Abstract
To unveil novel global changes associated with corpus luteum (CL) maturation, we analyzed transcriptome data for the bovine CL on days 4 and 11, representing the developing vs. mature gland. Our analyses revealed 681 differentially expressed genes (363 and 318 on day 4 and 11, respectively), with ≥2 fold change and FDR of <5%. Different gene ontology (GO) categories were represented prominently in transcriptome data at these stages (e.g. days 4: cell cycle, chromosome, DNA metabolic process and replication and on day 11: immune response; lipid metabolic process and complement activation). Based on bioinformatic analyses, select genes expression in day 4 and 11 CL was validated with quantitative real-time PCR. Cell specific expression was also determined in enriched luteal endothelial and steroidogenic cells. Genes related to the angiogenic process such as NOS3, which maintains dilated vessels and MMP9, matrix degrading enzyme, were higher on day 4. Importantly, our data suggests day 11 CL acquire mechanisms to prevent blood vessel sprouting and promote their maturation by expressing NOTCH4 and JAG1, greatly enriched in luteal endothelial cells. Another endothelial specific gene, CD300LG, was identified here in the CL for the first time. CD300LG is an adhesion molecule enabling lymphocyte migration, its higher levels at mid cycle are expected to support the transmigration of immune cells into the CL at this stage. Together with steroidogenic genes, most of the genes regulating de-novo cholesterol biosynthetic pathway (e.g HMGCS, HMGCR) and cholesterol uptake from plasma (LDLR, APOD and APOE) were upregulated in the mature CL. These findings provide new insight of the processes involved in CL maturation including blood vessel growth and stabilization, leucocyte transmigration as well as progesterone synthesis as the CL matures.
Introduction
The ovulatory surge of gonadotropins triggers extensive structural, cellular, and molecular changes in the preovulatory follicle, leading to ovulation and corpus luteum (CL) formation [1, 2]. The new CL develops from cells that remain in the follicular wall following ovulation but is eventually composed of multiple, distinctive cell types including luteal steroidogenic cells (LSC; small and large cells that originate from theca and granulosa, respectively) and non-steroidogenic cells (luteal endothelial cells—LEC; pericytes, fibrocytes, and immune cells) [3–6]. Various immune cells such as lymphocytes, macrophages, neutrophils, eosinophils, and dendritic cells are identified in the mature CL [7–11]. Yet, the molecules responsible for this massive infiltration of immune cells, much before luteolysis, are still poorly characterized. Small and large luteal cells have distinct characteristics [12, 13] but they are both engaged in progesterone production. Progesterone dramatically increases after the LH surge and CL formation reaching a plateau at the mid luteal stage that is maintained until days 15–16 of the cycle [14]. Cholesterol is the precursor for steroid hormone synthesis including progesterone; it can be derived from the diet through low-density lipoprotein receptor (LDLR) mediated uptake or is synthesized de novo [15, 16], but only few studies examined cholesterol synthesis in the CL [17–19] Along with increased steroidogenesis in the developing CL, robust angiogenesis takes place resulting in a highly vascular gland with LEC comprising the larger part of its cells, more than 50% [20–22]. The development of an elaborate network of blood vessels in the CL endows this gland with one of the highest blood flow per unit mass in the body [23], which guarantees the necessary supply of nutrients and hormones allowing for its proper function. The short period of angiogenesis is later followed by maintenance and stabilization of the vasculature in the fully active CL. The information described above is derived from numerous studies analyzing morphometric, biochemical and molecular changes associated with the maturation of the CL [5, 13, 24].
To unveil novel regulatory mechanisms and gain more comprehensive knowledge of the changes that accompany CL maturation, we analyzed transcriptome data for the bovine CL on early vs mature CL (days 4 and 11, respectively). Based on bioinformatic analyses, the profile of select gene expression in these CL and in isolated luteal cell types (endothelial and steroidogenic) was validated with quantitative real-time PCR (qPCR).
Materials and methods
Bioinformatic analyses
Our previously published dataset (accession no. GSE23348; Gene Expression Omnibus) was re-analyzed in order to compare the transcriptomes of early and mid-stage bovine corpora lutea. Microarray analysis was performed using the PartekGenomics Suite, version 6.5, 2010 (http://www.partek.com). Data was normalized and summarized using the robust multi-average method, followed by analysis of variance (ANOVA) for differentially expressed genes (DEG). Cluster analysis of the DEG (cutoffs: p-value ≤ 0.05 and absolute fold-change ≥ 2) was also performed. GO (Gene Ontology) analysis of the DEG was performed using the DAVID online application: https://david.ncifcrf.gov/. In order to obtain the most meaningful clusters, the threshold of EASE score (a modified Fisher exact p-value) for gene enrichment analysis was set to ≤ 0.05. The p-values were corrected for multiple comparisons using the Benjamini and Hochberg [25] method. An additional GO analysis was performed with Ontologizer 2.0 [26], using the term-for-term algorithm. (Term-for-Term), GO annotation files (gene_association.goa_ref_cow.gz and gene_association.goa_cow.g) were downloaded from http://beta.geneontology.org/page/download-mappings in October 2014. Ontologizer recognized 19,930 gene symbols from the background and 336 and 294 gene symbols of up- and down-regulated (p < 0.05; fold-change ≥ 2, Benjamini–Hochberg <5%) gene sets, respectively. For additional functional analysis we used Ingenuity pathway analysis (IPA): A list of DEG with associated fold change values (fold change |≥ 2|) was uploaded into the IPA server (http://www.ingenuity.com/). Using information stored in the Ingenuity Knowledge Base (IKB), genes were mapped to networks and pathways. The significance of the association between genes/networks/pathways was evaluated by right-tailed Fischer’s exact test (p<0.05). The IPA networks are ranked by a score derived from a p-value and indicates the likelihood of the focus genes appearance in a network, computed as p-score = -log10 (p-value).
If not otherwise mentioned biochemical were from Sigma-Aldrich Israel Ltd and tissue culture materials were from Biological Industries, Kibbutz Beit Haemeek, Israel.
Animals and CL collection
All animal procedures were approved by the All University Committee on Animal Use and Care at Michigan State University. Thirty Angus crossbred heifers (average body wt 395 ± 7 kg) were synchronized for estrus with two 25-mg injections of PG (Lutalyse; Pfizer, Kalamazoo, MI) 11 days apart. Heifers were monitored four times daily for behavioral estrus (day 0), and ovulation was verified by transrectal ultrasonography (Aloka 500V with a 7.5-MHz linear transducer; Aloka, Wallingford, CT) on day 1. CL collection was described in detail in our previous publication [27, 28]. Briefly, CL were collected after epidural anesthesia on Day 4 or Day 11 (n = 5 for each stage) of the estrous cycle. CL were diced and snap frozen in liquid nitrogen and stored at −80°C until RNA extraction with RNAeasy kits. Total RNA was subjected to reverse transcription for synthesis of cDNA, which served as a template for synthesis of full-length biotin labeled cRNA with the GeneChip HT One-Cycle Target Labeling and Controls Kit (Affymetrix, Santa Clara, CA.).
Isolation and culture of luteal cells
Luteal cells were dispersed and enriched as previously described [27, 29, 30]. Briefly, corpora lutea at the mid-luteal phase (day 9–14) were dispersed using collagenase. Dispersed cells were suspended in 1% BSA in M-199, mixed with magnetic tosyl activated beads pre-coated with BS-1 lectin (0.15 mg/ml) from bovine endothelial cells and incubated for 25 min at 4 °C on a rocking platform. The adherent cells (BS-1 positive) were washed with M-199 containing 1% BSA and concentrated using a magnet until the supernatant was free of BS-1 negative cells. The adherent cells were subsequently eluted by 0.2 M lactose solution in PBS. Freshly isolated BS-1-positive cells (enriched LEC) and non-adherent cells (BS-1 negative-enriched luteal steroidogenic cells; LSC) were collected for RNA extraction.
Isolation and culture of granulosa cells
Bovine granulosa cells were isolated from ovaries collected at a local slaughterhouse as previously described [12]. Only large follicles (>10 mm in diameter) containing ≥ 4 million viable cells were used. Granulosa cells were enzymatically dispersed using a mixture of collagenase type IA (5000 units), hyaluronidase III (1440 units), DNase I (390 units), and cultured overnight in DMEM/F12 containing 3% fetal calf serum (FCS), 2mM L-glutamine, penicillin (100U/ml) /streptomycin (1mg/ml) solution.
RNA extraction and qRT-PCR
Total RNA was isolated from enriched luteal cells and granulosa cells using TriFast reagent (Peqlab Biotechnologie GmbH) according to the manufacturer’s instructions. 1μg of total RNA was reverse transcribed using M-MuLV Reverse Transcriptase (200units/μl), M-MuLV RT Buffer (New England Biolabs, Ipswich, MA, USA), random primer (100nM), oligo-dT (100 μM) and dNTPs mix (100 mM) (Bioline Reagents Limited, London, UK). qRT-PCR were performed using the LightCycler 96 SW 1.1 software (Roche Diagnostics Corporation, Indianapolis, IN, USA) and Platinum SYBR Green (SuperMix; Invitrogen) as previously described [31]. Gene expression (NOTCH4, MMP9, JAG1, CD300LG, HMGCS1, HMGCR, SC5DL, and NOS3) was analyzed by qRT-PCR. The Ribosomal Protein S26 (RPS26) gene was used as the normalizing gene. Sequences of primers used for qRT-PCR are listed in Table 1. All primers were designed to have single-product melting curves, as well as consistent amplification efficiencies of >96% [32]. The threshold cycle number (Ct) was used to quantify the relative abundance of the gene; arbitrary units were calculated as 2−ΔCt = 2− (Ct target gene−Ct housekeeping gene).
Table 1. Genes, sequences and Genbank accessions of the primers used in qPCR.
| Gene symbol | Primer | Sequence | Accession # |
|---|---|---|---|
| CD300LG | Forward | gatgaagagcccggcctct | XM_002696037 |
| Reverse | cttgtgctcccaggttacg | ||
| HMGCS1 | Forward | agctcttgggatggacggtatg | NM_001206578 |
| Reverse | cggctccaactccacctgta | ||
| SC5DL | Forward | tcccgttacacaagacatcctgg | NM_001035356 |
| Reverse | cgtgcatcctgtacacgtggctg | ||
| HMGCR | Forward | tggcatcacctgacctggac | NM_001105613 |
| Reverse | tggcttgagacgcctgaagg | ||
| MMP9 | Forward | gagagggtcgcaatgatg | NM_174744 |
| Reverse | ctggcacggaggtgtgatcta | ||
| JAG1 | Forward | tcctacactttgctcgtggag | NM_001191178 |
| Reverse | acttattgcagccgaagcc | ||
| NOTCH4 | Forward | caggccatctctgtgaaattc | NM_001206948 |
| Reverse | ggtggcaggtgcagttgtctt | ||
| NOS3 | Forward | cctcaccgctacaatatcct | NM_181037 |
| Reverse | tgctcgttgtccaggtgcttc | ||
| RPS26 | Forward | ccaactgtgcccgatgtg | NM_001015561 |
| Reverse | ttcccgagagcgattcctga |
Statistical analyses
All statistical analyses were conducted using GraphPad Prism version 4.03 Software (GraphPad Software). Data are presented as the means of ±S.E.M; cell culture experiments were repeated at least three times; Asterisks represent significant differences between groups. *P<0.05, **P<0.01, ***P<0.001. Additional information is provided in figure legends.
Results
Identification of differentially expressed genes and functional analyses in day 4 and day 11 CL
To identify the gene expression that differ in the early (day 4) and mid-luteal (day 11) stages we reanalyzed a previously described microarray dataset [27, 28]. Initially hierarchical clustering (Fig 1) of the microarray results was carried out using the Partek Genomics Suite. High uniformity was displayed between the Affymetrix chips interrogated with samples within each time point (n = 5 per day). 681 DEG, with |≥ 2| fold change and FDR of <5% were found. About half of these genes (363) were expressed at a higher level in the early luteal stage compared to the mid luteal stage, while 318 genes had a lower expression level in the early luteal stage (day 4 of the cycle) as compared to the mid luteal stage (day 11 of the cycle).
Fig 1. Clustering of bovine CL DEG on day 4 vs. day 11 using hierarchical clustering performed with Partek Genomics Suite.
The Hierarchical clustering of 681 expressed genes (fold-change |≥ 2|, FDR of <5%) in day 4 and day 11 CL. Each column represents an Affymetrix chip (n = 10) and each row represents a gene. The deep red color represents relative upregulated expression, while the deep blue color represents relative down regulated expression.
Next, we performed functional enrichment analysis using David clustering and Ontologizer 2.0 tools on each gene list (day 4 and day 11). DEG were classified into 70 and 37 enriched GO terms in the early and mid-luteal stages, respectively (S1 File). On day 4, the cluster enriched at the highest significance level, including 38 genes (>10% of the tested genes), was cell cycle (GO: 0007049) with a p-value of 3.10 e-20 (all p-values presented here are after correction according to Benjamini and Hochberg [25]). Other selected clusters that were significantly enriched in day 4 CL data were: chromosome (GO: 0005694), DNA metabolic process (GO: 0006259), nucleoside binding (GO: 0001882) and DNA replication (GO: 0006260). Genes with higher mRNA abundance in day 11 CL were clustered into the following GO terms: pattern binding (GO: 0001871), immune response (GO: 0006955), lipid metabolic process (GO: 0006629), lysosome (GO: 0005764) and complement activation (GO: 0006956) (Fig 2).
Fig 2. Enriched Gene-Ontology (GO terms) in day 4 vs. day 11 bovine CL.
GO term analysis (using DAVID) of DEG (fold-change |≥ 2|, FDR of <5%) was carried out. The X-axis are selected GO terms and the Y-axis are −log10 (p-value) enrichment score of the GO terms. The numbers above bars are the numbers of DEG assigned to each GO terms.
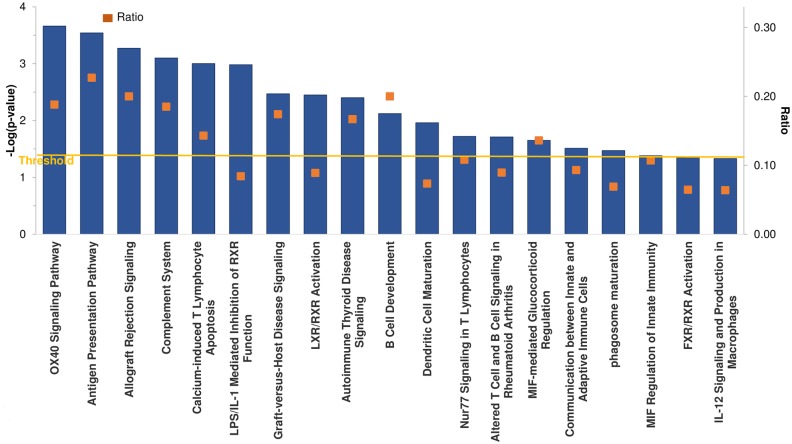
Pathway analysis of array data was performed using IPA software. From the 318 DEG identified in day 11 CL, 41 significant (p< 0.05) canonical pathways were found; ~50% were immune related. They included pathways such as the OX40 (member of the TNF receptor family, expressed on activated CD4+ T cells and CD8+ T cells) signaling pathway, antigen presentation pathway, B cell development, complement system, and dendritic cell maturation (Fig 3).
Fig 3. Up-regulated immune canonical pathways in day 11 CL, performed using Ingenuity Pathway Analysis (IPA) for immune pathways in day 11 vs. day 4 CL.
The vertical axis (left) shows the −log of the p-value calculated based on Fisher’s exact test. The ratio (vertical axis, right) is calculated by the number of genes in a given pathway that meet cutoff criteria, divided by the total number of genes that make up that pathway. The orange line stands for the threshold above which there are statistically significantly values (by default P<0.05).
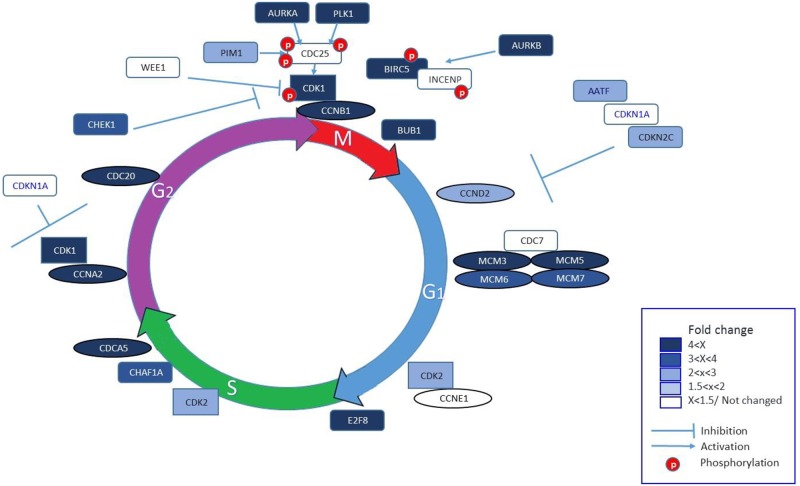
To demonstrate DEG related to cell cycle of greater abundance in day 4 CL, selected genes that were significantly upregulated using Ontologizer 2.0 (fold change >2; p<0.05) were marked on cell cycle diagram and colored according to their fold change (Fig 4). It shows genes involved in the G1/S transition, progression through S, G2 and M- phases. Genes include cyclins and their CDKs (Cyclin-dependent kinases), CDKs inhibitors, MCMs (mini-chromosome maintenances), CDCs (cell division cycles), cell cycle associated kinases (PLK1, AURKA, AURKB, PIM1), and survival/apoptosis factors (BIRC5/survivin, AAFT) (Fig 4).
Fig 4. Illustration of differentially expressed cell cycle genes in early CL.
The diagram depicts up-regulated genes that were differentially expressed (p<0.05) in day 4 vs. 11 CL. Genes appear in the diagram in relation to cell cycle steps. Intensity of the shading increases with the magnitude of the change (see inset).
Pattern of endothelial/blood vessel gene expression in day 4 and 11 CL
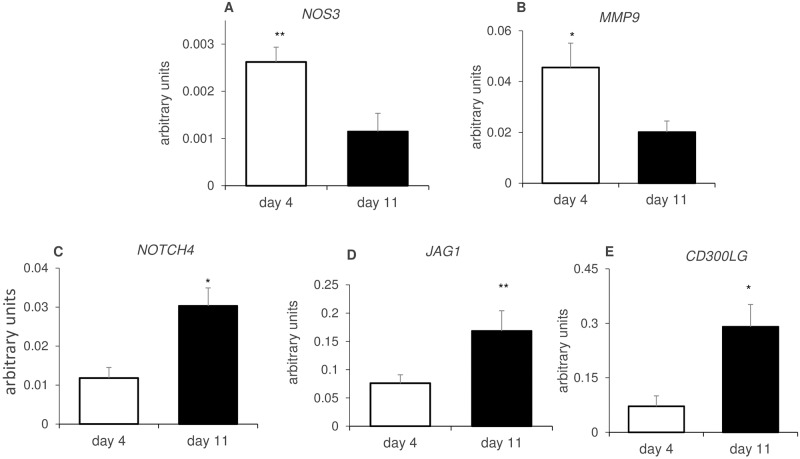
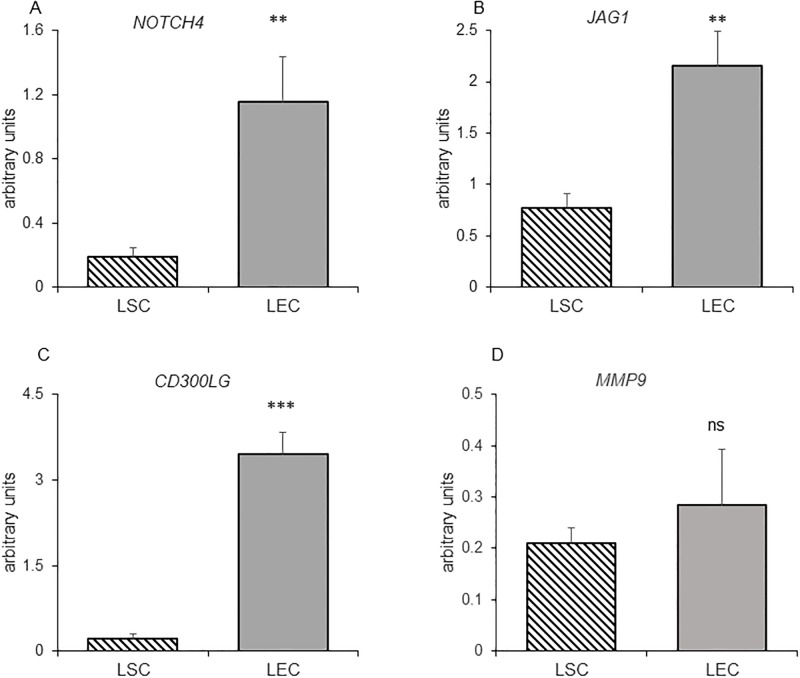
Microarray results revealed genes related to endothelial/blood vessel development and function that were highly expressed during the early luteal phase such as: NOS3 (2.4 fold), SELE (4.7 fold), MMP9 (2.4 fold) while others were highly expressed at mid-luteal stage: CD300LG (3.2 fold), TIE1 (2.1 fold), NOTCH4 (2.3 fold) and JAG1 (2 fold). The expression profile of several of these genes were validated by qRT-PCR, it was found that fold changes measured by qPCR were in excellent agreement with microarray results (Fig 5). The mRNA levels of NOS3 (Fig 6A) and MMP9 (Fig 6B) were significantly higher (2.3 and 2.3 fold, respectively) on day 4 as compared to day 11, again in accordance with microarray analyses (Fig 5). Oppositely, the mRNA levels of NOTCH4, JAG1 and CD300LG (Fig 6C, 6D and 6E) were significantly higher (2.7, 2.4 and 4.1 fold, respectively) in day 11 CL. Then the expression of these genes was determined in two isolated luteal cell types: endothelial and steroiodgenic cells representing major cell populations of the CL. These three genes showed preferential endothelial cell expression, with LEC/LSC ratio of 6.4, 2.8 and 16.4, respectively (Fig 7A–7C). MMP9 did not show any preferential cell distribution (Fig 7D), while endothelial gene markers SELE and NOS3 were localized to EC, as reported before [33–35].
Fig 5. Validation of microarray data with qPCR analysis.
Fold change (day 4 vs day 11 CL) of DEG (microarray analyses, dark bars) compared with qPCR results (open bars). Positive and negative values represent up and down-regulated genes, respectively.
Fig 6. Differential expression of endothelial/blood vessel genes during CL development.
mRNA levels of (A) NOS3, (B) MMP9, (C) NOTCH4, (D) JAG1 and (E) CD300LG in the early (day 4) and mid (day 11) luteal stages. Levels of mRNA were measured by qRT-PCR and normalized to RPS26 in the same samples. The results are presented as means ± SEM. Data were obtained from 5 cows for each luteal stage. Asterisks indicate significant differences between day 4 and day 11; *P<0.05, **P < 0.01, ***P < 0.001. The arbitrary units (Y axis) were multiplied by 100.
Fig 7. Luteal cell specific expression of NOTCH4, JAG1, CD300LG and MMP9.
(A) NOTCH4, (B) JAG1 (C) CD300LG and (D) MMP9 mRNA in LSC -luteal steroidogenic cells and LEC–luteal endothelial cells enriched from mid cycle CL. Levels of mRNA were measured by qRT-PCR and normalized to RPS26 in the same samples. The results are presented as means ± SEM; n = 9. Asterisks indicate significant differences between days 4 and 11; *P<0.05, **P < 0.01, ***P < 0.001. The arbitrary units (Y axis) were multiplied by 100.
Steroidogenic and cholesterol biosynthetic genes upregulated in day 11 vs day 4 CL
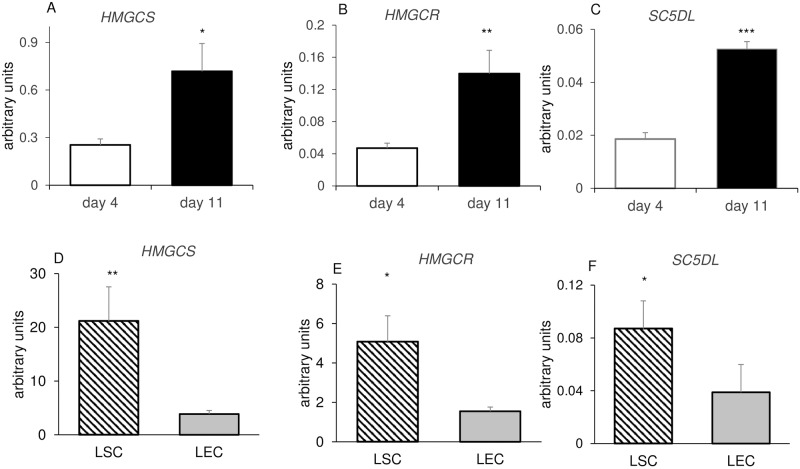
Data presented in Fig 2 showed significant enrichment of the lipid metabolic process GO term on day 11. We noted that the DEG list on day 11 contained many genes involved in the cholesterol biosynthetic pathway. Some of those genes had 1.5–2 fold difference, therefore, our analysis was expanded to include genes whose fold change was ≥1.5. The cholesterol biosynthetic pathway contains 20 genes [36, 37], 13 of which were upregulated significantly on day 11 vs. day 4 of the cycle based on microarray data analysis (Fig 8). These enzymes include TM7SF2 (4 fold), CYP51A1 (1.8 fold), NSDHL (1.7 fold), FDFT1 (1.7 fold), LSS (1.7 fold), SQLE (1.7 fold), MVK (1.7 fold), MVD (1.6 fold), IDI1 (1.6 fold), as well as the rate-limiting enzyme of cholesterol biosynthesis HMG-CoA reductase (HMGCR 1.9 fold), the enzyme that catalyzes the first step of cholesterol biosynthesis HMG-CoA synthase (HMGCS1 2.2 fold) and Sterol-C5-Desaturase (SC5DL 2.2 fold) associated with lethal cholesterol disorders [38]. We verified the expression levels of these last three genes with qPCR as shown in 9A-C. Again, in accordance with microarray analyses HMGCS, HMGCR and SC5DL were significantly higher (2.8, 3.0 and 2.8 fold, respectively) in mid luteal stage (day 11) as compared with the early stage (day 4; Fig 9A–9C). Examining luteal cell-specific expression of HMGCS, HMGCR and SC5DL, we found that these genes were expressed predominantly in luteal steroidogenic cells (Fig 9D–9F) with LSC/LEC ratio of 5.5, 3.3 and 2.2 folds, respectively.
Fig 8. Illustration of differentially expressed cholesterol biosynthetic genes in midcycle CL.
The diagram shows up-regulated, DEG (p<0.05) on day 11 vs. day 4 CL. Intensity of the shading increases with the magnitude of the change (see inset).
Fig 9. Expression of cholesterol biosynthetic genes in day 4 and 11 CL and their luteal cell specific expression.
mRNA expression of (A) HMGCS, (B) HMGCR and (C) SC5DL in the early (day 4) and mid (day 11) luteal stages and cell specific expression of (D) HMGCS1, (E) HMGCR and (F) SC5DL were measured in LEC–luteal endothelial cells and LSC–luteal steroidogenic cells enriched from mid cycle CL. Levels of mRNA were measured by qRT-PCR and normalized to RPS26 in the same samples. The results are presented as means ± SEM. Data were obtained from 5 cows /CL stage. Asterisks indicate significant differences between day 4 and day 11; *P<0.05, **P < 0.01, ***P < 0.001. The arbitrary units (Y axis) were multiplied by 100.
Next, to examine if the genes involved in cholesterol synthesis are elevated in granulosa cells as a result of luteinazation, cells were cultured in the absence or presence of forskolin (adenylyl cyclase activator) as detailed in Table 2. A significant stimulation of HMGCS1, HMGCR and SC5DL was observed after 24h of forskolin treatment (Table 2). The three main steroidogenic genes (CYP11A1, STAR and HSD3B1) were also determined by qPCR, showing the expected elevation in the mature CL with STAR being the most prominently induced, 3.6 fold higher at midcycle as compared with early CL (Fig 10A–10C).
Table 2. Effect of 24h treatment with forskolin (10μM) on cholesterol biosynthetic gene expression in granulosa cells.
| Gene | Control † | Forskolin † | Significance |
|---|---|---|---|
| HMGCS1 | 3.91±1.05 | 19.87 ±3.81 | * |
| HMGCR | 1.78±0.38 | 6.28±1.59 | ** |
| SC5DL | 0.01±0.002 | 0.03±0.004 | * |
The results are presented as means ± SEM.
Data were obtained from 4 different experiments.
Asterisks indicate significant differences from their respective controls;
*P<0.05.
**P < 0.01.
***P < 0.001.
† The arbitrary units of gene expression were multiplied by 100.
Fig 10. STAR, HSD3B1 and CYP11A1 expression in day 4 and day 11 CL.
mRNA expression of STAR, HSD3B1 and CYP11A1 (A-C). Levels of mRNA were measured by qRT-PCR and normalized to RPS26 in the same samples. The results are presented as means ± SEM; n = 9. Asterisks indicate significant difference between day 4 and day 11; *P<0.05, **P < 0.01, ***P < 0.001. The arbitrary units (Y axis) were multiplied by 100.
Molecular interactions in day 4 and 11 CL
To further decipher the molecular interactions of the 681 differentially expressed genes, network analysis was performed using the Ingenuity Pathway Analysis (IPA) tool. Twenty five networks were found, 17 of which had 20 or more focus genes in each network (all networks are available in S2 File). Four networks were merged and adapted to form a compound network representing the main underlying biological processes related to CL from these two developmental stages (Fig 11). The compound network shows genes in pivotal positions and the intricate crosstalk between genes and gene complexes. Specifically, the analysis shows NOS complex genes, IGFBP, PTX3-TNFA1P6, PGR, ACTG2 and ITGAV genes being upregulated on day 4 (red). In the mature (day 11) CL, NOTCH, IGF, LDL, HDL and NF-κB complexes together with APOD, APOE, CD74, STARD13, CX3CL and complement system genes were upregulated (green). The compound network strengthens results presented in previous figures.
Fig 11. Compound network representing the main underlying biological processes related to day 4 and day 11 CL.
Four IPA biological networks [network 6 (score 35), network 8 (score 31), network 10 (score 28) and network 12 (score 26) see S2 File] of the DEG (|fold change ≥2|, P<0.05) between day 4 and day 11 CL were merged and adopted to a larger biological network, indicating the mutual relationship between networks. The network is displayed graphically as nodes (gene/gene products) and edges (the biological relationship between nodes). Red nodes indicate genes that were upregulated in day 4 CL; green nodes indicate genes that were upregulated in day 11 CL. White nodes indicate genes that are not differentially expressed but related to this network. Intensity of the shading increases with the magnitude of the change. † Genes added manually with fold change >1.9.
Discussion
Using various bioinformatics tools we analyzed the transcriptomes of bovine CL on days 4 and 11. 681 genes (363 and 318 for day 4 and 11, respectively) were differentially expressed. The analysis revealed profound differences in networks, pathways and specific genes expressed in CL on days 4 and 11, representing the early, developing gland vs. the mature bovine CL at its plateau phase.
Previous studies using proliferation markers mainly to the S phase of the cell cycle, such as Bromodeoxyuridine (BrdU), PCNA and Ki67, showed an increased labeling index in the early luteal phase. Using the power of genomic technologies and collection of CL at precise days of cycle we were able to confirm and extend these findings, demonstrating that numerous genes, acting during all phases of the cell cycle (G1, S, G2, and M) were elevated in day 4 versus d 11 CL. These include CCNB1, CCNA1, CCNA2, CDK1, AURKA, PLK1 and the MCM complex genes. In fact, DEG on day 4 were greatly dominated by processes such as cell cycle, DNA replication and DNA metabolic process. Most studies comparing early vs. mature CL suggest that the bulk of proliferating cells were LEC or/and pericytes [20, 39, 40].
Our results show that NOS3 which is involved in maintaining dilated vessels and MMP9 implicated in the angiogenic process by promoting degradation of the extracellular matrix were elevated on day 4 as compared to day 11. It is noteworthy that MMP9, unlike NOS3, did not show preferential luteal cell localization, implying that endothelial and non-endothelial cells participate in ECM remodeling. Another novel aspect of the results suggest that endothelial cells on day 11 CL employ a mechanism to prevent further blood vessel sprouting and drive their maturation by expressing NOTCH4 and JAG1. NOTCH4 is a member of the NOTCH transmembrane receptor family that is expressed primarily on endothelial cells. NOTCH signaling is crucial for developmental processes, and is important for many aspects of vascular biology [41]. Using an in vitro endothelial-sprouting assay it was suggested that constitutive NOTCH4 activation in endothelial cells inhibits angiogenesis by various mechanisms [42]. More recently NOTCH4 was discovered to be an inhibitor of the NOTCH1 receptor which is positively correlated with angiogenesis [43]. JAG1 is one of the five notch ligands that include also DLL1, DLL3, DLL4, and JAG2. JAG1 is expressed in endothelial cells (here and [44]) and in vascular smooth muscle cells [44, 45]. It was shown to be indispensable for the development of vascular smooth muscle cells, a fact that may be highly relevant for blood vessel maturation in the CL. Together results presented here suggest that the presence of NOTCH4 and JAG1 in blood vessels may inhibit angiogenesis at mid cycle. Previous information suggest a role for Dll4/Notch-1 in luteal blood vessel development [46–50] yet NOTCH4 and JAG1 were not studied in CL before. VEGFR-1 (FLT1) was also elevated significantly on day 11 (2.3 fold). The negative role of VEGFR-1 in angiogenesis was suggested in several reports [51, 52] reinforcing the idea that VEGFR-1 may act as a VEGF-trap to inhibit the pro-angiogenic VEGFR-2 function. Its expression in day 11 CL may act in concert with JAG1 and NOTCH4 to curb angiogenesis.
Another novel finding from described studies relates to expression of CD300LG. While the family of CD300 signaling molecules are detected mostly in myeloid and lymphoid cells, the CD300LG gene is exclusively expressed in endothelial cells [53, 54] as also observed here for luteal endothelial cells. The CD300LG gene (also known as Nepmucin and CLM-9) encodes for an adhesion molecule that supports L-selectin-dependent lymphocyte migration via its Ig domain. Elevated expression of the CD300LG gene at mid cycle would therefore support the transmigration of immune cells into the CL at this stage. Interestingly, monocyte chemoattractant protein 1 (MCP-1/CCL2), a chemokine that regulates migration and infiltration of monocytes/macrophages, previously shown to be elevated in day 12 bovine CL [55], was not upregulated in our dataset on day 11. The findings of this study suggest a potential important role for CD300LG in promoting immune cell recruitment into the CL as means to support luteal function and/or in preparation for luteal regression.
In agreement with numerous studies published during last 2 decades, our study detected an abundance of immune related genes, enriched in categories such as regulation of immune system process, complement activation, B cell mediated immunity and acute inflammatory response, in day 11 CL. In addition, immune cell specific marker genes were identified on day 11, both of lymphocytes (CD74, CD52 and CD151), and of macrophages (CD68, CD302).
In cows, plasma progesterone concentrations increase from the early luteal phase and remain high up to mid-late luteal stage [56–58]. CYP11A1, the first rate-limiting enzyme that catalyzes the conversion of cholesterol to pregnenolone, and HSD3B, the enzyme that catalyzes the synthesis of progesterone from pregnenolone [59] were elevated in the mature gland in agreement with previous findings [60, 61]. STAR is crucial for transport of cholesterol to mitochondria and mediates the acute steroidogenic response [62]. We found that it was highly induced at mid cycle CL as also observed as in other studies [61, 63].
However, the findings presented here imply that cholesterol biosynthesis and utilization may constitute complementary mechanism to obtain high progesterone levels. We observed that genes involved in cholesterol biosynthesis pathway and utilization were significantly increased in the day 11 CL. In fact two thirds of the genes in this pathway were elevated in the mid luteal stage, including crucial enzymes for cholesterol production like HMGCR (here and also as reported by Rodgers et al, 1987 [17]), HMGCS and SC5DL. In addition genes encoding proteins for cholesterol uptake and transport from plasma, such as LDLR, APOD and APOE [64, 65] were also significantly enriched in day 11 versus d 4 CL (S1 File). We observed that key cholesterol synthesizing genes (HMGCR, HMGCS and SC5DL) were indeed localized to steroidogenic cells and were responsive to cAMP elevation, further implying their relevance to luteinized steroidogenic cells. Our data therefore strongly suggests that cholesterol synthesis (de novo and from exogenous sources) plays a major role in sustaining luteal progesterone levels. This proposition is supported by the study of Mares et al. [14, 18] in which they showed that luteal cholesterol content was elevated throughout most of the bovine CL lifespan (until day 15).
In summary, this study examined global changes associated with cow’s CL development; it reveals profound differences in networks, pathways and specific genes expressed in the CL. By analyzing the transcriptomes at exact days during CL development and specific luteal cell types, the potential roles of select novel genes were revealed. Only regulation of genes was studied here, the determination of protein products of these genes awaits future research. It is well known that angiogenesis in early CL is enhanced by growth factors and other factors [66]. This study suggests that angiogenesis can also be negatively regulated at midcycle by expressing JAG1, NOTCH4 and VEGFR-1. Endothelial gene (CD300LG) detected here for the first time in the CL, may play a significant role in immune cell recruitment into the mature CL. Finally, this study suggests the importance of cholesterol availability to the steroidogenically active CL as a means to maintain progesterone synthesis for an extended time during the plateau phase of the cycle.
Supporting information
(XLSX)
(XLSX)
Data Availability
All microarray data can be accessed at Gene Expression Omnibus (accession no. GSE23348). Other relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by grant No. IS-3987-07 from the BARD (Binational Agricultural Research & Development Fund). The grant was awarded to RM and funding played no role in the planning, writing, and decision to submit this manuscript.
References
- 1.Robker RL, Russell DL, Yoshioka S, Sharma SC, Lydon JP, O’Malley BW, et al. Ovulation: a multi-gene, multi-step process. Steroids. 2000;65(10–11):559–70. Epub 2000/12/08. . [DOI] [PubMed] [Google Scholar]
- 2.Robinson RS, Nicklin LT, Hammond AJ, Schams D, Hunter MG, Mann GE. Fibroblast growth factor 2 is more dynamic than vascular endothelial growth factor A during the follicle-luteal transition in the cow. Biology of reproduction. 2007;77(1):28–36. Epub 2007/03/16. doi: 10.1095/biolreprod.106.055434 . [DOI] [PubMed] [Google Scholar]
- 3.Meidan R, Girsh E. Role of endothelial cells in the steroidogenic activity of the bovine corpus luteum. Seminars in reproductive endocrinology. 1997;15(4):371–82. Epub 1997/01/01. doi: 10.1055/s-2008-1068375 . [DOI] [PubMed] [Google Scholar]
- 4.Meidan R, Levy N, Kisliouk T, Podlovny L, Rusiansky M, Klipper E. The yin and yang of corpus luteum-derived endothelial cells: balancing life and death. Domestic animal endocrinology. 2005;29(2):318–28. Epub 2005/06/02. doi: 10.1016/j.domaniend.2005.04.003 . [DOI] [PubMed] [Google Scholar]
- 5.O’Shea JD, Rodgers RJ, D’Occhio MJ. Cellular composition of the cyclic corpus luteum of the cow. Journal of reproduction and fertility. 1989;85(2):483–7. Epub 1989/03/01. . [DOI] [PubMed] [Google Scholar]
- 6.Wiltbank MC. Cell types and hormonal mechanisms associated with mid-cycle corpus luteum function. Journal of animal science. 1994;72(7):1873–83. Epub 1994/07/01. . [DOI] [PubMed] [Google Scholar]
- 7.Shirasuna K, Shimizu T, Matsui M, Miyamoto A. Emerging roles of immune cells in luteal angiogenesis. Reproduction, fertility, and development. 2013;25(2):351–61. Epub 2012/09/07. doi: 10.1071/RD12096 . [DOI] [PubMed] [Google Scholar]
- 8.Nio-Kobayashi J, Kudo M, Sakuragi N, Kimura S, Iwanaga T, Duncan WC. Regulated C-C motif ligand 2 (CCL2) in luteal cells contributes to macrophage infiltration into the human corpus luteum during luteolysis. Molecular human reproduction. 2015. Epub 2015/05/25. doi: 10.1093/molehr/gav028 . [DOI] [PubMed] [Google Scholar]
- 9.Walusimbi SS, Pate JL. Luteal cells from functional and regressing bovine corpora lutea differentially alter the function of gamma delta T cells. Biology of reproduction. 2014;90(6):140 Epub 2014/05/16. doi: 10.1095/biolreprod.114.117564 . [DOI] [PubMed] [Google Scholar]
- 10.Bauer M, Reibiger I, Spanel-Borowski K. Leucocyte proliferation in the bovine corpus luteum. Reproduction (Cambridge, England). 2001;121(2):297–305. Epub 2001/02/28. . [PubMed] [Google Scholar]
- 11.Penny LA, Armstrong D, Bramley TA, Webb R, Collins RA, Watson ED. Immune cells and cytokine production in the bovine corpus luteum throughout the oestrous cycle and after induced luteolysis. Journal of reproduction and fertility. 1999;115(1):87–96. . [DOI] [PubMed] [Google Scholar]
- 12.Meidan R, Girsh E, Blum O, Aberdam E. In vitro differentiation of bovine theca and granulosa cells into small and large luteal-like cells: morphological and functional characteristics. Biology of reproduction. 1990;43(6):913–21. Epub 1990/12/01. . [DOI] [PubMed] [Google Scholar]
- 13.Hansel W, Alila HW, Dowd JP, Milvae RA. Differential origin and control mechanisms in small and large bovine luteal cells. Journal of reproduction and fertility Supplement. 1991;43:77–89. Epub 1991/01/01. . [PubMed] [Google Scholar]
- 14.Mares SE, Zimbelman RG, Casida LE. Variation in Progesterone Content of the Bovine Corpus Luteum of the Estrual Cycle. Journal of animal science. 1962;21:266–71. [Google Scholar]
- 15.Kapourchali FR, Surendiran G, Goulet A, Moghadasian MH. The Role of Dietary Cholesterol in Lipoprotein Metabolism and Related Metabolic Abnormalities: A Mini-review. Critical reviews in food science and nutrition. 2015:0 Epub 2015/06/10. doi: 10.1080/10408398.2013.842887 . [DOI] [PubMed] [Google Scholar]
- 16.Chen HW. Role of cholesterol metabolism in cell growth. Federation proceedings. 1984;43(1):126–30. Epub 1984/01/01. . [PubMed] [Google Scholar]
- 17.Rodgers RJ, Mason JI, Waterman MR, Simpson ER. Regulation of the synthesis of 3-hydroxy-3-methylglutaryl coenzyme A reductase in the bovine ovary in vivo and in vitro. Molecular endocrinology (Baltimore, Md). 1987;1(2):172–80. Epub 1987/02/01. doi: 10.1210/mend-1-2-172 . [DOI] [PubMed] [Google Scholar]
- 18.Rizzo A, Stefani AL, Piccinno M, Roncetti M, D’Onghia G, Sciorsci RL. Dynamics of the progesterone and cholesterol concentrations within the bovine corpus luteum cavity. Res Vet Sci. 2016;109:56–8. doi: 10.1016/j.rvsc.2016.08.006 . [DOI] [PubMed] [Google Scholar]
- 19.Rainey WE, Rodgers RJ, Mason JI. The role of bovine lipoproteins in the regulation of steroidogenesis and HMG-CoA reductase in bovine adrenocortical cells. Steroids. 1992;57(4):167–73. . [DOI] [PubMed] [Google Scholar]
- 20.Wulff C, Dickson SE, Duncan WC, Fraser HM. Angiogenesis in the human corpus luteum: simulated early pregnancy by HCG treatment is associated with both angiogenesis and vessel stabilization. Human reproduction (Oxford, England). 2001;16(12):2515–24. Epub 2001/12/01. . [DOI] [PubMed] [Google Scholar]
- 21.Sugino N, Suzuki T, Sakata A, Miwa I, Asada H, Taketani T, et al. Angiogenesis in the human corpus luteum: changes in expression of angiopoietins in the corpus luteum throughout the menstrual cycle and in early pregnancy. The Journal of clinical endocrinology and metabolism. 2005;90(11):6141–8. Epub 2005/08/25. doi: 10.1210/jc.2005-0643 . [DOI] [PubMed] [Google Scholar]
- 22.Fraser HM, Wulff C. Angiogenesis in the primate ovary. Reproduction, fertility, and development. 2001;13(7–8):557–66. Epub 2002/05/10. . [DOI] [PubMed] [Google Scholar]
- 23.Wiltbank MC, Dysko RC, Gallagher KP, Keyes PL. Relationship between blood flow and steroidogenesis in the rabbit corpus luteum. Journal of reproduction and fertility. 1988;84(2):513–20. Epub 1988/11/01. . [DOI] [PubMed] [Google Scholar]
- 24.Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. Mechanisms controlling the function and life span of the corpus luteum. Physiological reviews. 2000;80(1):1–29. Epub 2000/01/05. doi: 10.1152/physrev.2000.80.1.1 . [DOI] [PubMed] [Google Scholar]
- 25.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological). 1995:289–300. [Google Scholar]
- 26.Bauer S, Grossmann S, Vingron M, Robinson PN. Ontologizer 2.0—a multifunctional tool for GO term enrichment analysis and data exploration. Bioinformatics (Oxford, England). 2008;24(14):1650–1. Epub 2008/05/31. doi: 10.1093/bioinformatics/btn250 . [DOI] [PubMed] [Google Scholar]
- 27.Zalman Y, Klipper E, Farberov S, Mondal M, Wee G, Folger JK, et al. Regulation of angiogenesis-related prostaglandin f2alpha-induced genes in the bovine corpus luteum. Biology of reproduction. 2012;86(3):92 Epub 2011/12/17. doi: 10.1095/biolreprod.111.095067 . [DOI] [PubMed] [Google Scholar]
- 28.Mondal M, Schilling B, Folger J, Steibel JP, Buchnick H, Zalman Y, et al. Deciphering the luteal transcriptome: potential mechanisms mediating stage-specific luteolytic response of the corpus luteum to prostaglandin F(2)alpha. Physiological genomics. 2011;43(8):447–56. Epub 2011/02/18. doi: 10.1152/physiolgenomics.00155.2010 . [DOI] [PubMed] [Google Scholar]
- 29.Levy N, Gordin M, Smith MF, Bolden-Tiller OU, Meidan R. Hormonal regulation and cell-specific expression of endothelin-converting enzyme 1 isoforms in bovine ovarian endothelial and steroidogenic cells. Biology of reproduction. 2003;68(4):1361–8. Epub 2003/02/28. doi: 10.1095/biolreprod.102.009134 . [DOI] [PubMed] [Google Scholar]
- 30.Levy N, Gordin M, Mamluk R, Yanagisawa M, Smith MF, Hampton JH, et al. Distinct cellular localization and regulation of endothelin-1 and endothelin-converting enzyme-1 expression in the bovine corpus luteum: implications for luteolysis. Endocrinology. 2001;142(12):5254–60. Epub 2001/11/20. doi: 10.1210/endo.142.12.8550 . [DOI] [PubMed] [Google Scholar]
- 31.Klipper E, Tatz E, Kisliouk T, Vlodavsky I, Moallem U, Schams D, et al. Induction of heparanase in bovine granulosa cells by luteinizing hormone: possible role during the ovulatory process. Endocrinology. 2009;150(1):413–21. Epub 2008/09/27. doi: 10.1210/en.2008-0697 . [DOI] [PubMed] [Google Scholar]
- 32.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8. . [DOI] [PubMed] [Google Scholar]
- 33.Collins T, Williams A, Johnston GI, Kim J, Eddy R, Shows T, et al. Structure and chromosomal location of the gene for endothelial-leukocyte adhesion molecule 1. The Journal of biological chemistry. 1991;266(4):2466–73. Epub 1991/02/15. . [PubMed] [Google Scholar]
- 34.Marsden PA, Schappert KT, Chen HS, Flowers M, Sundell CL, Wilcox JN, et al. Molecular cloning and characterization of human endothelial nitric oxide synthase. FEBS letters. 1992;307(3):287–93. Epub 1992/08/03. . [DOI] [PubMed] [Google Scholar]
- 35.Klipper E, Gilboa T, Levy N, Kisliouk T, Spanel-Borowski K, Meidan R. Characterization of endothelin-1 and nitric oxide generating systems in corpus luteum-derived endothelial cells. Reproduction (Cambridge, England). 2004;128(4):463–73. doi: 10.1530/rep.1.00271 . [DOI] [PubMed] [Google Scholar]
- 36.Wilcox CB, Feddes GO, Willett-Brozick JE, Hsu LC, DeLoia JA, Baysal BE. Coordinate up-regulation of TMEM97 and cholesterol biosynthesis genes in normal ovarian surface epithelial cells treated with progesterone: implications for pathogenesis of ovarian cancer. BMC cancer. 2007;7:223 Epub 2007/12/12. doi: 10.1186/1471-2407-7-223 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byrnes RW, Cotter D, Maer A, Li J, Nadeau D, Subramaniam S. An editor for pathway drawing and data visualization in the Biopathways Workbench. BMC systems biology. 2009;3:99 Epub 2009/10/06. doi: 10.1186/1752-0509-3-99 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krakowiak PA, Wassif CA, Kratz L, Cozma D, Kovarova M, Harris G, et al. Lathosterolosis: an inborn error of human and murine cholesterol synthesis due to lathosterol 5-desaturase deficiency. Human molecular genetics. 2003;12(13):1631–41. Epub 2003/06/19. . [DOI] [PubMed] [Google Scholar]
- 39.Jablonka-Shariff A, Grazul-Bilska AT, Redmer DA, Reynolds LP. Growth and cellular proliferation of ovine corpora lutea throughout the estrous cycle. Endocrinology. 1993;133(4):1871–9. Epub 1993/10/01. doi: 10.1210/endo.133.4.8404629 . [DOI] [PubMed] [Google Scholar]
- 40.Christenson LK, Stouffer RL. Proliferation of microvascular endothelial cells in the primate corpus luteum during the menstrual cycle and simulated early pregnancy. Endocrinology. 1996;137(1):367–74. Epub 1996/01/01. doi: 10.1210/endo.137.1.8536637 . [DOI] [PubMed] [Google Scholar]
- 41.Kume T. Ligand-dependent Notch signaling in vascular formation. Advances in experimental medicine and biology. 2012;727:210–22. Epub 2012/03/09. doi: 10.1007/978-1-4614-0899-4_16 . [DOI] [PubMed] [Google Scholar]
- 42.Leong KG, Hu X, Li L, Noseda M, Larrivee B, Hull C, et al. Activated Notch4 inhibits angiogenesis: role of beta 1-integrin activation. Molecular and cellular biology. 2002;22(8):2830–41. Epub 2002/03/23. doi: 10.1128/MCB.22.8.2830-2841.2002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.James AC, Szot JO, Iyer K, Major JA, Pursglove SE, Chapman G, et al. Notch4 reveals a novel mechanism regulating Notch signal transduction. Biochimica et biophysica acta. 2014;1843(7):1272–84. Epub 2014/03/29. doi: 10.1016/j.bbamcr.2014.03.015 . [DOI] [PubMed] [Google Scholar]
- 44.Bridges E, Oon CE, Harris A. Notch regulation of tumor angiogenesis. Future Oncology. 2011;7(4):569–88. doi: 10.2217/fon.11.20 [DOI] [PubMed] [Google Scholar]
- 45.Villa N, Walker L, Lindsell CE, Gasson J, Iruela-Arispe ML, Weinmaster G. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mechanisms of development. 2001;108(1–2):161–4. Epub 2001/10/02. . [DOI] [PubMed] [Google Scholar]
- 46.Accialini P, Hernandez SF, Bas D, Pazos MC, Irusta G, Abramovich D, et al. A link between Notch and progesterone maintains the functionality of the rat corpus luteum. Reproduction (Cambridge, England). 2015;149(1):1–10. Epub 2014/11/30. doi: 10.1530/rep-14-0449 . [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Pascual CM, Zimmermann RC, Ferrero H, Shawber CJ, Kitajewski J, Simon C, et al. Delta-like ligand 4 regulates vascular endothelial growth factor receptor 2-driven luteal angiogenesis through induction of a tip/stalk phenotype in proliferating endothelial cells. Fertility and sterility. 2013;100(6):1768–76.e1. Epub 2013/10/01. doi: 10.1016/j.fertnstert.2013.08.034 . [DOI] [PubMed] [Google Scholar]
- 48.Fraser HM, Hastings JM, Allan D, Morris KD, Rudge JS, Wiegand SJ. Inhibition of delta-like ligand 4 induces luteal hypervascularization followed by functional and structural luteolysis in the primate ovary. Endocrinology. 2012;153(4):1972–83. Epub 2012/02/16. doi: 10.1210/en.2011-1688 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hernandez F, Peluffo MC, Stouffer RL, Irusta G, Tesone M. Role of the DLL4-NOTCH system in PGF2alpha-induced luteolysis in the pregnant rat. Biology of reproduction. 2011;84(5):859–65. Epub 2011/01/07. doi: 10.1095/biolreprod.110.088708 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vorontchikhina MA, Zimmermann RC, Shawber CJ, Tang H, Kitajewski J. Unique patterns of Notch1, Notch4 and Jagged1 expression in ovarian vessels during folliculogenesis and corpus luteum formation. Gene expression patterns: GEP. 2005;5(5):701–9. Epub 2005/06/09. doi: 10.1016/j.modgep.2005.02.001 . [DOI] [PubMed] [Google Scholar]
- 51.Rahimi N. VEGFR-1 and VEGFR-2: two non-identical twins with a unique physiognomy. Frontiers in bioscience: a journal and virtual library. 2006;11:818–29. Epub 2005/09/09. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kearney JB, Kappas NC, Ellerstrom C, DiPaola FW, Bautch VL. The VEGF receptor flt-1 (VEGFR-1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood. 2004;103(12):4527–35. Epub 2004/02/26. doi: 10.1182/blood-2003-07-2315 . [DOI] [PubMed] [Google Scholar]
- 53.Takatsu H, Hase K, Ohmae M, Ohshima S, Hashimoto K, Taniura N, et al. CD300 antigen like family member G: A novel Ig receptor like protein exclusively expressed on capillary endothelium. Biochemical and biophysical research communications. 2006;348(1):183–91. Epub 2006/08/01. doi: 10.1016/j.bbrc.2006.07.047 . [DOI] [PubMed] [Google Scholar]
- 54.Umemoto E, Tanaka T, Kanda H, Jin S, Tohya K, Otani K, et al. Nepmucin, a novel HEV sialomucin, mediates L-selectin-dependent lymphocyte rolling and promotes lymphocyte adhesion under flow. The Journal of experimental medicine. 2006;203(6):1603–14. Epub 2006/06/07. doi: 10.1084/jem.20052543 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Townson DH, O’Connor CL, Pru JK. Expression of monocyte chemoattractant protein-1 and distribution of immune cell populations in the bovine corpus luteum throughout the estrous cycle. Biology of reproduction. 2002;66(2):361–6. Epub 2002/01/24. . [DOI] [PubMed] [Google Scholar]
- 56.Singh J, Pierson RA, Adams GP. Ultrasound image attributes of the bovine corpus luteum: structural and functional correlates. Journal of reproduction and fertility. 1997;109(1):35–44. Epub 1997/01/01. . [DOI] [PubMed] [Google Scholar]
- 57.Hauger RL, Karsch FJ, Foster DL. A new concept for control of the estrous cycle of the ewe based on the temporal relationships between luteinizing hormone, estradiol and progesterone in peripheral serum and evidence that progesterone inhibits tonic LH secretion. Endocrinology. 1977;101(3):807–17. Epub 1977/09/01. doi: 10.1210/endo-101-3-807 . [DOI] [PubMed] [Google Scholar]
- 58.Johansson ED. Progesterone levels in peripheral plasma during the luteal phase of the normal human menstrual cycle measured by a rapid competitive protein binding technique. Acta endocrinologica. 1969;61(4):592–606. Epub 1969/08/01. . [DOI] [PubMed] [Google Scholar]
- 59.Miller WL. Molecular biology of steroid hormone synthesis. Endocrine reviews. 1988;9(3):295–318. Epub 1988/08/01. doi: 10.1210/edrv-9-3-295 . [DOI] [PubMed] [Google Scholar]
- 60.Rodgers RJ, Waterman MR, Simpson ER. Levels of messenger ribonucleic acid encoding cholesterol side-chain cleavage cytochrome P-450, 17 alpha-hydroxylase cytochrome P-450, adrenodoxin, and low density lipoprotein receptor in bovine follicles and corpora lutea throughout the ovarian cycle. Molecular endocrinology (Baltimore, Md). 1987;1(3):274–9. Epub 1987/03/01. doi: 10.1210/mend-1-3-274 . [DOI] [PubMed] [Google Scholar]
- 61.Jiang YF, Tsui KH, Wang PH, Lin CW, Wang JY, Hsu MC, et al. Hypoxia regulates cell proliferation and steroidogenesis through protein kinase A signaling in bovine corpus luteum. Animal reproduction science. 2011;129(3–4):152–61. Epub 2012/01/10. doi: 10.1016/j.anireprosci.2011.12.004 . [DOI] [PubMed] [Google Scholar]
- 62.Clark BJ, Soo SC, Caron KM, Ikeda Y, Parker KL, Stocco DM. Hormonal and developmental regulation of the steroidogenic acute regulatory protein. Molecular endocrinology (Baltimore, Md). 1995;9(10):1346–55. Epub 1995/10/01. doi: 10.1210/mend.9.10.8544843 . [DOI] [PubMed] [Google Scholar]
- 63.Pescador N, Soumano K, Stocco DM, Price CA, Murphy BD. Steroidogenic acute regulatory protein in bovine corpora lutea. Biology of reproduction. 1996;55(2):485–91. Epub 1996/08/01. . [DOI] [PubMed] [Google Scholar]
- 64.Driscoll DM, Getz GS. Extrahepatic synthesis of apolipoprotein E. Journal of lipid research. 1984;25(12):1368–79. Epub 1984/12/01. . [PubMed] [Google Scholar]
- 65.Rassart E, Bedirian A, Do Carmo S, Guinard O, Sirois J, Terrisse L, et al. Apolipoprotein D. Biochimica et biophysica acta. 2000;1482(1–2):185–98. Epub 2000/11/04. . [DOI] [PubMed] [Google Scholar]
- 66.Woad KJ, Robinson RS. Luteal angiogenesis and its control. Theriogenology. 2016;86(1):221–8. doi: 10.1016/j.theriogenology.2016.04.035 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
Data Availability Statement
All microarray data can be accessed at Gene Expression Omnibus (accession no. GSE23348). Other relevant data are within the paper and its Supporting Information files.