Summary
Background
The effectiveness of drug-free interventions in controlling human cysticercosis is not well known. We aimed to estimate the effectiveness of a community-based educational intervention in reducing the frequency of human cysticercosis in Burkina Faso.
Methods
We did a cluster-randomised controlled trial between 2011 and 2014. 60 eligible villages from three provinces (Boulkiemdé, Sanguié, and Nayala) were randomly allocated to the intervention or control group. Villages raising pigs, that were not a regional capital or located on a main road, that were more than 20 km from Ouagadougou or 5 km from one another, were eligible. In each village, 60 participants were asked for blood samples at baseline, 18 months later (before randomisation), and 18 months after randomisation. Villages were block randomised (1:1) by pig-raising department immediately after the pre-randomisation visit. The intervention aimed to improve knowledge of Taenia solium transmission and control through screening and structured discussion of a 52-min movie, and to increase community self-efficacy through a Self-esteem, Associative strengths, Resourcefulness, Action planning, Responsibility (SARAR) approach via the Participatory Hygiene and Sanitation Transformation (PHAST) model. The primary outcome was active cysticercosis, defined as the presence of circulating antigens detected by use of B158/B60 ELISA. Effectiveness measured at the village level was estimated by use of three Bayesian hierarchical models. This study is registered with ClinicalTrials.gov, number NCT0309339.
Findings
Two villages in the same randomisation block were excluded, resulting in a final sample size of 58 villages. Overall, the intervention tended towards a decrease in the cumulative incidence of active cysticercosis from baseline to after randomisation (adjusted cumulative incidence ratio 0·65, 95% Bayesian credible interval [95% CrI] 0·39–1·05) and a decrease in active cysticercosis prevalence from baseline to after randomisation (adjusted prevalence proportion ratio 0·84; 95% CrI 0·59–1·18). The intervention was shown to be effective in Nayala and Sanguié but not in Boulkiemdé.
Interpretation
Community-engaged participatory interventions can be effective at reducing the incidence and prevalence of cysticercosis in some low-resource settings.
Funding
US National Institutes of Health (National Institute of Neurological Disorders and Stroke, Fogarty International Center, and National Institute of General Medical Sciences).
Introduction
Taenia solium is a zoonotic cestode transmitted between human beings and pigs, causing serious disease and financial burden in countries where the parasite is endemic.1–5
Few large-scale cluster-randomised controlled trials have been done to assess the effectiveness of control strategies for human cysticercosis. A systematic review6 of the literature, in 2014, found a shortage of solid evidence on the effectiveness of community-based approaches to control cysticercosis. Only two of 13 studies reviewed were cluster-randomised controlled trials,7,8 one of which8 followed CONSORT guidelines.9 Since our review, evaluations of five community-based interventions to control cysticercosis have been published,10–15 with one following CONSORT guidelines.10 Together, these studies used a range of intervention types, alone or in combination, including mass drug administration in pigs or human beings, pig vaccination, education, and sanitation improvement, but the small number of cluster-randomised controlled trials makes it difficult to conclude which approach might be best. Evidence from transmission dynamics models tends to support the effectiveness of drug-focused and vaccine-focused interventions in the short term,16,17 but such interventions are unlikely to be sustainable and cost-effective in the long term, especially in resource-poor areas.6,16,18
In addition to the shortage of evidence on the effectiveness of these interventions, the control of taeniasis and cysticercosis through mass drug administration is especially challenging in sub-Saharan Africa because of limited access to drug and vaccine treatment for pigs and inconsistent provision of community-wide mass drug administration programmes. Indeed, in Burkina Faso, such programmes were gradually interrupted after the goals of the lymphatic filariasis control programme were reached in 2012.19 Moreover, the importance of using implementation research methods and grounding interventions in social and behavioural theory are increasingly being recognised as important means for maximising potential programme effectiveness as well as ultimate dissemination, scale-up, and sustainability.20 Theory-based interventions have been found to be effective21 and theory, when combined with appropriate evaluation designs, enhances our understanding of the effectiveness of interventions.22 An implementation research method has been used in one cluster-randomised controlled trial to control porcine cysticercosis,8 but human cysticercosis was not studied.
The aim of our study was to assess the effectiveness of an educational intervention, developed with an implementation research method, in reducing the frequency, at the village-level, of human cysticercosis in 60 villages of Burkina Faso.
Methods
Study design
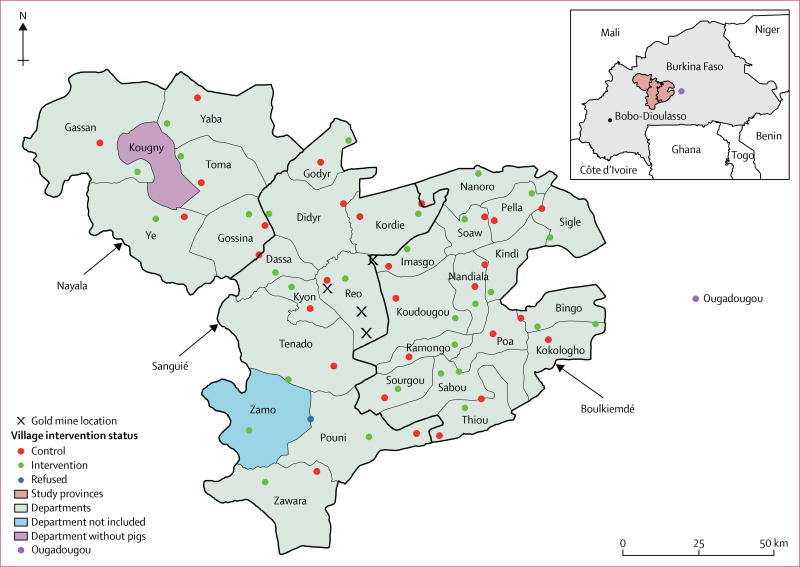
Évaluation du Fardeau Économique de la Cysticercose au Burkina (EFECAB) was a cluster-randomised controlled trial done in Burkina Faso between 2011 and 2014. The study was done in three provinces of Burkina Faso: Boulkiemdé (Centre-Ouest Region), which had the largest pig population in the country in 2009;23 Sanguié (Centre-Ouest Region), which had the third largest pig population in the country in 2009;23 and the neighbouring province of Nayala (Boucle du Mouhoun Region), where pigs were reportedly penned but fed human faeces. All pig-raising departments were selected and two eligible villages per department were randomly selected. Figure 1 depicts the locations of the selected provinces, departments, and villages.
Figure 1. Location of major gold mines and of the 60 participating villages.
This study was reviewed and approved by the University of Oklahoma Health Sciences Center Institutional Review Board (IRB#1419) and the Centre Muraz Ethical Review Board (14-0027-AFRICSANTE/DR). This study is registered with ClinicalTrials.gov, number NCT0309339.
Participants
The population of Boulkiemdé is primarily of the Mossi ethnic group and has a highly vertical structure with a central authority in charge of most community decisions. The population of Sanguié is primarily of the Lyéla ethnic group, whereas the population of Nayala is primarily of the Samo ethnic group. In contrast to Boulkiemdé, there is no central authority in Sanguié and Nayala, and decisions are made by community members. Another notable difference between the provinces is that women are responsible for raising pigs in more than 99% of households in Nayala and Sanguié, whereas this percentage is 94% in Boulkiemdé. Communities in Boulkiemdé also had access to safer sources of water than did those in Nayala and Sanguié.24
Villages with a population of at least 1000 people at the 2006 census, present on the 2000 map from the Institut Géographique du Burkina and separated from another participating village by at least 5 km, were eligible for inclusion. Villages that were located on a national or provincial road or within 20 km of Ouagadougou or that were the regional or provincial capital were excluded. Only one village was eligible in the department of Zamo (Sanguié). Therefore, an eligible village from Pouni, located on the border with Zamo, was selected. If a village was found to be ineligible during the first visit, it was replaced by another randomly selected eligible village in the same department.
One individual was sampled per concession (compound). A concession is a group of households living in a residential development, often fenced, where the authority of a concession chief is recognised. Detailed descriptions of the sampling approach in each village are provided elsewhere.24,25 Briefly, 80 concessions were randomly selected and stratified by the presence of reproductive sows, piglets, and others as strata. This approach resulted in the inclusion of ten concessions raising sows, 30 concessions raising piglets, and 40 concessions with or without pig raising. One concession whose chief refused participation was replaced by another concession in the village. The chief of one randomly selected household per concession was asked for his consent. One household chief refused participation and asked that his father’s household be sampled.
One individual aged at least 5 years, who had lived in the village for at least 12 months and was not planning to move in the next 3 years, was randomly selected from each concession. Participants, or their caregivers if the participants were younger than 16 years, were asked to answer a sociodemographic and screening questionnaire about epilepsy (appendix p 1),26 which was adapted to also include headaches often associated with neurocysticercosis,27 and were asked to provide three blood samples during a 3-year period until 60 participants consented in each village. The mothers and chiefs of the participants’ households were asked further sociobehavioural questions (appendix p 1).28 Participants unwilling to provide three blood samples or those selected after 60 participants had agreed to the serological follow-up were invited to participate in the questionnaire-based study. Individuals refusing to participate in the serological follow-up were included in the questionnaire-only follow-up, for which there were no refusals. Individuals who screened positive for single seizures, epilepsy, or severe chronic headache confirmed by the study neurologist were excluded from the follow-up and replaced by another person randomly selected from the same household who screened negative. This approach allowed estimation of the incidence of epilepsy and severe chronic headaches in this population. Hence, only individuals free of neurological symptoms were included in the follow-up.
For various reasons, including temporary absenteeism and the discovery of gold in the area after the study was initiated (figure 1), some participants were absent or had unexpectedly emigrated at the time of the two follow-up visits. To increase sample size, participants who had not been selected for the serological follow-up at baseline but who were willing to provide a blood sample were invited to do so. Moreover, in households where the participant was absent, a randomly selected individual from the same household was asked for consent to participate in the study and to provide a blood sample. Individuals selected at baseline who were absent for the pre-randomisation sampling but present for the post-randomisation sampling were eligible.
Oral consent was sought at three stages. First, village chiefs were asked for their consent to have the village participate in the study. Second, household chiefs were asked for their consent. Third, individual consent was sought each time a new participant was asked to provide a blood sample. All participants were asked for oral consent to answer the questionnaire. Parents consented for all children younger than 18 years, but children older than 10 years were also asked for their assent.
Randomisation and masking
Villages located in pig-raising departments of the three provinces were block randomised (1:1) at the department level to the intervention or control group. Block randomisation was done to balance the potential effect of departmental-level mass drug administration policy and delivery, cultural differences among ethnicities and tribes, as well as water and sanitation programmes. The village was the unit of randomisation and level of intervention. Each village was sampled three times: at baseline, 18 months later and immediately before allocation of the intervention (pre-randomisation), and 18 months after allocation of the intervention (post-randomisation). Data were collected by a team of seven trained fieldworkers. Block randomisation of villages was done by the principal investigator by use of the RANDOM() command in Excel. All residents of a village had the opportunity to receive the intervention allocated to their village. Intervention allocation took place after the baseline visit. After training the field team in the delivery of the educational intervention, the principal investigator sent the intervention allocation to the local team. This information was used to schedule the pre-randomisation visits and delivery of the intervention 18 months after the baseline visit in each village.
Given the nature of this study, masking of the participants and fieldworkers was not possible. However, the laboratory staff doing the serological tests were unaware of the allocations of villages to the intervention or control groups.
Intervention
The community-based intervention was developed with the implementation research framework PRECEDE-PROCEED (Predisposing, Reinforcing, and Enabling Constructs in Educational Diagnosis and Evaluation-Policy, Regulatory, and Organizational Constructs in Educational and Environmental Development).29 PRECEDE-PROCEED comprises nine planning phases designed to explain health-related behaviours and environments and to design and assess appropriate interventions to change health behaviours and their related determinants.29 The integration of social and behavioural theory and the PRECEDE-PROCEED framework enhances the potential appropriateness and effectiveness of interventions.29 A detailed description of our application of PRECEDE-PROCEED to understanding risk behaviours and risk contexts for cysticercosis in the study areas has been provided elsewhere.24 Data describing factors associated with the prevalence of active cysticercosis in pigs and human beings during a pilot project in three villages25,30 and baseline data on human active cysticercosis28 from the participating villages were part of the PRECEDE-PROCEED assessment.
PRECEDE-PROCEED and a well established social and behavioural theory, the Health Belief Model,31 guided the development of a multicomponent educational intervention that targeted the general population while taking the zoonotic aspect of the infection into account.24 The intervention was based on our PRECEDE assessment24 of the importance of, and potential for, change in behavioural and environmental factors as well as of the predisposing, reinforcing, and enabling factors related to open defecation, drinking of unboiled water, and consumption of infected pork. The intervention aimed to improve knowledge about T solium transmission and the benefits of its control; and to increase community self-efficacy in implementation of cysticercosis control measures. To increase knowledge, we produced a 52-min movie and comic book that was shown and discussed during village events. To increase community self-efficacy, we used Participatory Hygiene and Sanitation Transformation (PHAST),32 a community-level model that uses a Self-esteem, Associative strengths, Resourcefulness, Action-planning and Responsibility (SARAR) approach. The SARAR approach recognises that people have an innate ability to work as a collective to bring sufficient information and experience to solve problems on their own. We adapted the PHAST model to the context of Burkina Faso and cysticercosis transmission and control (examples of PHAST training cards are available in the appendix, pp 2–4). Fieldworkers were trained on PHAST by experts from Water and Sanitation for Africa (WSA), a Pan African Inter-governmental Agency headquartered in Ouagadougou.
Five trained fieldworkers spent 2 days delivering PHAST in each village (5–6 h per session, with a maximum of four sessions per village). Only fieldworkers fluent in the local languages (primarily Mooré and Dioula, but also Lélé) delivered PHAST. The intervention movie was publicised during the PHAST sessions and shown in a common place in the village (generally a school yard or the market place) in the early evening following the PHAST process. At the end of the movie, trained fieldworkers fluent in the local language facilitated a structured discussion with the men, women, and children who attended. These discussions lasted 2–4 h and focused on summarising the movie and identifying and clarifying its key messages. Supervisory visits were conducted by the WSA staff and by the local principal investigators (AM and RG) during implementation of the intervention. The field staff wrote a report following implementation of the intervention in each village.
Procedures
Blood samples were collected on three occasions: at baseline (from Feb 11, 2011, to Jan 12, 2012), at the pre-randomisation sampling (from Sept 25, 2012, to Sept 19, 2013) immediately before the intervention, and at the post-randomisation sampling (from March 20, 2014, to Nov 24, 2014).
At baseline, a physician went to each village to take blood samples from 60 participants who consented to the serological component of the study. Blood samples were taken at the same time as recruitment and up to a few weeks later. For the pre-randomisation sampling, the villages were visited in the same order by the field team and the study phlebotomist. However, although the fieldworkers spent 3–4 days in each village to collect questionnaire data and offer the educational intervention to the villages allocated to receive the intervention, the phlebotomist visited one to two villages per day following appointments convened with a key leader in the village (eg, with the councillor). Despite convening of the appointments, several individuals were absent at the time of sampling because of various reasons such as social events, markets, and gold mining. A physician collected all blood samples in villages of Nayala and completed missed blood samples during the last 2 months of the pre-randomisation visits.
During the post-randomisation visits, blood samples were collected at the same time as the field visits. This approach allowed for blood samples to be collected among most serologically followed individuals who were present at the time of the field visit.
Blood samples were drawn by venepuncture with 10 mL Venosafe serum gel tubes. The tubes were placed in a cooler, left to decant, and the serum samples collected and placed into two pre-labelled tubes. The serum samples were placed in freezers (−20°C) and brought by batch to the Institut de Recherche en Sciences de la Santé (IRSS) in Bobo Dioulasso and kept at −20°C.
Outcomes
The primary outcomes were the prevalence and cumulative incidence of human active cysticercosis measured at the village level. Active cysticercosis was defined as the presence of excretory-secretory circulating antigens of the metacestode of T solium measured with B158/B60 ELISA.33 Secondary outcomes were evidence of construction of a new latrine or pig pens during the post-randomisation visit and measured at the concession level. These outcomes were measured by observations from the field team.
Statistical analysis
The initial sample size was based on estimation of the change in the incidence rate of active cysticercosis in human beings. On the basis of a pilot study done in the same region in 2008,25 as well as evidence from three cohort studies that had been published when the present study was designed,34 we assumed that we would observe six seroconversions per 100 person-years in this population. Further assuming that monitoring alone would lead to a decrease of 10% in the incidence rate of infection and assuming that a 50% reduction in the incidence rate in the intervention group would be worth detecting, we estimated that 60 villages would be required to detect a difference in incidence rate of 2·4 per 100 person-years. Unfortunately, one visit had to be eliminated from our study design because of budget cuts after the baseline study had been done, so incidence rates could no longer be estimated. Incidence rate per 12 person-months was replaced by cumulative incidence per 18 months.
The effectiveness of the intervention was measured at the village level with three analytical samples and three effectiveness measures. The first analytical sample consisted of participants providing a blood sample at all three visits; the second consisted of all participants providing at least one blood sample during the study; and the third consisted of participants providing a blood sample at baseline and at the post-randomisation visit. Descriptive statistics were first used to compare participants’ characteristics in the intervention and control groups for all three samples. The significance of differences in the distribution of characteristics between the intervention and control groups was assessed by χ² tests for categorical variables and t-tests for continuous variables at an alpha of 0·05. Descriptive statistics were done in Stata 14.
The first effectiveness measure was defined as the prevalence proportion ratio of active cysticercosis at the post-randomisation visit in the intervention group compared with the control group while taking into consideration the change in prevalence of active cysticercosis in the 18 months before randomisation. The first effectiveness measure was estimated with the first and second analytical samples. The second effectiveness measure estimated the post-randomisation cumulative incidence ratio of active cysticercosis between the intervention and control villages, while taking into consideration the cumulative incidence of active cysticercosis before randomisation. This analysis was run with the first analytical sample. The third effectiveness measure was estimated as the 36-month cumulative incidence ratio of active cysticercosis in the intervention group compared with the control group with the first and third analytical samples.
Details of the three distinct Bayesian models used to estimate each effectiveness measure can be found in the appendix (p 5). In frequentist statistics, the data are the only source for making inferences about the parameters of interest. In Bayesian statistics, information from prior knowledge, reflected by a prior density distribution, is updated with data from the study to obtain a posterior density distribution of the estimate of interest (eg, the effectiveness measure). Features of the posterior density distribution, such as the mean, median, and the interval between the 2·5th and 97·5th percentile (ie, 95% Bayesian credible interval [CrI]), can be used to summarise the estimate of interest. The 95% CrI can be interpreted as the limits within which 95% of the posterior density of the estimate of interest lies. Here, all models were run with non-informative priors (ie, a distribution not favouring any particular estimate and allowing for a wide range of estimates) to avoid unduly influencing the results with prior beliefs.
The prevalence proportion ratio and cumulative incidence ratio were first estimated will all provinces combined. We then did analyses stratified by province (appendix p 6). Since Sanguié and Nayala had similar sociocultural structures unlike those seen in Boulkiemdé, all further analyses also assessed the effectiveness of the intervention in these two groups of provinces. This decision was taken because of the presence of effect modification of the intervention due to context, an effect that must be explored in community-based cluster-randomised controlled trials.35
Secondary outcomes were measured as prevalence proportion ratios of evidence of recent construction of latrines (completely built or under construction) and pig pens (completely built or under construction) in the intervention villages compared with the control villages among all concessions where such information was available. The same model structure as that used for the third effectiveness measure estimate was used. Results for each province are provided in the appendix (p 7).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data, did all the data analyses, and had final responsibility for the decision to submit for publication.
Results
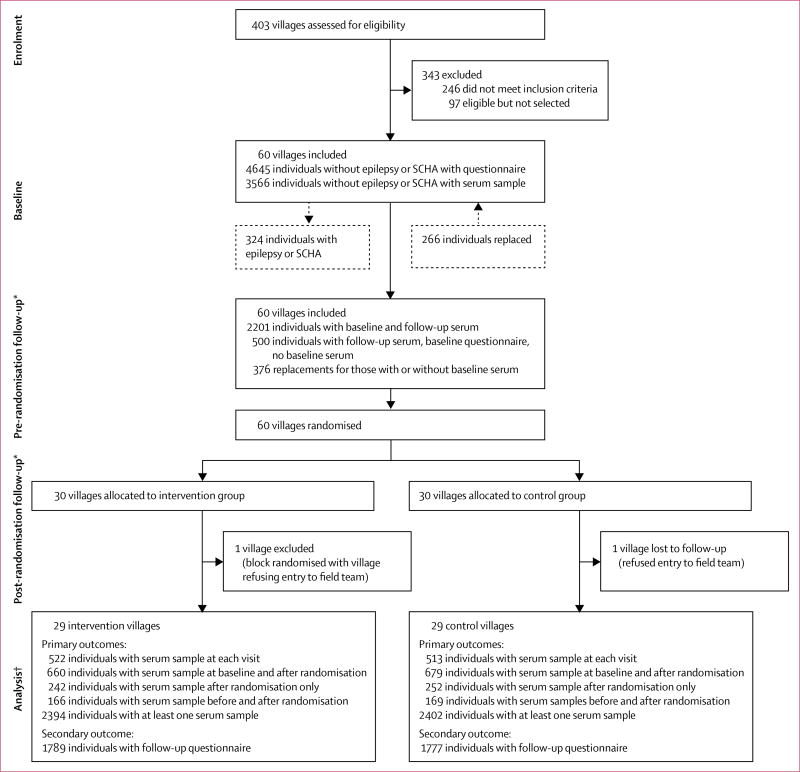
Figure 2 shows the participating villages and the number of individuals who provided a serum sample at each visit. At enrolment, 403 villages were assessed for eligibility, of which 157 were eligible and 60 were selected. At baseline, 4645 individuals without epilepsy or severe chronic headaches answered the questionnaires, of whom 3566 also provided a serum sample. One village allocated to the control group refused entry to the field team at the post-randomisation visit. This village and the village randomised in the same block were excluded, leaving 58 villages in the final analysis in which 522 participants from the intervention villages and 513 from the control villages provided blood samples at all visits. Details of the number of individuals who provided serum samples at each visit are provided in figure 2 and table 1.
Figure 2. Flow chart of participating villages and individuals.
No villages were lost to follow-up. SCHA=severe chronic headaches. *Individuals absent at the pre-randomisation follow-up but who returned for the post-randomisation follow-up are not included. †There were six concessions in the intervention group and three concessions in the control group where two individuals were sampled at the same visit. Only data from the originally sampled individuals were kept.
Table 1.
Baseline characteristics of villages and their residents randomised to the intervention and control groups
| Intervention group |
Control group | |
|---|---|---|
| All analytical samples | ||
|
| ||
| Number of villages by province | ||
| Boulkiemdé | 15 | 15 |
| Nayala | 5 | 5 |
| Sanguié* | 9 | 9 |
| Total | 29 | 29 |
| Prevalence of active cysticercosis at baseline among all eligible participants (ie, no confirmed epilepsy or severe chronic headaches) | ||
| Boulkiemdé | 44/891 (4·9%) | 32/888 (3·6%) |
| Nayala | 11/300 (3·7%) | 7/294 (2·4%) |
| Sanguié | 10/538 (1·9%) | 8/537 (1·5%) |
| Total | 65/1729 (3·8%) | 47/1719 (2·7%) |
|
| ||
| Analytical sample 1: baseline characteristics of eligible participants with serum samples at all visits | ||
|
| ||
| Number of participants per group | 522 | 513 |
| Mean age (SD) | 31·4 (19·1) | 31·5 (18·4) |
| Sex | ||
| Men (%) | 240 (46·0%) | 233 (45·4%) |
| Women (%) | 282 (54·0%) | 280 (54·6%) |
| Mean number of days between baseline and pre-randomisation serum collection (SD) | 583·8 (87·3) | 589·6 (60·9) |
| Mean number of days between pre- and post-randomisation serum collection (SD) | 469·5 (46·5) | 467·7 (54·5) |
| Mean number of days between baseline and post-randomisation serum collection (SD) | 1056 (30·9) | 2068 (33·5) |
| Attended some years of school? | ||
| Yes (%) | 170 (32·9%) | 171 (33·6%) |
| No (%) | 347 (67·1%) | 338 (66·4%) |
| Data missing | 5 | 4 |
| Reported using toilet to defecate? | ||
| Yes (%) | 70 (13·5%) | 79 (15·5%) |
| Data missing | 5 | 4 |
| Pork eating behaviour | ||
| Never eats pork | 117 (22·6%) | 90 (17·7%) |
| Eats at home only | 201 (38·9%) | 211 (41·5%) |
| Eats at other households | 50 (9·7%) | 68 (13·4%) |
| Eats at village market | 83 (16·1%) | 84 (16·5%) |
| Eats at other village market | 26 (5·0%) | 26 (5·1%) |
| Ate pork before, not anymore | 40 (7·7%) | 30 (5·9%) |
| Data missing | 5 | 4 |
| Knowledge about tapeworm | ||
| Never heard of tapeworm infection | 200 (38·7%) | 187 (36·7%) |
| Has heard of tapeworm infection, was never infected | 265 (51·3%) | 265 (52·1%) |
| Had a tapeworm infection | 52 (10·1%) | 57 (11·2%) |
| Data missing | 5 | 4 |
| Ever seen or heard of nodules in pigs? | ||
| Yes (%) | 339 (65·6%) | 338 (66·4%) |
| Data missing | 5 | 4 |
| Primary source of drinking water† | ||
| Open well or river | 135 (25·9%) | 170 (33·1%) |
| Laid stone dug or covered well | 126 (24·1%) | 142 (27·7%) |
| Drilled well or tap | 261 (50·0%) | 201 (39·2%) |
| Wealth quintile | ||
| 1–3 | 294 (56·3%) | 302 (58·9%) |
| 4–5 | 228 (43·7%) | 211 (41·1%) |
| Do household members have access to a latrine? | ||
| Yes (%) | 73 (14·0%) | 62 (12·1%) |
| Data missing | 3 | 4 |
| Type of concession sampled | ||
| Sow | 87 (16·7%) | 70 (13·7%) |
| Piglet | 208 (39·9%) | 214 (41·7%) |
| Any | 227 (43·5%) | 229 (44·6%) |
| Does the household own pigs? | ||
| Yes (%) | 388 (74·3%) | 400 (78·0%) |
| Pork cooking by mother of the household | ||
| Does not cook pork | 175 (33·7%) | 141 (27·6%) |
| Well cooked | 318 (61·3%) | 344 (67·3%) |
| Medium or rare | 26 (5·1%) | 26 (5·1%) |
| Data missing | 3 | 2 |
|
| ||
| Analytical sample 2: demographic and baseline household characteristics of eligible participants with at least one serum sample | ||
|
| ||
| Number of participants per group | 2394 | 2402 |
| Mean age (SD) | 31·6 (19·8) | 32·7 (19·9) |
| Data missing (participant age) | 12 | 22 |
| Sex | ||
| Men (%) | 1119 (46·9%) | 1072 (44·8%) |
| Women (%) | 1267 (53·1%) | 1321 (55·2%) |
| Data missing | 8 | 9 |
| Primary source of drinking water† | ||
| Open well or river | 672 (28·2%) | 753 (31·5%) |
| Laid stone dug or covered well | 631 (26·5%) | 661 (27·6%) |
| Drilled well or tap | 1082 (45·4%) | 980 (40·9%) |
| Data missing | 9 | 8 |
| Did household members have access to a latrine at baseline?† | ||
| Yes (%) | 320 (13·6%) | 255 (10·7%) |
| Data missing | 34 | 24 |
| Wealth quintile | ||
| 1–3 | 1376 (57·5%) | 1442 (60·1%) |
| 4–5 | 1017 (42·5%) | 958 (39·9%) |
| Data missing | 2 | 2 |
| Type of concession sampled | ||
| Sow | 307 (12·8%) | 312 (13·0%) |
| Piglet | 860 (35·9%) | 904 (37·6%) |
| Any | 1228 (51·3%) | 1186 (49·4%) |
| Did the household own pigs at baseline?† | ||
| Yes (%) | 1627 (68·1%) | 1794 (74·8%) |
| Data missing | 5 | 5 |
| Pork cooking by mother of the household at baseline† | ||
| Does not cook pork | 906 (38·2%) | 661 (27·7%) |
| Well cooked | 1347 (56·8%) | 1597 (67·0%) |
| Medium or rare | 117 (4·9%) | 125 (5·3%) |
| Data missing | 21 | 20 |
|
| ||
| Analytical sample 3: baseline characteristics of eligible participants with serum samples at baseline and after randomisation | ||
|
| ||
| Number of participants per group | 660 | 679 |
| Mean age (SD) | 30·4 (18·3) | 31·0 (18·2) |
| Sex | ||
| Men (%) | 297 (45·0%) | 307 (45·2%) |
| Women (%) | 363 (55·0%) | 372 (54·8%) |
| Mean number of days between baseline and post-randomisation serum collection (SD) | 1056 (29·9) | 1055 |
| Attended some years of school? | ||
| Yes | 204 (31·2%) | 223 (33·0%) |
| Data missing | 6 | 4 |
| Reported using toilet to defecate | ||
| Yes | 91 (13·9%) | 97 (14·4%) |
| Data missing | 6 | 4 |
| Pork eating behaviour | ||
| Never eats pork | 166 (25·4%) | 132 (19·6%) |
| Eats at home only | 239 (36·5%) | 272 (40·3%) |
| Eats at other households | 69 (10·6%) | 85 (12·6%) |
| Eats at village market | 100 (15·3%) | 107 (15·8%) |
| Eats at other village market | 35 (5·4%) | 34 (5·0%) |
| Ate pork before, not anymore | 45 (6·9%) | 45 (6·7%) |
| Data missing | 6 | 4 |
| Knowledge about tapeworm | ||
| Never heard of tapeworm infection | 256 (39·1%) | 255 (37·8%) |
| Has heard of tapeworm infection, was never infected | 338 (51·7%) | 341 (50·5%) |
| Had a tapeworm infection | 60 (9·2%) | 79 (11·7%) |
| Data missing | 6 | 4 |
| Ever seen or heard of nodules in pigs? | ||
| Yes (%) | 414 (63·3%) | 438 (64·9%) |
| Data missing | 6 | 4 |
| Primary source of drinking water† | ||
| Open well or river | 193 (29·3%) | 214 (31·6%) |
| Laid stone dug or covered well | 152 (23·1%) | 193 (28·5%) |
| Drilled well or tap | 313 (47·6%) | 271 (40·0%) |
| Data missing | 2 | 1 |
| Wealth quintile | ||
| 1–3 | 379 (57·5%) | 392 (57·7%) |
| 4–5 | 280 (42·5%) | 287 (42·3%) |
| Data missing | 1 | 0 |
| Do household members have access to a latrine? | ||
| Yes (%) | 93 (14·2%) | 83 (12·3%) |
| Data missing | 5 | 4 |
| Type of concession sampled | ||
| Sow | 95 (14·4%) | 90 (13·3%) |
| Piglet | 262 (39·7%) | 265 (39·0%) |
| Any | 303 (45·9%) | 324 (47·7%) |
| Does the household own pigs? | ||
| Yes (%) | 481 (73·1%) | 512 (75·5%) |
| Data missing | 2 | 1 |
| Pork cooking by mother of the household† | ||
| Does not cook pork | 231 (35·2%) | 195 (28·8%) |
| Well cooked | 396 (60·4%) | 447 (66·0%) |
| Medium or rare | 29 (4·4%) | 35 (5·2%) |
| Data missing | 4 | 2 |
Data are n (%) unless otherwise stated. The displayed percentages do not include missing values.
One village from Sanguié randomly assigned to the intervention refused to be sampled at the last follow-up. The village in the same department was excluded.
Significant difference between the intervention and control group (assessed by χ² tests).
Table 1 shows the distribution of potential confounders in the intervention and control villages according to the analytical sample used. Only baseline participants’ age and sex, and baseline household characteristics were explored for the second analytical sample. Variables likely to be influenced by the intervention were not explored. The randomisation process was generally successful at balancing potential confounders in the three analytical samples. However, some factors were less balanced. For all analytical samples, the main source of drinking water as reported by the household chief differed between the intervention and control groups. However, this variable was not associated with the cumulative incidence of infection during the pre-randomisation or post-randomisation period, nor with the prevalence of active cysticercosis at any visit and was therefore not assessed further. No other variable was imbalanced between the two groups in analytical sample 1. The proportions of household chiefs declaring owning pigs and of mothers’ pork cooking behaviours were different in analytical samples 2 and 3. However, neither variable was associated with the cumulative incidence of infection between the baseline and post-randomisation visit, and these variables were therefore not considered confounders for analytical sample 3. For analytical sample 2, these variables as well as access to a latrine were all associated with prevalence of active cysticercosis at the different visits and were included in the analyses.
Table 2 describes estimates of the prevalence of active cysticercosis at each visit for analytical sample 1 and 2 as well as an estimate of the cumulative incidence during the pre-randomisation and post-randomisation periods and between the baseline and post-randomisation visits. Overall, the prevalence of active cysticercosis at baseline was higher in the intervention villages than in the control villages. This difference was significant for analytical sample 1 for Nayala and Sanguié, but not for any other comparisons. However, no such difference between intervention and control villages was observed during the pre-randomisation visit, and the cumulative incidence of infection during the pre-randomisation period was similar in the two groups. The prevalence of active cysticercosis in the control villages increased during the course of the study, reaching 7·4% (for all provinces) in analytical sample 2 at the post-randomisation visit. In the control villages, the cumulative incidence of infection (for all provinces) from baseline to after randomisation was almost as high, at 5·9% in analytical sample 3 (table 2). By contrast, after randomisation the prevalence of infection in the intervention villages among all three provinces either increased to a lesser extent than in the control villages (eg, in analytical sample 2) or remained the same (eg, in analytic sample 1). Estimates of the cumulative incidence of infection were similar in the intervention and control groups during the pre-randomisation and post-randomisation periods (analytical sample 1), but the cumulative incidence of infection from baseline to after randomisation was lower in the intervention villages than in the control villages in both analytical samples 1 and 3.
Table 2.
Proportion of participants with active cysticercosis in each analytical sample in the intervention and control villages by province group according to different measures of frequency and periods of measurement
| Analytical sample 1
|
Analytical sample 2
|
Analytical sample 3
|
||||
|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | |
| Prevalence at baseline | ||||||
|
| ||||||
| All provinces | 27/522 (5·2%) | 14/513 (2·7%) | 65/1729 (3·8%) | 47/1719 (2·7%) | NA | NA |
| Boulkiemdé | 20/309 (6·5%) | 13/308 (4·2%) | 44/891 (4·9%) | 32/888 (3·6%) | NA | NA |
| Nayala and Sanguié | 7/213 (3·3%) | 1/205 (0·5%) | 21/838 (2·5%) | 15/831 (1·8%) | NA | NA |
|
| ||||||
| Prevalence before randomisation | ||||||
|
| ||||||
| All provinces | 36/522 (6·9%) | 30/513 (5·9%) | 78/1493 (5·2%) | 78/1509 (5·2%) | NA | NA |
| Boulkiemdé | 25/309 (8·1%) | 21/308 (6·8%) | 51/784 (6·5%) | 53/771 (6·9%) | NA | NA |
| Nayala and Sanguié | 11/213 (5·2%) | 9/205 (4·4%) | 27/709 (3·8%) | 25/738 (3·4%) | NA | NA |
|
| ||||||
| Prevalence after randomisation | ||||||
|
| ||||||
| All provinces | 36/522 (6·9%) | 35/513 (6·8%) | 71/1102 (6·4%) | 83/1118 (7·4%) | NA | NA |
| Boulkiemdé | 28/309 (9·1%) | 22/308 (7·1%) | 54/625 (8·6%) | 58/671 (8·6%) | NA | NA |
| Nayala and Sanguié | 8/213 (3·8%) | 13/205 (6·3%) | 17/477 (3·6%) | 25/447 (5·6%) | NA | NA |
|
| ||||||
| Cumulative incidence from the baseline to pre-randomisation period | ||||||
|
| ||||||
| All provinces | 21/495 (4·2%) | 20/499 (4·0%) | NA | NA | NA | NA |
| Boulkiemdé | 12/289 (4·2%) | 12/295 (4·1%) | NA | NA | NA | NA |
| Nayala and Sanguié | 9/206 (4·4%) | 8/204 (3·9%) | NA | NA | NA | NA |
|
| ||||||
| Cumulative incidence from the pre-randomisation to post-randomisation period | ||||||
|
| ||||||
| All provinces | 16/486 (3·3%) | 16/483 (3·3%) | NA | NA | NA | NA |
| Boulkiemdé | 12/284 (4·2%) | 9/287 (3·1%) | NA | NA | NA | NA |
| Nayala and Sanguié | 4/202 (2·0%) | 7/196 (3·6%) | NA | NA | NA | NA |
|
| ||||||
| Seroconversion from the baseline to post-randomisation period | ||||||
|
| ||||||
| All provinces | 19/495 (3·8%) | 25/499 (5·0%) | NA | NA | 25/660 (3·8%) | 40/679 (5·9%) |
| Boulkiemdé | 14/289 (4·8%) | 13/295 (4·4%) | NA | NA | 18/370 (4·9%) | 23/407 (5·7%) |
| Nayala and Sanguié | 5/206 (2·4%) | 12/204 (5·9%) | NA | NA | 7/290 (2·4%) | 17/272 (6·3%) |
Data are n/N (%). NA=not applicable.
However, these observations differed by province group. In Nayala and Sanguié, prevalence decreased (analytical sample 1) or stayed at a similar level (analytical sample 2) in the intervention villages, but it increased for intervention villages in Boulkiemdé. The difference in cumulative incidence from baseline to after randomisation between the intervention and control groups was also more marked in Sanguié and Nayala than in Boulkiemdé (table 2).
When we used effectiveness measures 1 and 3 with analytic samples 2 and 3, the intervention tended to decrease the prevalence (adjusted prevalence proportion ratio 0·84, 95% CrI 0·59–1·18) and the baseline to post-randomisation cumulative incidence (adjusted cumulative incidence ratio 0·65, 95% CrI 0·39–1·05) of active cysticercosis in all provinces (table 3). However, such decreasing tendency was less marked when analytical sample 1 was used for effectiveness measure 3, and no reduction in cumulative incidence was observed with effectiveness measure 2 (table 3).
Table 3.
Intervention effectiveness measure estimates with three analytical samples comparing intervention villages and control villages in all provinces and by province group
| Effectiveness measure 1
|
Effectiveness measure 2
|
Effectiveness measure 3
|
||||
|---|---|---|---|---|---|---|
| Crude PPR (95% CrI) |
Adjusted PPR (95% CrI) |
Crude CIR (95% CrI) |
Adjusted CIR (95% CrI) |
Crude CIR (95% CrI) |
Adjusted CIR (95% CrI) |
|
| Analytical sample 1 | ||||||
|
| ||||||
| All provinces | 0·83 (0·50–1·39) | 0·85 (0·51–1·42)* | 0·97 (0·46–2·02) | 0·97 (0·47–2·03)* | 0·73 (0·41–1·31) | 0·75 (0·42–1·35)* |
| Boulkiemdé | 1·00 (0·57–170) | 1·00 (0·58–1·70)* | 1·25 (0·56–2·73) | 1·26 (0·56–2·73)* | 1·10 (0·51–2·29) | 1·13 (0·54–2·38)* |
| Nayala and Sanguié | 0·52 (0·22–1·15) | 0·54 (0·22–1·13)* | 0·54 (0·14–1·58) | 0·54 (0·15–1·56)* | 0·40 (0·13–1·06) | 0·38 (0·12–1·02)* |
|
| ||||||
| Analytical sample 2 | ||||||
|
| ||||||
| All provinces | NA | 0·84 (0·59–1·18)† | NA | NA | NA | NA |
| Boulkiemdé | NA | 0·98 (0·67–1·42)† | NA | NA | NA | NA |
| Nayala and Sanguié | NA | 0·57 (0·32–0·98)† | NA | NA | NA | NA |
|
| ||||||
| Analytical sample 3 | ||||||
|
| ||||||
| All provinces | NA | NA | NA | NA | 0·63 (0·38–1·02) | 0·65 (0·39–1·05)‡ |
| Boulkiemdé | NA | NA | NA | NA | 0·86 (0·46–1·59) | 0·86 (0·46–1·56)‡ |
| Nayala and Sanguié | NA | NA | NA | NA | 0·38 (0·15–0·86) | 0·38 (0·15–0·88)‡ |
PPR=prevalence proportion ratio. CIR=cumulative incidence ratio. NA=not applicable. Effectiveness measure 1=PPR adjusted for the change from baseline to the pre-randomisation period to the post-randomisation period and for clustering by village. Effectiveness measure 2=CIR adjusted for the change from seroconversion from the baseline to the pre-randomisation period and clustering by village. Effectiveness measure 3=CIR from baseline to the post-randomisation visit. Analytic sample 1=participants who provided a blood sample at all three visits. Analytic sample 2=participants who provided a blood sample at least once. Analytic sample 3=participants who provided blood samples at baseline and at the post-randomisation visit.
Adjusted for baseline age, sex, and type of concession sampled.
Adjusted for baseline age, sex, household members having access to a latrine, pork cooked by the mother of the household, wealth index of the household, and household owning pigs.
Adjusted for baseline age, sex, household members having access to a latrine, pork cooked by the mother of the household, wealth index of the household, and type of concession sampled.
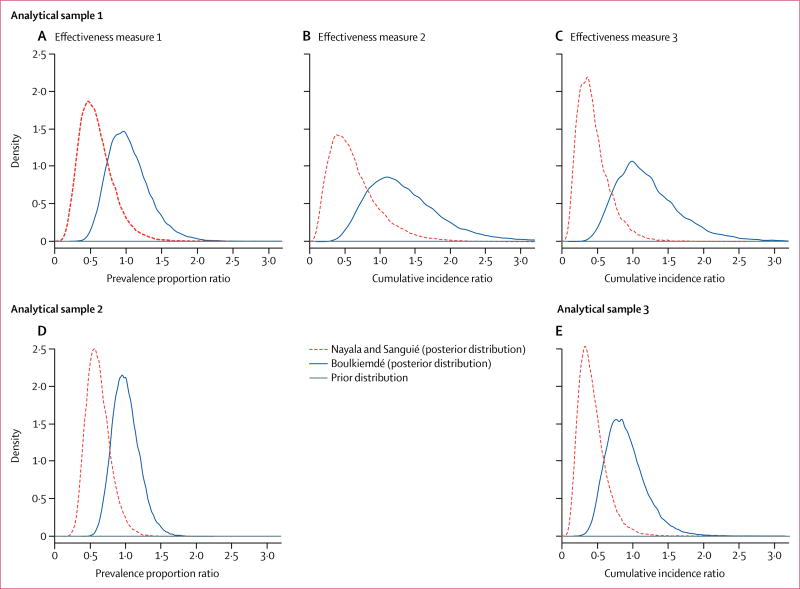
However, the Bayesian models suggested the presence of effect modification by province group. Indeed, the intervention was effective in reducing the prevalence and the cumulative incidence of active cysticercosis in Nayala and Sanguié but not in Boulkiemdé (table 3). The prior and posterior distributions of the intervention effects by province group are shown in figure 3. The estimated effectiveness of the intervention in Nayala and Sanguié was quite robust across the analytical samples and effectiveness measures; the crude cumulative incidence ratio from baseline to after randomisation for effectiveness measure 3, analytical sample 3 was 0·38 (95% CrI 0·15–0·86), while the adjusted prevalence proportion ratio of active cysticercosis for effectiveness measure 1, analytical sample 2, was 0·57 (95% CrI 0·32–0·98; table 3). These results were robust when the same effectiveness measure was estimated with different analytical samples. For example, the estimated prevalence proportion ratio in Nayala and Sanguié was 0·54 (95% CrI 0·22–1·13) with analytical sample 1 and 0·57 (0·32–0·98) with analytical sample 2 for effectiveness measure 1 (table 3). The results for all provinces were also similar for all effectiveness measures with analytical sample 1, although the uncertainly was larger than that for analytical samples 2 and 3 because of the smaller sample size of this subgroup. Results of all effectiveness measures and all analytical samples suggest that the intervention did not contribute to a reduction in the frequency of active cysticercosis in Boulkiemdé.
Figure 3. Prior and posterior distributions of the adjusted effect of the intervention in Boulkiemdé and in Nayala and Sanguié.
Variables adjusted for in the different models are reported in table 1. (A) Effectiveness measure 1 (prevalence proportion ratio adjusted for the change from baseline to the pre-randomisation period to the post-randomisation period and for clustering by village) and analytical sample 1 (participants provided a blood sample at all three visits). (B) Effectiveness measure 2 (cumulative incidence ratio adjusted for the change from seroconversion from the baseline to the pre-randomisation period and clustering by village) and analytical sample 1. (C) Effectiveness measure 1 and analytical sample 3 (participants provided blood samples at baseline and at the post-randomisation visit). (D) Effectiveness measure 1 and analytical sample 2 (participants provided a blood sample at least once). (E) Effectiveness measure 3 (cumulative incidence ratio from baseline to the post-randomisation visit) and analytical sample 3.
With regard to secondary outcomes, we observed a larger proportion of concessions with evidence of recently built latrines, or new latrines under construction, in the intervention villages than in the control villages in all provinces, but the prevalence proportion ratio was higher in Nayala and Sanguié than in Boulkiemdé (table 4). This effect was primarily driven by a stronger effect in Nayala than in Sanguié (appendix p 7). The effect was even stronger when only newly built latrines were assessed. The intervention showed no evidence of an effect on pig pens being built or under construction.
Table 4.
Comparison of secondary outcomes in intervention villages and control villages
| New latrine built or under construction |
New latrine built | New pig pen built or under construction* |
|
|---|---|---|---|
| Number (%) of concessions | |||
|
| |||
| All provinces | |||
| Intervention | 194/2022 (9·6%) | 155/1983 (7·9%) | 39/705 (5·5%) |
| Control | 157/1990 (7·9%) | 108/1941 (5·6%) | 43/832 (5·2%) |
| Boulkiemdé | |||
| Intervention | 117/1034 (11·4%) | 99/1016 (9·8%) | 24/380 (6·3%) |
| Control | 103/1045 (9·9%) | 76/1018 (7·5%) | 27/439 (6·2%) |
| Nayala and Sanguié | |||
| Intervention | 77/988 (7·8%) | 56/967 (5·8%) | 15/325 (4·6%) |
| Control | 54/945 (5·7%) | 32/923 (3·5%) | 16/393 (4·1%) |
|
| |||
| Prevalence proportion ratio (95% CrI) | |||
|
| |||
| All | 1·21 (1·00–1·48) | 1·40 (1·11–1·80) | 1·10 (0·72–1·67) |
| Boulkiemdé | 1·14 (0·89–1·46) | 1·30 (0·98–1·73) | 1·07 (0·62–1·81) |
| Nayala and Sanguié | 1·37 (0·98–1·93) | 1·68 (1·11–2·61) | 1·13 (0·56–2·29) |
These estimates are among concessions where the interviewee declared that pigs were being raised by someone in their household at follow-up.
Discussion
The EFECAB study is, to our knowledge, the first cluster-randomised controlled trial done in sub-Saharan Africa to assess the effect of a community-based intervention developed with an implementation research method on control of human cysticercosis. To the best of our knowledge, this study is also the first to measure the effectiveness of the PHAST model in accordance with CONSORT guidelines. The results show that the intervention tended towards a reduction in active cysticercosis when all provinces were analysed together and when larger samples (analytical samples 2 or 3) were used. However, weaker to no effects were observed when the smaller database of analytical sample 1 was used. This observation could be explained by the apparent effect modification by province group. Indeed, although done within a relatively small geographical area, the intervention was effective in two of three provinces. Although we have no objective evidence to explain this difference, the difference in ethnic groups and social structures mentioned above might have had a role. The Mossi ethnic group of Boulkiemdé is a more hierarchical society where decisions are taken by the authorities and then strongly recommended to the general population. By contrast, the Lyéla and Samo ethnic groups of Nayala and Sanguié tend to be less hierarchical and value more individual decision making. Additionally, observations made by the field team can help raise further hypotheses. First, the field team noted that additional effort and time was required to motivate participants from Boulkiemdé to participate in the different study components (eg, answering questionnaires and providing blood samples). Moreover, we noted more frequent refusals in Boulkiemdé than elsewhere to provide blood samples and, among those with confirmed neurological disorders, to obtain a CT scan (these scans were done to diagnose neurocysticercosis and results have not yet been reported). Anecdotal data show that participants from Boulkiemdé often complained that the incentive, which was soap, was not sufficient for a blood sample. This complaint was never raised in villages of Sanguié and Nayala. Unmeasured factors that resulted in a general unwillingness of participants from Boulkiemdé to participate enthusiastically could have also influenced the community’s response to, and the subsequent effectiveness of, our intervention. The difficulty of convincing people to improve latrine use and sanitation in Burkina Faso was recently emphasised in an article36 published in August, 2017, in the country’s national newspaper. This article reported on the difficulty of getting the populations of four villages in Boulkiemdé, two of which were part of our study, to improve sanitation and to use latrines.36 Further studies are needed to not only confirm these hypotheses but also to tailor interventions to sociocultural and regional contexts to maximise intervention effectiveness across populations, even within a small area of the same country.
A noteworthy finding is that the intervention was effective at increasing the proportion of concessions that built latrines, with a stronger effect in Nayala than in Sanguié and Boulkiemdé. Convincing people to build latrines is challenging, particularly in resource-poor communities, but our intervention of combining PHAST with the movie and structured discussion seemed to be effective. Although substantial additional work is needed to make improved sanitation available at a wider scale, particularly in sub-Saharan Africa, the effectiveness of a relatively low-cost intervention is promising. Indeed, development of the intervention cost US$31 538, which included the costs of movie production, comic book production, training of field team members in SARAR-PHAST, and adaptation of the SARAR-PHAST approach to human cysticercosis. These costs did not include the fees associated with delivery of the intervention.
Two articles recently reviewed the effectiveness of interventions to control cysticercosis,6,18 with one focusing on interventions implemented at the community level.6 Few cluster intervention studies done to date have used an appropriate study design or analyses to obtain a valid effectiveness estimate. Nonetheless, our findings agree with those of another cluster-randomised controlled trial, which used PRECEDE-PROCEED to develop a training programme targeting pig farmers in Tanzania.8 In that study, the incidence of porcine cysticercosis was reduced by 43% over a median follow-up of 4 months. Our study showed an effect on human cysticercosis in the longer term and, in contrast to the study in Tanzania,8,37 we found an increase in latrine construction in the intervention villages. This result is not surprising since our study emphasised sanitation, reinforced by the use of PHAST to build self-efficacy in implementation of T solium control measures. By contrast, the intervention in Tanzania used an information, education, and communication approach and did not seek to increase community self-efficacy.32 Similarly, a study in Zambia assessed the effectiveness of community-led total sanitation in reducing prevalence of porcine cysticercosis through a pre-post intervention assessment. Full results are yet to be published, but a preliminary assessment indicated a non-significant increase in the prevalence of porcine cysticercosis and decrease in latrine use after the intervention and a significant increase in the presence of latrines (p=0·027).13 These results are similar to those of our study since we observed an increase in the prevalence of human cysticercosis from baseline to after randomisation, even in the intervention group. However, neither the Tanzania study nor the Zambia study measured the effect of the intervention on human cysticercosis. Future analyses will estimate the effectiveness of our intervention on porcine cysticercosis.
A limitation of our study was that the baseline prevalence of active cysticercosis was imbalanced between the control and intervention villages among individuals providing serum samples at all three visits. However, this imbalance was substantially less marked among all participants providing a blood sample at baseline. Additionally, the results were robust when data on seroconversion were analysed, for which the pre-randomisation data were well balanced between the two groups. It is therefore unlikely that this imbalance biased the results. Another limitation was the large number of individuals lost to follow-up during the 3 years of the study because of the discovery of gold in the area. To avoid losing too many participants, we replaced those who had moved away by another member of the same household. We were able to analyse data from all individuals who provided serum samples at least once, and the results were similar to those from the group providing three serum samples. This observation suggests that little bias was introduced by replacement of participants. Finally, we were unable to spend additional time in each intervention village because of budgetary and personnel constraints, despite requests from the villagers. However, we believe this approach could be implemented at a reasonably low cost on a larger scale and be sustainable in areas where the community has a similar structure to those of Nayala and Sanguié. Elsewhere, further implementation research will be needed to assess how best to work with the community to control cysticercosis.
Several lessons were learnt during the conduct of this large cluster-randomised controlled trial in one of the poorest countries of the world. First, finding personnel willing to spend 3 years in rudimentary conditions while getting salaries on par with those for a small research project was a challenge. Qualified personnel have become accustomed to receiving larger salaries38 and on a daily basis for any type of travel39 for large international projects, which initially hampered our ability to find competent personnel. However, we were fortunate to eventually find a group of individuals dedicated to improving the health of these rural populations. Second, we were denied access to one of the villages during the last visit because of infighting among the local authorities. Although we did involve traditional leadership (eg, chiefs and religious leaders) to overcome some hurdles in the conduct of the study and implementation of the intervention, perhaps stronger involvement from these leaders could have further improved participation and the effectiveness of our intervention. Indeed, involvement of such traditional leadership was shown to improve the effectiveness of community-led total sanitation in Zambia.40 However, this involvement needs to be managed carefully so as not to create further infighting among local authorities. Third, we were unprepared for the substantial sociocultural variations among provinces and the different ways in which the villagers responded to the field teams. In hindsight, there should have been more careful consideration of the observed variations in knowledge, attitudes, and practices, in the qualitative perceptions and beliefs, and the risk factors for cysticercosis prevalence28 among the three provinces24 to adapt the intervention to each context. Finally, future cluster-randomised controlled trials of this scale done in resource-poor settings should consider the use of a pre-post block randomised design such as ours to control for the potential spatial and temporal change of various factors which could affect the results. One element that we had not planned for was the discovery of gold in the area and the subsequent large movement of the local populations. Future studies should carefully plan for the need to replace individuals who migrate unexpectedly. One must be willing to make incremental compromises to conduct such a study while minimising the effect on validity. Bayesian methods were helpful in dealing with such replacements, as this approach ensures that outcome information can be imputed from risk factors collected at baseline.
Supplementary Material
Research in context.
Evidence before this study
We used two review papers published in 2014 and 2017 to assess the available evidence about the effectiveness of community-based interventions in controlling cysticercosis. We re-ran the MEDLINE search that we had used in the 2014 publication using MeSH terms “Taenia solium” or “cysticercosis” and publication type “randomised controlled trial” without restriction for date or language on Aug 17, 2017. We also re-ran a search keeping the MeSH terms combined with the keywords (“control” OR “intervention” OR “treatment” OR “vaccination” OR “trial”) AND (“community” OR “village”* OR “municipality” OR “household” OR “farm”). We found 18 original studies assessing the effect of an intervention implemented at the community level on the frequency of porcine or human cysticercosis, neurocysticercosis, or human taeniasis. Only two trials followed CONSORT guidelines in the design and reporting of their studies, and none reported on the effectiveness of their approach in controlling human cysticercosis. The available literature generally does not have adequate control groups and randomisation, and does not have large numbers of clusters, making the evidence weak.
Added value of this study
Our study used a rigorous study design and included 60 randomised villages, of which 58 were included in the analysis. To our knowledge, this is the largest cluster-randomised controlled trial done to date for the control of human cysticercosis. We found that a community-based drug-free intervention tended towards a reduction in the cumulative incidence and prevalence of human cysticercosis overall. The intervention had a stronger effect in two of the three provinces studied, but almost no effect in the third. Moreover, we showed that our intervention led to an increase in the proportion of households with recently built latrines.
Implications of all the available evidence
We developed a low-cost, culturally appropriate intervention that was successful in controlling human cysticercosis in two of the three study provinces. Furthermore, our results indicate that the intervention was effective in promoting the building of latrines in households. This outcome in itself is noteworthy given the ongoing global challenge of finding appropriate and low-cost interventions that will lead to community investments in rural sanitation, particularly in low-resource settings such as Burkina Faso. Because of its low cost and low technological nature, our intervention could be disseminated and implemented at a larger scale. Further research is needed to investigate the cultural and contextual adaptations essential for ensuring the effectiveness of this intervention at a broader scale and to test strategies for its dissemination and translation into local programmes and policy. Future studies that examine the effectiveness of this intervention on human cysticercosis and rural sanitation outcomes at a larger scale could provide much-needed evidence for policy makers and the global health community at large.
Acknowledgments
ACK reports grants from Institut de Recherche en Sciences de la Santé/Direction Régionale de l’Ouest (IRSS/DRO). J-BO reports personal fees from IRSS-DRO during the conduct of the study.
This work was done with support from the National Institute of Neurological Disorders and Stroke and the Fogarty International Center of the US National Institutes of Health under the Brain Disorders in the Developing World: Research Across the Lifespan programme (grant R01NS064901). HC received partial funding from the National Institute of General Medical Sciences (U54 GM104938). IS received funding from the National Institute of Neurological Disorders and Stroke at the US National Institutes of Health (grant F31NS093983). We are indebted to all participants in this study who took time to answer our many questions and who consented to provide blood samples over a period of 3 years. We also thank all members of the field team who lived in very basic conditions for 36 months while completing all components of this study. Finally, this analysis would have not been possible without the support of Henri Somé who programmed all personal digital assistants and carefully managed all databases to minimise any possible errors.
Footnotes
Contributors
HC, AM, LDC, P-MP, J-BO, RC, M-PB-M, and RG designed the study, interpreted the results, and reviewed the manuscript (LDC died at the end of 2014 and hence was only involved in the design phase). HC, AM, and RG managed the study. HC and CB developed the Bayesian models and HC ran all models. HC cleaned and analysed all descriptive statistics. RG and AM oversaw the consent process, data collection, and management. ACK did all serological analyses. SG, PD, and ZT oversaw all serological analyses. HAN, HC, AM, TS, and RG interpreted the pilot study and knowledge, attitudes, and practices data to develop key messages for the movie and adaptation of SARAR PHAST. ALS helped with interpretation of the implementation research method. VD helped with data cleaning, literature review, and interpretation of results. IS developed the maps. All authors reviewed and approved the final version of the manuscript.
Declaration of interests
All other authors declare no competing interests.
References
- 1.Torgerson PR, Devleesschauwer B, Praet N, et al. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Med. 2015;12:e1001920. doi: 10.1371/journal.pmed.1001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carabin H, Krecek RC, Cowan LD, et al. Estimation of the cost of Taenia solium cysticercosis in Eastern Cape Province, South Africa. Trop Med Intl Health. 2006;11:906–16. doi: 10.1111/j.1365-3156.2006.01627.x. [DOI] [PubMed] [Google Scholar]
- 3.Praet N, Speybroeck N, Manzanedo R, et al. The disease burden of Taenia solium cysticercosis in Cameroon. PLoS Negl Trop Dis. 2009;3:e406. doi: 10.1371/journal.pntd.0000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattarai R, Budke CM, Carabin H, et al. Quality of life in patients with neurocysticercosis in Mexico. Am J Trop Med Hyg. 2011;84:782–86. doi: 10.4269/ajtmh.2011.10-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattarai R, Budke CM, Carabin H, et al. Estimating the non-monetary burden of neurocysticercosis in Mexico. PLoS Negl Trop Dis. 2012;6:e1521. doi: 10.1371/journal.pntd.0001521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carabin H, Traore AA. Taenia solium taeniasis and cysticercosis control and elimination through community-based interventions. Curr Trop Med Rep. 2014;1:181–93. doi: 10.1007/s40475-014-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia HH, Gonzalez AE, Gilman RH, et al. Combined human and porcine mass chemotherapy for the control of T. solium. Am J Trop Med Hyg. 2006;74:850–55. [PubMed] [Google Scholar]
- 8.Ngowi HA, Carabin H, Kassuku AA, Mlozi MRS, Mlangwa JED, Willingham AL. A health-education intervention trial to reduce porcine cysticercosis in Mbulu District, Tanzania. Prev Vet Med. 2008;85:52–67. doi: 10.1016/j.prevetmed.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10:28–55. doi: 10.1016/j.ijsu.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Mwidunda SA, Carabin H, Matuja WB, Winkler AS, Ngowi HA. A school based cluster randomised health education intervention trial for improving knowledge and attitudes related to Taenia solium cysticercosis and taeniasis in Mbulu district, northern Tanzania. PLoS One. 2015;10:e0118541. doi: 10.1371/journal.pone.0118541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia HH, Gonzalez AE, Tsang VC, et al. Elimination of Taenia solium transmission in Northern Peru. N Engl J Med. 2016;374:2335–44. doi: 10.1056/NEJMoa1515520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Neal SE, Moyano LM, Ayvar V, et al. Ring-screening to control endemic transmission of Taenia solium. PLoS Negl Trop Dis. 2014;8:e3125. doi: 10.1371/journal.pntd.0003125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bulaya C, Mwape KE, Michelo C, et al. Preliminary evaluation of community-led total sanitation for the control of Taenia solium cysticercosis in Katete district of Zambia. Vet Parasitol. 2015;207:241–48. doi: 10.1016/j.vetpar.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 14.Okello AL, Thomas L, Inthavong P, et al. Assessing the impact of a joint human-porcine intervention package for Taenia solium control: results of a pilot study from northern Lao PDR. Acta Trop. 2016;159:185–91. doi: 10.1016/j.actatropica.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Braae UC, Magnussen P, Ndawi B, Harrison W, Lekule F, Johansen MV. Effect of repeated mass drug administration with praziquantel and track and treat of taeniosis cases on the prevalence of taeniosis in Taenia solium endemic rural communities of Tanzania. Acta Trop. 2017;165:246–51. doi: 10.1016/j.actatropica.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Kyvsgaard NC, Johansen MV, Carabin H. Simulating transmission and control of Taenia solium infections using a Reed-Frost stochastic model. Int J Parasitol. 2007;37:547–58. doi: 10.1016/j.ijpara.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Winskill P, Harrison WE, French MD, Dixon MA, Abela-Ridder B, Basanez MG. Assessing the impact of intervention strategies against Taenia solium cysticercosis using the EPICYST transmission model. Parasit Vectors. 2017;10:73. doi: 10.1186/s13071-017-1988-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabriël S, Dorny P, Mwape KE, et al. Control of Taenia solium taeniasis/cysticercosis: the best way forward for sub-Saharan Africa? Acta Trop. 2017;165:252–60. doi: 10.1016/j.actatropica.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Drabo F, Ouedraogo H, Bougma R, et al. Successful control of soil-transmitted helminthiasis in school age children in burkina faso and an example of community-based assessment via lymphatic filariasis transmission assessment survey. PLoS Negl Trop Dis. 2016;10:e0004707. doi: 10.1371/journal.pntd.0004707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabak RG, Khoong EC, Chambers DA, Brownson RC. Bridging research and practice: models for dissemination and implementation research. Am J Prev Med. 2012;43:337–50. doi: 10.1016/j.amepre.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO Regional Officie of the Eastern Mediterranean. Health education: theoretical concepts, effective strategies and core competencies: a foundtion document to guide capacity development of health educators. Cairo, Egypt: World Health Organization, Regional Office for the Eastern Mediterranean; 2012. [Google Scholar]
- 22.Rimer BK, Glanz K, Rasband G. Searching for evidence about health education and health behavior interventions. Health Educ Behav. 2001;28:231–48. doi: 10.1177/109019810102800208. [DOI] [PubMed] [Google Scholar]
- 23.Ministère des Ressources Animales. Annuaire Statistique du Secteur de l’Élevage. Burkina Faso: Ministère des Ressources Animales; 2010. [Google Scholar]
- 24.Ngowi H, Ozbolt I, Millogo A, et al. Development of a health education intervention strategy using an implementation research method to control taeniasis and cysticercosis in Burkina Faso. Infect Dis Poverty. 2017;6:95. doi: 10.1186/s40249-017-0308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carabin H, Millogo A, Praet N, et al. Seroprevalence to the antigens of Taenia solium cysticercosis among residents of three villages in Burkina Faso: a cross-sectional study. PLoS Negl Trop Dis. 2009;3:e555. doi: 10.1371/journal.pntd.0000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nitiema P, Carabin H, Hounton S, et al. Prevalence case-control study of epilepsy in three Burkina Faso villages. Acta Neurol Scand. 2012;126:270–78. doi: 10.1111/j.1600-0404.2011.01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sing G, Prabhakar S. Taenia solium cysticercosis: from basic to clinical science. Wallingford, NY: CABI; 2002. [Google Scholar]
- 28.Carabin H, Millogo A, Cisse A, et al. Prevalence of and factors associated with human cysticercosis in 60 villages in three provinces of Burkina Faso. PLoS Negl Trop Dis. 2015;9:e0004248. doi: 10.1371/journal.pntd.0004248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green LW, Kreuter M. Health program planning: an educational and ecological approach. 4. New York, NY: McGraw-Hill Higher Education; 2005. [Google Scholar]
- 30.Ganaba R, Praet N, Carabin H, et al. Factors associated with the prevalence of circulating antigens to porcine cysticercosis in three villages of Burkina Faso. PLoS Negl Trop Dis. 2011;5:e927. doi: 10.1371/journal.pntd.0000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skinner C, Tiro J, Champion VL. The health belief model. In: Glanz K, Rimer B, Viswanath K, editors. Health behavior. San Francisco, CA: Jossey-Bass; 2015. [Google Scholar]
- 32.WHO, World Bank Water and Sanitation Program. A new approach to working with communities. Geneva: World Health Organization; 1997. The PHAST initiative. Participatory hygiene and sanitation transformation. [Google Scholar]
- 33.Dorny P, Phiri IK, Vercruysse J, et al. A Bayesian approach for estimating values for prevalence and diagnostic test characteristics of porcine cysticercosis. Int J Parasitol. 2004;34:569–76. doi: 10.1016/j.ijpara.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Garcia HH, Gonzalez AE, Gilman RH, et al. Short report: transient antibody response in Taenia solium infection in field conditions— a major contributor to high seroprevalence. Am J Trop Med Hyg. 2001;65:31–32. doi: 10.4269/ajtmh.2001.65.31. [DOI] [PubMed] [Google Scholar]
- 35.Fuller D, Potvin L. Context by treatment interactions as the primary object of study in cluster randomized controlled trials of population health interventions. Int J Public Health. 2012;57:633–36. doi: 10.1007/s00038-012-0357-x. [DOI] [PubMed] [Google Scholar]
- 36.Compaoré J. Rapport à la saleté, stratégies de communication et adoption des latrines dans la commune urbaine de Koudougou au Burkina Faso. [accessed Aug 30, 2017];Lefasonet. 2017 Aug 24; http://lefaso.net/spip.php?article79010.
- 37.Ngowi HA, Mlangwa JED, Mlozi MRS, et al. Implementation and evaluation of a health-promotion strategy for control of Taenia solium infections in northern Tanzania. Int J Health Promot Educ. 2009;47:24–34. [Google Scholar]
- 38.Lavigne Delville PA, Abdelkader A. Les mécanismes et les impacts de l’aide vus par des practiciens Nigériens. Niamey, Niger: Laboratoire d’Études et Recherches sur les Dynamiques Sociales et le Développement Local (LASDEL); 2010. A cheval donné, on ne regarde pas les dents. [Google Scholar]
- 39.Ridde V. Per diems undermine health interventions, systems and research in Africa: burying our heads in the sand. Trop Med Int Health. 2010;15:e1–4. doi: 10.1111/tmi.2607. [DOI] [PubMed] [Google Scholar]
- 40.Tiwari A, Russpatrick S, Hoehne A, et al. Assessing the Impact of leveraging traditional leadership on access to sanitation in rural Zambia. Am J Trop Med Hyg. 2017;97:1355–61. doi: 10.4269/ajtmh.16-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.