi.12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Silvestri JM, et al. Autonomic nervous system dysregulation: breathing and heart rate perturbation during wakefulness in young girls with Rett syndrome. Pediatr Res. 2006;60:443–9. doi: 10.1203/01.pdr.0000238302.84552.d0. [DOI] [PubMed] [Google Scholar]
- 18.De Felice C, Maffei S, Signorini C, Leoncini S, Lunghetti S, Valacchi G, et al. Subclinical myocardial dysfunction in Rett syndrome. Eur Heart J Cardiovasc Imaging. 2012;13:339–45. doi: 10.1093/ejechocard/jer256. [DOI] [PubMed] [Google Scholar]
- 19.Ramirez J-M, Ward CS, Neul JL. Breathing challenges in Rett syndrome: lessons learned from humans and animal models. Respir Physiol Neurobiol. 2013;189:280–7. doi: 10.1016/j.resp.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papandreou A, Schneider R, Augustine E, Ng J, Mankad K, Meyer E, et al. Delineation of the movement disorders associated with FOXG1 mutations. Neurology. 2016;86:1794–800. doi: 10.1212/WNL.0000000000002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bahi-Buisson N. Genetically determined encephalopathy: Rett syndrome. Handb Clin Neurol. 2013;111:281–6. doi: 10.1016/B978-0-444-52891-9.00031-2. [DOI] [PubMed] [Google Scholar]
- 22.Kumakura A, Takahashi S, Okajima K, Hata D. A haploinsufficiency of FOXG1 identified in a boy with congenital variant of Rett syndrome. Brain Dev. 2014;36:725–9. doi: 10.1016/j.braindev.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Das DK, Jadhav V, Ghattargi VC, Udani V. Novel mutation in forkhead box G1 (FOXG1) gene in an Indian patient with Rett syndrome. Gene. 2014;538:109–12. doi: 10.1016/j.gene.2013.12.063. [DOI] [PubMed] [Google Scholar]
- 24.Lee BH, Smith T, Paciorkowski AR. Autism spectrum disorder and epilepsy: disorders with a shared biology. Epilepsy Behav. 2015;47:191–201. doi: 10.1016/j.yebeh.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayley N. Bayley scales of infant and toddler development. 3. San Antonio (TX): Harcourt Assessment Inc; 2006. [Google Scholar]
- 26.Sparrow S, Cicchetti D, Balla D. Vineland-II: Vineland adaptive behavior scales. 2. Minneapolis (MN): Pearson Assessments; 2005. [Google Scholar]
- 27.Bruininks R, Woodcock R, Weatherman R, Hill B. Scales of independent behavior—revised: comprehensive manual. Riverside: Itasca; 1996. [Google Scholar]
- 28.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–45. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paciorkowski AR, Thio LL, Rosenfeld JA, Gajecka M, Gurnett CA, Kulkarni S, et al. Copy number variants and infantile spasms: evidence for abnormalities in ventral forebrain development and pathways of synaptic function. Eur J Hum Genet. 2011;19:1238–45. doi: 10.1038/ejhg.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Psychiatric Association, American Psychiatric Association. DSM-5 task force. Diagnostic and statistical manual of mental disorders: DSM-5. Arlington (VA): American Psychiatric Association; 2013. [Google Scholar]