Abstract
Background
Little is known about real-world outcomes for new interferon-free treatment for hepatitis C virus (HCV) among underserved and diverse communities.
Objective
To identify predictors of treatment prescription and evaluate outcomes as measured by sustained virologic response (SVR) with HCV RNA testing three months after therapy completion.
Design
Retrospective cohort at a safety-net health care system.
Participants
Patients with (1) at least one clinical visit between December 6, 2013, and December 31st 2014; and (2) at least three months follow-up.
Key results
Predominantly non-White cohort (61%). Of 1,284 HCV-infected patients 121 prescribed sofosbuvir-based therapy. Severe liver fibrosis (OR 1.66, 95% CI 1.05, 2.64) independently associated with treatment prescription. In those with evaluable HCV RNA, SVR was 99%.
Conclusion
Cure rates similar to clinical trial data can be achieved in diverse underserved communities.
Keywords: Treatment, hepatitis C, outcome, safety-net hospital
The approval of a highly effective and well-tolerated therapy for hepatitis C (HCV) has revolutionized the care of HCV-infected patients.1 As a result, HCV cure can be achieved with three months of therapy and minimal side effects.2,3 New HCV drugs have recorded high rates of sustained virologic response (SVR) in randomized, controlled clinical trials, but data about real-world outcomes are only now emerging.
Given the growing enthusiasm for the goal of eradicating HCV in the United States,4 it is particularly important to understand treatment effectiveness within urban, safety-net health care systems, where HCV prevalence is high, new infections are being transmitted, the patient population is ethnically diverse, and high rates of both drug use and poverty complicate therapy.5,6 Until now, HCV treatment initiation and SVR have been rare events among urban patients. In a study including 293 HCV-infected patients at an urban Ohio county hospital, 28% (83) were treated and only 13% (11) of those treated achieved SVR.7 The availability of interferon-free treatment options will likely lead to more treatment initiations and SVR at safety-net centers, but there are currently no data available to test that hypothesis. Data from national registries or integrated health systems provide information from large populations of treated patients,8–11 but they are not generalizable to safety-net centers caring for uninsured or underinsured populations and individuals who are disproportionally affected by HCV.
We therefore sought to evaluate HCV treatment outcome at a large, urban safety net health care system during the new interferon free era. The objectives of the study were (1) to identify predictors of treatment prescription and (2) to evaluate outcomes as measured by SVR with HCV RNA testing three months after therapy completion. We also sought to describe the demographics of HCV-infected patients prescribed sofosbuvir-based therapy, and to compare individuals prescribed sofosbuvir to HCV-infected patients who were followed during the same time period, but were not started on treatment. We hypothesized that during the early interferon-free era HCV-infected patients with more severe liver fibrosis would be prioritized for treatment.
Methods
Overview
We used the electronic medical record (EMR) at Boston Medical Center (BMC) to create a retrospective cohort of patients diagnosed with HCV between January 1st, 2004, and December 31st, 2014. We included patients with reactive HCV antibody or individuals with negative antibody, but with documented detectable HCV viral load at least six months after serology testing. The latter cases were likely patients of acute HCV infection who were identified prior to seroconversion.
Site
Boston Medical Center is a non-profit academic medical center. It is the largest safety-net hospital in New England and 70% of the patients at BMC are from underserved groups including ethnic/racial minorities, immigrants and the elderly.12 Safety net hospitals provide care to low-income as well as uninsured or underinsured populations. Boston Medical Center is the only academic medical center in Massachusetts also classified as a Disproportionate Share Hospital (DSH). As a DSH, BMC serves a large proportion of low-income patients and receives Centers for Medicaid and Medicare Services payments to cover the care provided to uninsured patients. In 2013, the hospital’s overall payer mixed was 23% commercial insurance, 27% Medicare and other federal programs and 49% state programs. 13
Primary outcome
Our primary outcome was sofosbuvir prescription during the first year of interferon-free treatment availability. The FDA approved sofosbuvir on December 6th, 2013. We included patients who were prescribed a sofosbuvir-based regimen between December 6th, 2013, and December 31st, 2014.
Secondary outcomes
Our secondary outcome was as-treated sustained virologic response (SVR) defined as an undetectable viral load measured three months after HCV treatment completion. SVR has been demonstrated to be a durable indicator (and best indicator) of successful treatment.14
Study population
We used the following inclusion criteria: (1) reactive HCV antibody, or negative antibody and detectable viral load as noted above between January 1st 2004 and December 31st, 2014; (2) at least one clinical visit between December 6th, 2013, and December 31st, 2014; (3) at least three months of follow-up time during the time period of interest.
Data collection
We extracted the following data elements from the EMR: (1) demographic information; (2) laboratory values; (3) dates and location of clinical visits; and (4) prescription information and refills. HIV status was assessed either by HIV antibody testing or HIV infection documented on the problem list.
Independent variables
We included the following covariates in the analysis: age at baseline, gender, race/ethnicity, insurance, HIV status, birthplace, number of follow-up visits during the study period (dichotomized at the median), and liver fibrosis staging as measured by fibrosis 4 (FIB 4)-index. FIB-4 is a non-invasive measurement of hepatic fibrosis calculated using the following formula: age (years) X AST level [U/L] / (platelets [109/L] X (ALT [U/L])1/2. FIB-4 index of 3.25 or greater is an indication of severe fibrosis.15
Statistical analysis
We used descriptive statistics to estimate the proportion of patients who were prescribed sofosbuvir-based therapy and the proportion of individuals with SVR. We computed as-treated SVR, determined using the proportion of patients who had available RNA testing three months after treatment completion. Additionally, we also estimated and tested a multivariate logistic regression including variables significant in univariate analysis and potential confounders. We estimated odds ratios with 95% confidence intervals and all p-value significance levels were two-sided. Statistical analyses were performed with STATA 12 (STATA, College Station, TX).
Regulatory approvals
The Boston University Medical Center Institutional Review Board approved this study.
Results
Cohort characteristics
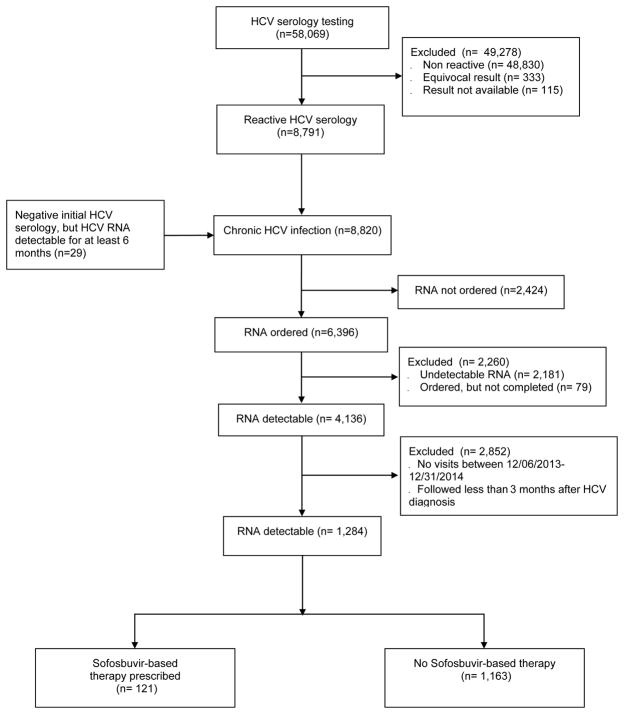
Nearly 60,000 (58,069) patients were tested for HCV between January 1st 2004 and December 31st, 2014. Of those, 8,791 (15%) had a reactive HCV antibody test. Individuals with detectable viral load for a least six months, but negative HCV antibody (29) between January 1st 2004 and December 31st, 2014 were added to the 8,791 with reactive antibody to create a cohort of 8,820 HCV-infected patients. Of those, 6,396 had HCV RNA ordered, and 4,136 (65%) had detectable HCV RNA. We then constructed a cohort of 1,284 HCV-infected patients by excluding individuals who were not seen during the study period, and patients who had less than three months follow-up between December 6th, 2013, and December 31st, 2014 (Figure 1).
Figure 1.
Study flow diagram
HCV: Hepatitis C; RNA: Ribonucleotide Acid
The mean age was 47 years and the cohort was predominantly male (64%), and non-White (43% Black, 10% Latino, 2% Asian, 6% other). More than half (56%) had public insurance including Medicaid and Medicare, and 14% were covered by Health Safety Net, the Massachusetts state sponsored program covering health services for uninsured or underinsured Massachusetts residents. Eleven percent of the cohort was HIV-coinfected, but 524 (41%) had no record of HIV testing. A majority (755, 59%) had a history of substance use recorded as an International Classification of Diseases (ICD)-9 code for either heroin, alcohol, or any other illicit drug use. Median follow-up time was nine months (range 3–13 months). Eighteen percent of the cohort had a calculated FIB-4 index suggestive of severe liver fibrosis, including 27% of individuals who initiated sofosbuvir-based therapy and 17% of those who were not treated during the time period of interest (p-value <.01) (Table 1). Of the 121 individuals who were started on sofosbuvir-based therapy 96 (79%) were treatment-naïve and 25 (2%) were treatment-experienced.
Table 1.
Baseline characteristics
| Characteristic | Total N = 1,284 N (%) |
Sofosbuvir-based therapy N = 121 N (%) |
No sofosbuvir-based therapy N = 1,163 N (%) |
p-value |
|---|---|---|---|---|
| Age at HCV diagnosis | ||||
| Mean | 47 | 48 | 47 | 0.55 |
| Median | 48 | 49 | 48 | |
| Range | 18 – 90 | 22 – 70 | 18 – 90 | |
| Age categories | 0.80 | |||
| 18–29 | 137 (11) | 13 (11) | 124 (11) | |
| 30–39 | 189 (15) | 14 (12) | 175 (15) | |
| 40–49 | 397 (31) | 37 (31) | 360 (31) | |
| 50–59 | 422 (33) | 41 (34) | 381 (33) | |
| 60–90 | 139 (11) | 16 (13) | 123 (11) | |
| Male | 828 (64) | 82 (68) | 746 (64) | 0.43 |
| Race/Ethnicity | ||||
| White | 513 (40) | 52 (43) | 461 (40) | 0.23 |
| Black | 546 (43) | 42 (35) | 504 (43) | |
| Latino | 130 (10) | 13 (11) | 117 (10) | |
| Asian | 23 (2) | 4 (3) | 19 (2) | |
| Other/unknown | 72 (6) | 10 (8) | 62 (5) | |
| HIV status | ||||
| HIV-uninfecteda | 622 (48) | 50 (41) | 572 (49) | 0.01 |
| HIV-infected | 138 (11) | 7 (6) | 131 (11) | |
| Unknown status | 524 (41) | 64 (53) | 460 (40) | |
| Insurance | ||||
| Public | 723 (56) | 61 (50) | 662 (57) | 0.41 |
| Private | 364 (28) | 40 (33) | 324 (28) | |
| Health Safety Net | 175 (14) | 19 (16) | 156 (13) | |
| Other/unknown | 22 (2) | 1 (1) | 21 (2) | |
| Substance use | <0.001 | |||
| Yesb | 755 (59) | 43 (36) | 712 (61) | |
| No | 529 (41) | 78 (64) | 451 (39) | |
| Birth place | ||||
| US | 1,037 (81) | 88 (73) | 949 (82) | 0.05 |
| Non US | 245 (19) | 33 (27) | 212 (18) | |
| Unknown | 2 (0.12) | 0 (0) | 2 (0.17) | |
| Seen by GI or ID | 648 (50) | 116 (96) | 532 (46) | <0.001 |
| Number of clinical visits c | ||||
| Mean | 13 | 13 | 13 | 0.70 |
| Median | 10 | 11 | 9 | |
| Range | (2 – 88) | (2 – 59) | (2 – 88) | |
| No. visits after diagnosis | ||||
| ≤ 10 | 689 (54) | 57 (47) | 632 (54) | 0.13 |
| > 10 | 595 (46) | 64 (53) | 531 (46) | |
| Follow-up time, months | ||||
| Mean | 9 | 9 | 9 | 0.08 |
| Median | 9 | 10 | 9 | |
| Range | (3 – 13) | (3 – 13) | (3 – 13) | |
| Time since HCV diagnosis, months | ||||
| Mean | 66 | 58 | 66 | 0.03 |
| Median | 64 | 53 | 65 | |
| Range | (3 – 131) | (4 – 128) | (3 – 131) | |
| FIB 4 | 0.17 | |||
| Mean | 2 | 3 | 2 | |
| Median | 1 | 2 | 1 | |
| Range | (0.22 – 86) | (0.35 – 14) | (0.22 – 86) | |
| FIB 4 | ||||
| <3.25 | 1,051 (82) | 88 (73) | 963 (83) | 0.01 |
| ≥3.25 | 233 (18) | 33 (27) | 200 (17) |
HCV: Hepatitis C; HIV: Human Immunodeficiency Virus; US: United States; GI: Gastroenterology specialist; ID: infectious diseases specialist; FIB 4: fibrosis 4.
HIV status was determined by HIV testing results or International Classification of Diseases (ICD)-9 code on the problem list. Individuals who did not have HIV testing performed were listed as unknown status.
History of substance use included heroin, alcohol and any other illicit drug use as recorded by ICD-9 code on the problem list.
Number of clinical visits during between December 6th, 2013 and December 31st, 2014.
Predictors of having a prescription for sofosbuvir-based therapy
Severe liver fibrosis (FIB-4 greater 3.25) was associated with increased odds of receiving a prescription for sofosbuvir-based therapy (OR 1.66, 95% CI 1.05, 2.64; p-value <.03) in multivariate analysis controlling for age, gender, race/ethnicity, insurance type, HIV status, birthplace, number of visits and liver fibrosis stage (Table 2).
Table 2.
Predictors of sofosbuvir-based therapy prescription (N= 1,284)
| Predictors | Univariate Odds Ratio (95% CI) | Univariate p-value | Adjusted Odds Ratio (95% CI) | Adjusted p-value |
|---|---|---|---|---|
| Age | ||||
| 18–29 | 0.97 [0.51 – 1.88] | 0.94 | 1.01 [0.49 – 2.07] | 0.98 |
| 30–39 | 0.74 [0.39 – 1.40] | 0.36 | 0.75 [0.39 – 1.47] | 0.40 |
| 40–49 | 0.96 [0.60 – 1.52] | 0.85 | 0.99 [0.61 – 1.60] | 0.95 |
| 50–59 | Reference | Reference | ||
| 60–89 | 1.21 [0.66 – 2.23] | 0.54 | 0.97 [0.51 – 1.85] | 0.93 |
| Male | 1.18 [0.79 – 1.75] | 0.43 | 1.16 [0.77 – 1.77] | 0.48 |
| Race/Ethnicity | ||||
| White | Reference | Reference | ||
| Black | 0.74 [0.48 – 1.13] | 0.16 | 0.68 [0.43 – 1.07] | 0.10 |
| Latino | 0.99 [0.52 – 1.87] | 0.96 | 0.71 [0.33 – 1.52] | 0.38 |
| Asian | 1.87 [0.61 – 5.70] | 0.27 | 1.03 [0.29 – 3.64] | 0.96 |
| Other/Unknown | 1.43 [0.69 – 2.96] | 0.34 | 1.00 [0.43 – 2.32] | 0.99 |
| Insurance | ||||
| Public | Reference | Reference | ||
| Private | 1.34 [0.88 – 2.04] | 0.17 | 1.31 [0.85 – 2.02] | 0.21 |
| Health Safety Net | 1.32 [0.77 – 2.28] | 0.32 | 1.27 [0.72 – 2.23] | 0.41 |
| Other/Unknown | 0.52 [0.07 – 3.91] | 0.52 | 0.53 [0.07 – 4.04] | 0.54 |
| HIV status | ||||
| HIV-uninfected or unknown HIV statusa | Reference | Reference | ||
| HIV-infected | 2.07 [0.94 – 4.53] | 0.07 | 2.08 [0.94 – 4.65] | 0.07 |
| Birth place | ||||
| US | Reference | Reference | ||
| Non US | 1.68 [1.10 – 2.57] | 0.02 | 1.67 [0.93 – 2.99] | 0.08 |
| Number of clinical visitsb (≥10 v. <10) | 1.34 [0.92 – 1.94] | 0.13 | 1.40 [0.96 – 2.06] | 0.08 |
| FIB 4 | ||||
| ≤3.25 | Reference | Reference | ||
| >3.25 | 1.81 [1.18 – 2.77] | 0.01 | 1.66 [1.05 – 2.64] | 0.03 |
HIV: Human Immunodeficiency Virus; US: United States; FIB 4: Fibrosis 4. This analysis controls for age, gender, ethnicity, insurance type, birthplace, number of clinical visits, and FIB-4 index.
HIV status was determined by HIV testing results or International Classification of Diseases (ICD)-9 code on the problem list. Individuals who did not have HIV testing performed were listed as unknown status
Number of clinical visits during the one-year period evaluated.
Treatment outcomes
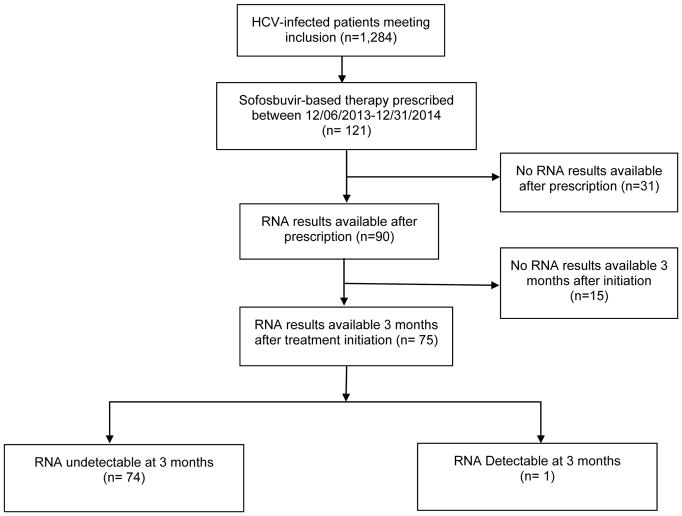
In an as-treated analysis, we identified 75 patients who had HCV RNA testing available three months after treatment. Of those, 74 (99%) achieved SVR (Figure 2). Of the 31 patients who did not have any HCV RNA testing, 53% had no visits with either a gastroenterology or an infectious diseases subspecialist after the prescription had been written. Of the 75 HCV-infected patients with available HCV RNA testing at three months, 61 (81%) were treatment-naïve. Sustained virologic response rates were 98% and 100% for treatment-naïve and treatment-experienced individuals, respectively.
Figure 2.
HCV treatment outcome
HCV: Hepatitis C, RNA: Ribonucleotide Acid
Discussion
Our findings provide much needed information about interferon-free treatment outcomes at an urban, safety-net hospital system. Similar to our prior studies and those of others, we found a high HCV detection rate of 15%.5,6 While most infected patients had not yet been treated by the end of the first year of sofosbuvir availability, it is encouraging that over 10% of known cases were treated within 12 months of sofosbuvir approval, especially when this treatment initiation rate is compared to the interferon era.
Sofosbuvir-based therapy was associated with more advanced liver fibrosis. It is possible that during the early interferon-free treatment era, providers and payers are focusing efforts on HCV-infected patients with more severe disease who are at high-risk for progression to decompensated cirrhosis or other HCV complications. This practice is consistent with The American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) guidelines identifying individuals with advanced disease as a priority group,16 and also reflects common restrictions on access to therapy.17
Less expected was the high proportion of patients who received HCV therapy without a documented HIV serology in the medical record. This finding is surprising, given that HIV and HCV share a common mode of transmission and that guidance clearly recommends that HCV-infected patients should also be tested for HIV co-infection.16 It is possible that individuals might have been tested for HIV elsewhere and that results were not available in our system.
Importantly, we found cure rates comparable to clinical trials, demonstrating that new HCV therapies are effective, even in a real-world setting characterized by a diverse patient population and a high prevalence of substance use. Additionally, cure rates were high in both treatment-naïve and treatment-experienced individuals. Our findings are similar to those reported from large national registries and from the Veterans Administration, and extend those findings to a new, high-priority treatment setting.8–10, 18, 19 These data should inform ongoing discussions about the appropriateness of cost-control strategies that limit treatment access only to specific centers of excellence, as well as debate about the ability to treat HCV among recent and active drug users.20
Prior studies have focused on treatment initiation during the interferon era. For example, in a retrospective case series of 327 patients referred for HCV evaluation at an urban teaching, county hospital in Ohio, 28% were treated and only 13% had SVR.7 In another urban cohort in Baltimore, including 597 HCV-infected individuals, only 26 initiated treatment and only 5 reached SVR.21 More recent data presented at national and international conferences revealed real-world outcomes of HCV treatment during the interferon-free era; however, the patient population is not representative of the diverse low-income population disproportionally affected by HCV.8–10, 19 One of those studies involved the Veteran Affairs (VA) population—a predominantly male population with access to care. The VA study included approximately 4,000 HCV-infected patients and reported lower SVR rates than in clinical trials. Sustained virologic response rates were between 70–80% depending on the genotype and the regimen administered. Another study, the HCV-TARGET International registry, included approximately 2000 HCV-infected patients and reported SVR rates similar to clinical trials data. For example, reported SVR rates with sofosbuvir/ledipasvir regimen of approximately 95% after three months of treatment, similar to outcomes in clinical trials. In this particular study, however, only 2% of the participants were HIV-infected, and 12% were Black, differing greatly from the demographic profile of the current real world study.
There are limitations to this study, including a single site and a retrospective study design which might limit generalizability. We were not able to control for other comorbidities that could influence treatment initiation and outcomes because of incomplete ICD-9 codes for history of substance use. We were also not able to assess if substance use was in remission or active. Another limitation is that we did not have genotype test results on most of the individuals included in the study.
In conclusion, our study provides important information on treatment initiation and completion during the early interferon-free era. In our study, HCV-infected patients with severe liver disease had a high chance of being prescribed sofosbuvir-based treatment. We also demonstrate that SVR rates similar to clinical trials results can be achieved in safety-net hospitals with a high proportion of Medicaid and underinsured patients. These findings suggest that HCV eradication might be possible during the interferon-free era, but measures are necessary to ensure access to treatment at safety-net hospitals. Interferon-free therapy has transformed the landscape of HCV management, but continued efforts are necessary to ensure that treatment reaches its maximum potential in high prevalence areas. Future studies are needed on long term outcomes of sofosbuvir-based treatment for HCV in safety net hospitals and to determine why some individuals prescribed treatment do not initiate it during this new HCV treatment era.
Article summary.
Little is known on real-world outcomes of new hepatitis C virus (HCV) treatment among the underserved and in diverse communities disproportionally affected by HCV. We found that cure rates similar to clinical trial data can be achieved in this population.
Acknowledgments
The authors thank Linda Rosen MSEE who extracted information from the electronic medical record.
Funding: This work was supported by the National Institutes of Health [5 R01 DA031059, 3R01DA031059-03S1, and R25DA035163] and a Boston University School of Medicine Department of Internal Medicine pilot grant.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Conflict of interest: None
References
- 1.US Food and Drug Administration. FDA approves Sovaldi for chronic hepatitis C. 2013 [Available from: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm377888.htm.
- 2.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. The New England journal of medicine. 2013;368(20):1878–87. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 3.Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, et al. Ledipasvir and Sofosbuvir for 8 or 12 Weeks for Chronic HCV without Cirrhosis. The New England journal of medicine. 2014 doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 4.Edlin BR, Winkelstein ER. Can hepatitis C be eradicated in the United States? Antiviral research. 2014;110:79–93. doi: 10.1016/j.antiviral.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Putka B, Mullen K, Birdi S, Merheb M. The disposition of hepatitis C antibody-positive patients in an urban hospital. Journal of viral hepatitis. 2009;16(11):814–21. doi: 10.1111/j.1365-2893.2009.01137.x. [DOI] [PubMed] [Google Scholar]
- 6.Assoumou SA, Huang W, Horsburgh CR, Jr, Drainoni ML, Linas BP. Relationship between hepatitis C clinical testing site and linkage to care. Open Forum Infect Dis. 2014;1(1):ofu009. doi: 10.1093/ofid/ofu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falck-Ytter Y, Kale H, Mullen KD, Sarbah SA, Sorescu L, McCullough AJ. Surprisingly small effect of antiviral treatment in patients with hepatitis C. Annals of internal medicine. 2002;136(4):288–92. doi: 10.7326/0003-4819-136-4-200202190-00008. [DOI] [PubMed] [Google Scholar]
- 8.Backus Lisa, Belperio Pamela, Shahoumian Troy, Mole Larry. Effectiveness of Sofosbuvir-Based Hepatitis C (HCV) Antiviral Regimens in a Large U.S. Veteran Cohort. Paper presented at Digestive Disease Week; 2015; Washington D.C., USA. 2015. May 16–19, [Accessed on July 8, 2015]. Available at http://www.natap.org/2015/DDW/DDW_09.htm. [Google Scholar]
- 9.Nelson David. HCV-TARGET International Registry Do Phase III Trials Translate into Real World. Paper presented at International Symposium on Viral Hepatitis and Liver Diseases; 2015; Berlin, Germany. 2015. Jun 26–28, [Accessed on July 8, 2015]. Available at http://www.natap.org/2015/HCV/070215_03.htm. [Google Scholar]
- 10.Terrault N, Zeuzem S, Di Bisceglie A, Lim J, Pockros P, Frazier L, et al. Treatment Outcomes With 8, 12 and 24 Week Regimens of Ledipasvir/Sofosbuvir for the Treatment of Hepatitis C Infection: Analysis of a Multicenter Prospective, Observational Study. 2015 [Google Scholar]
- 11.Trio Health platform website. [Available from: http://triohealth.com/
- 12.Boston Medical Center. [Accessed on August 27th 2015];What Make BMC Special brochure. Available at http://www.bmc.org/Documents/WhatMakes_BMC_Special_Brochure.pdf.
- 13.Center for Health Information and Analysis (CHIA) [Accessed on August 27, 2015];2013 hospital profile Boston Medical Center. Available at http://www.chiamass.gov/assets/docs/r/hospital-profiles/2013/bmc.pdf.
- 14.Saab S, Ng V. Effects of a Sustained Virologic Response on Outcomes of Patients With Chronic Hepatitis C. Clinical Gastroenterology and Hematology. 9(11):923–30. doi: 10.1016/j.cgh.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 15.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32–6. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 16.AASLD/IDSA/IAS–USA. Recommendations for Testing, Managing, and Treating Hepatitis C. 2014 [updated November 20, 2014. Available from: http://www.hcvguidelines.org/full-report/when-and-whom-initiate-hcv-therapy.
- 17.Illinois department of health care and family service. [Accessed June 19th, 2015];General criteria for prior approval of newer direct-acting antivirals (DAA) for hepatitis C. Available at http://www2.illinois.gov/hfs/SiteCollectionDocuments/HepatitisC_General_Criteria.pdf. [Available from: http://www2.illinois.gov/hfs/SiteCollectionDocuments/HepatitisC_General_Criteria.pdf.
- 18.Younossi ZM, Park H, Gordon SC, Ferguson JR, Ahmed A, Dieterich D, et al. Real-World Outcomes of Ledipasvir/Sofosbuvir in Treatment-Naïve Patients With Hepatitis. C2016;(5 Spec Issue No 6):SP205-SP11. Available from: http://www.ajmc.com/journals/issue/2016/2016-5-vol22-sp/real-world-outcomes-of-ledipasvir-sofosbuvir-in-treatment-naive-patients-with-hepatitis-c/P-1. [PubMed]
- 19.Curry MP, Bacon B, Dieterich D, Flamm SL, Guest L, Kowdley KV, et al. Effectiveness of 8 or 12 week LDV-SOF in Treatment-Naïve Patients with Non-Cirrhotic, Genotype 1 Hepatitis C: Real-World Experience from the TRIO Network. Presentation; 2015. In press. [Google Scholar]
- 20.Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid Reimbursement of Sofosbuvir for the Treatment of Hepatitis C Virus Infection in the United States. Annals of internal medicine. 2015;163(3):215–23. doi: 10.7326/M15-0406. [DOI] [PubMed] [Google Scholar]
- 21.Mehta SH, Genberg BL, Astemborski J, Kavasery R, Kirk GD, Vlahov D, et al. Limited uptake of hepatitis C treatment among injection drug users. Journal of community health. 2008;33(3):126–33. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]