Abstract
In adult mammalian testes, spermatids, most notably step 17–19 spermatids in stage IV–VIII tubules, are aligned with their heads pointing toward the basement membrane and their tails toward the tubule lumen. On the other hand, these polarized spermatids also align across the plane of seminiferous epithelium, mimicking planar cell polarity (PCP) found in other hair cells in cochlea (inner ear). This orderly alignment of developing spermatids during spermiogenesis is important to support spermatogenesis, such that the maximal number of developing spermatids can be packed and supported by a fixed population of differentiated Sertoli cells in the limited space of the seminiferous epithelium in adult testes. In this review, we provide emerging evidence to demonstrate spermatid PCP in the seminiferous epithelium to support spermatogenesis. We also review findings in the field regarding the biology of spermatid cellular polarity (e.g., head-tail polarity and apico-basal polarity) and its inter-relationship to spermatid PCP. Furthermore, we also provide a hypothetical concept on the importance of PCP in endocytic vesicle-mediated protein trafficking events to support spermatogenesis through protein endocytosis and recycling.
Keywords: Testis, planar cell polarity, spermatogenesis, Sertoli cell, blood-testis barrier
Introduction
Cell polarity plays a pivotal role in multiple cellular events to support tissues and organs during embryogenesis, post-natal development and cell homeostasis, including directional cell movement, cell proliferation, cell survival/apoptosis, and cell differentiation [1–6]. On the other hand, cell polarity is a distinguishable feature of highly differentiated cells in some organs, such as in elongating/elongated spermatids and spermatozoa in the testis. Elongated spermatids and spermatozoa are highly polarized cells with the head that contains the genetic materials in highly condensed chromosomes on one end, and a long tail constituted by actin- and microtubule (MT)-based cytoskeletal elements at the opposite end of the spermatid/sperm. The process from which spermatozoa are formed in the seminiferous epithelium of the seminiferous tubule is designated spermatogenesis. Spermatogenesis consists of three distinctive phases. Phase I includes the mitotic self-renewal and differentiation of spermatogonia stem cells at the stem cell niche located near the basement membrane of the seminiferous tubules at the site where seminiferous tubules meet and near the microvessels in the interstitium [7–9]. Phase II denotes development of spermatocytes, such as preleptotene spermatocytes derived from type B spermatogonia, which must be transported across the blood-testis barrier (BTB) to enter the adluminal compartment, and to differentiate into zygote, pachytene and diplotene spermatocytes to undergo meiosis I and II to generate haploid spermatids [10]. In phase III, post-meiotic haploid spermatids undergo a series of morphological, molecular, and cellular differentiation to form elongated spermatids via spermiogenesis (including steps 1–12, 1–16 and 1–6 spermatids in mouse, rat and human testes, respectively [11–16]), to be accompanied by their transport across the adluminal compartment of the seminiferous epithelium, thereby fully developed spermatids (i.e., spermatozoa) line-up at the luminal edge and be released into tubule lumen at spermiation [17–19]. It is of interest to note that during spermiogenesis, developing spermatids exhibit a unique dual-level of cell polarity. On a single cell level, the seemingly symmetric round spermatids (steps 1–7 spermatids in the rat testis) initiate unique polarization in step 8–19 spermatids wherein the head at the proximal end containing the cell nucleus is accompanied by the elongating flagellar at the tail (i.e., distal growing end), exhibiting head-tail polarity. On the multi-cellular level, those elongating and elongated spermatids are uniquely oriented with their heads point basally towards the basement membrane of the seminiferous epithelium while their tails extend apically to the tubule lumen of the seminiferous tubule as groups of cells (such as in stage V tubules) and the entire population of cells (such as in stage VIII tubules), displaying apico-basal polarity. These two levels of polarity thus guarantee functional specialization of the developing spermatids and to ensure that a maximal number of spermatids are packed in the limited space of the seminiferous epithelium along the seminiferous tubules. It is of interest to note that the length of all tubules in an adult rat testis was estimated to be ~26-meter in length and a diameter of ~280 μm [20, 21], thereby capable of producing as much as ~70 million sperm daily per testis pair [22]. Without the highly polarized orientation of spermatids in the tubules, it is not possible to maintain such an enormous output in adult animals.
It is noted that steps 17–19 spermatids during stage V–VIII of the epithelial cycle in the rat testis also reveal another type of polarity known as planar cell polarity (PCP), referring to the alignment of highly polarized cells (i.e., elongating/elongated spermatids) on the plane of seminiferous epithelium, analogous to cuticle cell hair or cell hair found in insects or inner ear (cochlea) of rodents and humans. Herein, we review some of the latest findings in the field, examining the functional relationship of PCP and spermatogenesis, including the likely underlying mechanism(s) that support PCP during spermiogenesis.
Planar cell polarity (PCP) of elongating/elongated spermatids during spermiogenesis
In adult rat testes, any typical cross-section of the seminiferous tubule along the longitudinal axis illustrates the alignment of polarized spermatids across the plane of polarized epithelial Sertoli cells which support spermatid development, differentiation, and transport across the epithelium, such as noted in Figure 1. Due to the unique association of developing spermatids with Sertoli cells in the seminiferous epithelium, spermatogenesis has been divided into 6, 12 and 14 stages in the human, mouse and rat testes, in which unique stages of developing spermatids and cellular events are associated with Sertoli cells in the epithelium [11, 13–15, 23, 24]. For instance, in the rat testis, spermiation takes place at the stage VIII of the epithelial cycle with the concomitant appearance of step 8 spermatids, plus transport of preleptotene spermatocytes across the BTB; while meiosis I/II takes place in stage XIV tubules [13]. Furthermore, step 5 round spermatids and step 17 elongating spermatids are found together in stage V tubules, whereas step 19 elongated spermatids are seen in the stage VII and VIII tubules.
Figure 1. The organized alignment of polarized spermatids across the plane of the seminiferous epithelium in adult rat testes, supporting the concept of spermatid planar cell polarity (PCP) during spermiogenesis.
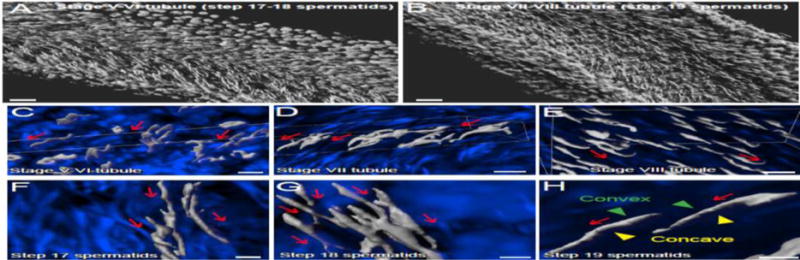
Isolated rat seminiferous tubules were obtained from adult testes as earlier described [84], stained with DAPI (4′,6-diamdino-2-phenylindole) and images were obtained by confocal microscopy, illustrating spermatid PCP across the seminiferous epithelium (A–B). Images were obtained using an inverted Zeiss LSM 880 NLO laser scanning confocal/multiphoton microscope (Carl Zeiss MicroImaging, Thornwood, NY). Optical sections of 20–100 μm of the seminiferous tubule were collected at 0.83-μm intervals along the z-axis to obtain Z-stack image series. Images were then deconvoluted using Autoquant X deconvolution software (Media cybernetics), and 3D images were subsequently obtained using Imaris (Bitplane) software package (Version 8.9), but using only the DAPI channel to show the alignment of polarized spermatid heads across the plane of seminiferous epithelium. White pseudo color was applied to the DAPI channel to obtain optimal image contrast (A–B). For other tubule images shown in C–H, deconvolved images of the Z-stack series were reconstructed in Imaris with the blue channel for DAPI. Red arrows annotate the directional (head-tail polarity) orientation of spermatid heads by pointing toward the basement membrane, also noted are the multicellular polarity in C–H. As noted in H, the convex (dorsal) and concave (ventral) sides of the spermatid head were also annotated. Scale bar, 100 μm in A, B; 15 μm in C–E; 3 μm in F–H.
When a cross-section of a seminiferous tubule, such as at stage VIII of the cycle, is closely examined, step 19 elongated spermatids exhibit typical apico-basal polarity in which their heads point basally to the basement membrane, whereas their tails extend apically to the tubule lumen (Figure 1). Furthermore, spermatid heads containing the condensed genetic materials in rodents are not round- or oval-shaped as noted in human sperm, instead they are curveshaped at the tip of the head, analogous to a hook-like structure, and the alignment of these hook-like structures are all uniform across the plane of Sertoli cell epithelium. We have used confocal microscopy and the Imaris software package (Version 9; Bitplane, Concord, MA) for subsequent 3D-reconstruction of the images obtained along the longitudinal axis of a stage V–VI (containing step 17 and 18 spermatids) (Figure 1A) and stage VII–VIII (containing step 19 spermatids) (Figure 1B) seminiferous tubule to assess the presence PCP regarding spermatid alignment in the epithelium. It was noted that polarized step 17, 18 and 19 spermatids in stage V, VI and VII–VIII tubules, respectively, displaying PCP (Figure 1A–H). Among step 17–19 spermatids, step 19 spermatid heads (Figure 1A) were shown to align with strict and uniform PCP across the plane of the seminiferous epithelium (Figure 1B, D, E & H vs. A, C, F & G). In short, polarized step 19 elongated spermatids in stage VII–VIII tubules display strict PCP by aligning onto the plane of the seminiferous epithelium, whereas PCP is also detected in stage V and VI tubules in step 17 and 18 spermatids as these spermatids progressively to assume their proper alignment to prepare for their release at spermiation.
In this context, it is of interest to note that step 19 spermatids displaying PCP are similar to other polarized epithelia. For instance, Drosophila wing hair also align and point strictly to a uniform direction along the tissue axis which is perpendicular to the apico-basal axis of the cell plane [25]. The cochlea (inner ear) in rodents and humans is another example of PCP in which the actin-based stereocilia on the apical surface of sensory hair cells are precisely oriented [26]. However, unlike Drosophila wing hair in wing cells, and the stereocilia of the sensory hair cells in cochlea, in which wing hair and stereocilia are integrated components of the corresponding hair cells, step 19 spermatids are not organelles of the Sertoli cells. Instead, spermatids are separate entities and independent cells per se even though they rely exclusively on Sertoli cells for nutritional, paracrine/hormonal and structural supports. Nonetheless, elongated spermatids exhibit PCP polarity and it is supported by the fact that numerous PCP proteins expressed by and Sertoli and germ cells [27] across the plane of the seminiferous epithelium.
Regulation of spermatid head-tail and apico-basal polarity
As briefly discussed above, spermatids exhibit polarity on both single- and multi-cellular levels. These two levels of polarities emerge as early as in the step 1 round spermatids during the onset of spermiogenesis in which the genetic materials begin to undergo condensation and tightly pack into the head region to form the nucleus, concomitant with the preparations of the: (i) genesis of the acrosome located in front of the spermatid nucleus and (ii) elongation of the tail in which the spermatid head also begin to orientate by pointing towards the basement membrane. However, spermatid PCP is not readily detectable until around step 17 spermatids at stage IV–V of the cycle in adult rat testes when polarized spermatids align across the plane of the seminiferous epithelium (Figure 1). By stage VII–VIII of the epithelial cycle, step 19 elongated spermatids display obvious PCP as noted in Figure 1. These observations also suggest that different regulatory machineries and/or signaling proteins may be involved to support these changes during the epithelial cycle. We fist briefly review literature regarding the regulation of the head-tail polarity in spermatids at the single cellular level, and also apico-basal polarity of spermatids at the multi-cellular level.
At the single cellular level, spermatid polarity is most obvious through its head-tail polarity in which spermatid heads align toward the basement membrane uniformly across the epithelium in step 9–19 spermatids from stages IX through VIII [12, 13]. It was shown that in mice deficient of junctional adhesion molecule-C (JAM-C), a spermatid-specific and also apical ES-specific integral membrane protein, spermatid elongation was blocked at the level of round spermatids [28]. Since the spermatid head-tail polarity is a prerequisite for the establishment of the apico-basal polarity, it was not surprising that spermatid apico-basal polarity in JAM-C deficient mice was also disrupted. However, it is of interest to note that in mice with deletion of nectin-2, another apical ES protein but expressed by both Sertoli and late staged spermatids in VI–VIII tubules, spermatozoa from these mice displayed aberrant morphology including deformed sperm heads and malformed mid-pieces, probably due to a disruption of actin microfilament organization at the apical ES [29]. While there were severe spermatid head and mid-piece malformation, the head-tail polarity persisted and maintained. Also, Drosophila spermatids lacking polarity protein atypical protein kinase C (aPKC) had seemingly normal elongation but with impaired alignment of spermatid heads [30]. In RA175 (also known as IGSF4a, SynCAM, or cell adhesion molecule 1 (Cadm1)) which is member of the immunoglobulin superfamily with a PDZ-binding domain at its C-terminus, similar to JAM-C) deficient male mice, Par3 was absent and JAM-C was either absent or abnormally localized, and these mice were infertile, displaying oligo-astheno-teratozoospermia [31, 32]. In contrast to the JAM-C−/− mice where formation of step1–8 spermatids was defective [28], round spermatids (steps 1–8) of RA175−/− mouse testes were morphologically normal – that is, head-tail polarity was detected in early phase of spermigoenesis [32]. However, subsequent spermiogenesis that form steps 9–16 spermatids was blocked, due to the lack of RA175 in RA175−/− testes, failing to recruit Par-3 and JAM-C to create the RA175/Par-3/JAM-C complex to support spermiogenesis [31]. As such, acpico-basal polarity was not detected in these RA175−/− mouse testis [31].
In this context, it is of interest to note that the establishment of spermatid head-tail polarity may rely on the polarized distributions of polarity proteins in developing spermatids. For instance, in the JAM-C-deficient model, all three members of the Par-based polarity protein complex including Par6, Cdc42 and aPKC had little or no polarized distribution in developing round spermatids vs. wild type round spermatids in which their polarized distribution was notably displayed [28]. Furthermore, the distribution of PATJ (pals1-associated tight junction protein), a member of the Crumbs (Crb)-based polarity protein complex was also significantly affected in JAM-C-deficient round spermatids [28]. This lack of polarized distribution of the Par- and Crumbs-based polarity proteins in developing round spermatids thus failed to support spermatid head-tail polarization in step 1–7 spermatids. During the establishment of spermatid apico-basal polarity in step 8–16 spermatids in the mouse testis, apical ES plays a major role because this is the only anchoring device at the Sertoli cell-spermatid interface when it arises in step 8 spermatids. Interestingly, in the nectin-2-deficient model, actin-bundling protein espin was absent, which led to a failure in apical ES assembly [29]. Thus, it is not surprising to note that a knockdown of actin nucleation protein formin1 in rat testes that induced disruptive changes in actin microfilament organization at the apical ES also impeded spermatid apico-basal polarity [33]. Additionally, in another rat model in which adjudin, known to target apical ES specifically when rats were treated with a single oral gavage dose at 50 mg/kg b.w., was found to considerably perturb spermatid apico-basal polarity, which was associated with a notably reduction in the expression of polarity protein Par 6 [34], and actin barbed-end capping and bundling protein Eps8 (epidermal growth factor receptor pathway substrate 8) [35].
Taking collectively, these findings thus support the notion that the establishment of spermatid head-tail polarity and apico-basal polarity require different regulatory machineries. For instance, the spermatid head-tail polarity requires the presence of JAM-C and aPKC, whereas spermatid apico-basal polarity requires the presence of RA175/Par-3/JAM-C complex to properly maintain the polarized distribution of the Par-, Crumbs- and Scribble-based polarity proteins at the apical ES.
Regulation of the spermatid PCP
As shown in Figure 1, polarized step 17–19 spermatids that align across the plane of the seminiferous epithelium in stage IV–VIII tubules, displaying PCP is obviously noted. However, it is obvious that the establishment of spermatid head-tail polarity and also spermatid apico-basal polarity are also crucial to contribute to PCP polarity. However, similar to PCP found in other epithelia in particular hair cells in Drosophila and cochlea, PCP is conferred and regulated by distinctive sets of PCP proteins other than the Par-, Crumbs- and Scribble-based polarity complexes. For instance, there is a core group of PCP proteins found in Drosophila. These include Frizzled (Fz), Flamingo (Fmi)/Starry Night (Stan), Dishevelled (Dsh), Diego (Dgo), Van Gogh (Vang)/Strabismus (Stbm), and Prickle (Pk) that localize asymmetrically at the proximal (e.g., Vang, Pk, and Fmi) vs. distal (e.g., Fz, Dgo, and Fmi) end of neighboring cells to regulate wing hair, bristle and eye ommatidial polarity in concert with atypical cadherins Fat and Dachsous (Dchs) signaling in Drosophila [36–41]. Interestingly, PCP proteins are well conserved from Drosophila to vertebrates. In vertebrates, the so-called PCP core protein include Frizzled, Van-Gogh like (Vangl), Celsr, Dishevelled (Dvl) and Prickle that are most notably to regulate convergent extension and neural tube closure during embryogenesis [42–45], and also orientation of stereociliary bundles of sensory hair cells in the inner ear in adult animals including humans [45, 46]. Several PCP proteins have been identified in the testis including the PCP core protein Vangl2 [27, 47, 48], Dvl2, Wnt5a, Fuzzy and Dchs1 [27]. Also, in mammals, Vangl2/Prickle and Frizzled/Dvl are two known PCP protein complexes, wherein Vangl2 and Frizzled are integral membrane proteins, and Prickle and Dvl are the corresponding binding adaptor partners to create the two PCP protein complexes [49, 50]. In adult rat testes, Vangl2 localizes at the apical ES and the basal ES at the blood-testis barrier (BTB). At the apical ES, its expression gradually reduces from stage V to IX, while it peaks at stage VIII at the basal ES at the BTB [27]. Interestingly, knockdown of Vangl2 in the testis in vivo disrupted the PCP-like polarity in step 19 spermatids since many spermatid heads had deviated by almost 90° from the intended orientation vs. control testes. Furthermore, secreted frizzled-related protein 1 (sFRP1) has been detected in the testis and shown to be a crucial regulatory of apical ES function [51]. Although not considered to be a PCP core protein, sFRP1 contains a cysteine-rich domain (CRD), homologous to the putative Wnt-binding site of Frizzled proteins, and can competitively bind to Wnts, thereby serving as a soluble modulator of PCP signaling [52–54]. Indeed, intratesticular injection of sFRP1 recombinant protein was shown to cause defects in spermatogenesis by delaying spermiation [51], illustrating a potential regulatory role of Wnt signaling in spermatogenesis.
In this context, it is of interest to note that the apical ES in the testis is typified by the presence of an array of actin filament bundles in the Sertoli cell that sandwiched in-between opposing plasma membranes of the Sertoli cell/spermatid and cisternae of the endoplasmic reticulum [55, 56]. These actin filament bundles thus serve as anchors that confer structural and mechanical support to ES. It is also conceivable that in steps 17–19 spermatids that interact with Sertoli cells via apical ES, which in turn confer spermatid PCP polarity, the actin network at the ES is playing a crucial role. In fact, emerging evidence has shown that PCP proteins exert their effects through changes in actin-based cytoskeletal organization. For instance, the PCP signaling pathways which involve both PCP core proteins and effectors to maintain PCP [57–59] have been shown to modulate actin network remodeling [60–62]. For instance, in Drosophila, Fz/Dsh-mediated PCP signaling that affects wing cell hair PCP is mediated by modulating the assembly of F-actin bundles through Rho-associated kinase [63]. One of the PCP effectors called Wdpcp has been shown to co-localize with actin filaments which induces actin filament stabilization through its interaction with Sept2 [64]. Thus, a loss of Wdpcp negatively affects Sept2 localization, thereby disrupting the actin cytoskeleton [64]. In kidneys, similar to the ES, slit diaphragms (CDs) are specialized actin-based anchoring junctions formed by neighboring foot processes (FPs) of glomerular podocytes which are also highly polarized cells [65, 66]. Many PCP core proteins are expressed in podocytes such as Vangl1, Vangl2, Dvl1, 2 and 3 and also Prickle. Activation of PCP signaling by Wnt5a/Dvl was shown to induce actin reorganization in podocytes in which more actin filaments were found in the cell cortical region [67]. On the other hand, a knockdown of Vangl2 led to a considerable reduction of actin filament bundles in podocytes [67]. This finding is consistent with phenotypes observed in the Sertoli cells in vitro and testis in vivo following Vangl2 knockdown. For instance, Vangl2 knockdown in Sertoli cells reduced actin bundling activity considerably with more actin filaments concentrated at the cortical zones of Sertoli cells. Furthermore, Vangl2 knockdown in the testis perturbed the spatiotemporal expression of actin nucleation protein Arp3 (actin-related protein 3) and actin barbed end capping protein Eps8, facilitating actin filament branching at the apical ES [27]. These changes thus destabilized the function of adhesion protein complexes such as nectin-3/afadin to promote apical ES degeneration [27]. In Drosophila wing hair cells, Vang/Prickle and Fz/Dsh localize at the opposing sides of the adjacent wing cells, and it is now generally accepted that the Vang/Prickle complex and the Fz/Dsh complex promotes F-actin reorganization and maintains actin organization, respectively, through their antagonizing roles in mediating PCP signaling [49, 50].
In the testis, step 19 spermatids are transformed to spermatozoa at late state VIII when the release of sperm takes places at spermiation, concomitant with the disruption of the spermatid PCP. It is likely that PCP proteins may be involved in the cellular events of spermatid release at spermiation through the regulatory role of Vangl2/Prickle vs. Fzd/Dvl on actin organization at the apical ES which are all expressed by Sertoli and/or germ cells in the testis [27]. On the other hand, on the concave side of spermatid heads (Figure 2), at stage VII to early VIII of the epithelial cycle, apical ES transforms into a testis-specific ultrastructure known as the apical tubulobulbar complex (apical TBC) [68–70]. The apical TBC represents a giant endocytic vesicle-based protein trafficking device known to be involved in the elimination of cytoplasm from the head region of late spermatids (i.e., residual bodies) but also for endocytosis and recycling of apical ES proteins (e.g., integrins, laminins, nectins) [69, 70]. Thus, proteins pertinent to modulate endocytic vesicle-mediated protein trafficking, such as clathrins, cortactin, N-WASP [71–74], Apr3 [75], and Eps8 [76] abundantly expressed at the apical TBC site. Since PCP protein Vangl2 has been robustly expressed at the concave side of spermatid heads, namely the site of apical TBC in stage VII tubules [27], plus the observation that Vangl2 is involved in promoting F-actin disorganization [27], we thus hypothesize that Vangl2 PCP signaling is involved in ES dynamics by promoting residual body elimination and recycling of apical ES proteins in late stage VII and earlier VIII tubules when the engulfment of residual body by Sertoli cells takes place, concomitant with the formation of step 8 spermatids which thus requires the assembly of new apical ES to support spermiogenesis (Figure 2). It is obvious that much work is indeed to confirm and expand this model during the events of spermiation, such as the involvement of Fzd and Dvl. In this context, it is noted that Dvl1 has been detected in the testis [47]. Although its localization at the apical ES requires confirmation, Dvl1 has been shown to interact with actin by co-immunoprecipitation [47]. Furthermore, disheveled-associated activator of morphogenesis I (DAAMI) was localized at the apical ES by immunofluorescence microscopy [48]. Moreover, DAAMI has been known to connect Dvl proteins with Rho GTPases during PCP signaling [48, 77], and Rho GTPases are known regulators of actin remodeling [78, 79]. Thus, activation of Dvl-mediated PCP signaling pathway induced by Vangl2 downregulation at stage VII–VIII of the cycle may trigger Rho GTPase-based activation to induce actin dynamics. These possibilities must be carefully evaluated in future studies.
Figure 2. A hypothetical model illustrating the role of PCP proteins (e.g., Vangl2) at the apical tubulobulbar complex (apical TBC) to support protein endocytic vesicle-mediated protein trafficking events.
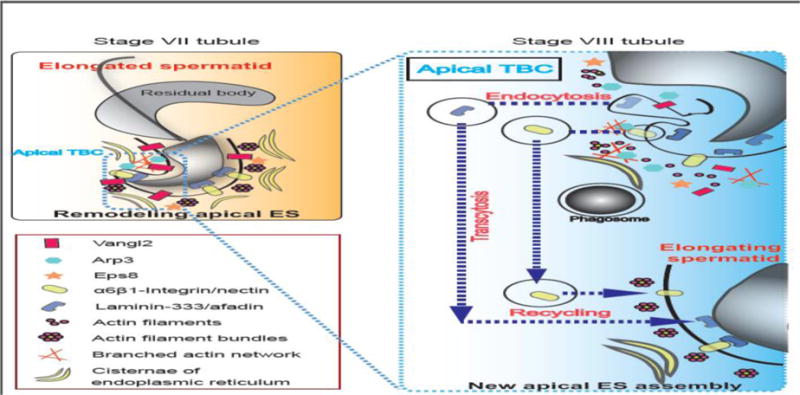
Studies have shown that there is a considerable reduction in the expression of Vangl2 on the apical ES in stage VII tubules, which appears to facilitate the conversion of the apical ES at the concave side of spermatid head to a transient ultrastructure known as apical TBC [19, 70, 85] (left panel). Apical TBC represents a giant endocytic vesicle machinery which supports endocytic-mediated protein trafficking event, facilitating protein endocytosis, transcytosis and recycling so that “old” apical ES proteins (e.g., integrins, nectins, laminins, afadin) can be recycled to assemble “new” apical ES when step 8 spermatids appear in stage VIII tubules (right panel). Abbreviations used: Vangl2, Van Gogh-like 2; Arp3, actin-related protein 3; Eps8, epidermal growth factor receptor pathway substrate 8.
Concluding remarks and future perspectives
In this review, we have reviewed findings in the field that support spermatid PCP in the testis in particular step 17–19 spermatids in stages IV–VIII of the epithelial cycle. Spermatid polarity in step 1–7 spermatids, namely head-tail polarity and apico-basal polarity, is supported by the Par, the Crumbs- and the Scribble-based polarity complexes. However, a total different set of PCP proteins may confer spermatid PCP during spermiogenesis from step 8–19 spermatids in the rat testis. Given the intimate relationship between the head-tail, apico-basal polarity and PCP polarity in step 17–19 spermatids, it is also possible that conventional polarity protein complex such as the Par-, Crb- and Scribble-based complex interact with the PCP proteins, at least functionally, to confer spermatid PCP. Furthermore, besides actin-based cytoskeleton, it remains to be established if microtubules (MTs) are also involved in spermatid polarity and PCP. In fact, emerging evidence supports this possible involvement of MTs in PCP. For example, PCP core protein Dvl has been shown to regulate axon formation by direct binding to aPKC, and by subsequently exerting its effects through an aPKC-based signaling on MTs [80]. Furthermore, Vangl2 and aPKC in Xenopus oocytes was shown to modulate MT stability [81]. Moreover, both Wnt5a and Dvl were involved in the re-orientation of the centrosome and Golgi apparatus during epithelial cell polarization, which is a MT-dependent cellular event, requiring the participation of Cdc42/Par6/aPKC to promote polarized MT re-organization [82, 83]. In short, much research is needed to better understand the role of polarity proteins and PCP proteins on MT dynamics since MTs are localized adjacent to actin filament bundles at the ES, which is also the crucial structure to support spermatid polarity and spermatid PCP. Nonetheless, we have provided a hypothetical model as depicted in Figure 2, regarding the role of PCP in endocytic vesicle-mediated protein trafficking to support protein endocytosis and recycling during spermiogenesis.
Table.
| PCP Component | Function in Wnt/PCP Signaling | Cancer Type | Contribution to Tumorigenesis | Reference |
|---|---|---|---|---|
| WNT5A | Secreted ligand, activates Wnt/PCP signaling | Promote or suppress in context dependent manner | ||
| Breast | Promote cell migration and invasiveness | [25],[26] | ||
| Gastric | Promote cell migration and invasiveness | [27] | ||
| Melanoma | Promote cell motility and invasiveness | [32] | ||
| Colon | Suppress cell proliferation and EMT | [36] | ||
| Hematopoeitic Malignancies | Suppress cell proliferation (inhibit canonical Wnt signaling) | [38] | ||
| Thyroid Carcinoma | Suppress cell proliferation, migration, and invasiveness (inhibits canonical Wnt signaling | [39] | ||
| WNT11 | Secreted ligand, activates Wnt/PCP signaling | Promote or suppress in context dependent manner | ||
| Breast | Promote cell motility and tumor metastasis | [22] | ||
| Colon | Promote cell proliferation, migration and invasiveness | [30] | ||
| Prostate | Promote cell differentiation, survival and migration | [31] | ||
| Hepatocellular Carcinoma | Supress cell proliferation and migration (inhibits canonical Wnt signaling | [37] | ||
| FZD7 | Transmembrane Wnt receptor | |||
| Ovarian | Promote cell migration and invasiveness | [42] | ||
| Hepatocellular Carcinoma | Promote cell migration | [44] | ||
| Colorectal | Promote cell migration | [45] | ||
| VANGL1 | Transmembrane scaffold for Wnt/PCP signaling | |||
| Glioblastoma | Promotes cell migration, scaffold for PCP negative regulator Nrdp1 | [43] | ||
| Colon | Promote cell migration | [46] | ||
| Head and Neck Squamous Cell Carcinoma | Promote cell migration and metastasis | [47] | ||
| Breast | Promote cell migration | [48] | ||
| VANGL2 | Transmembrane scaffold for Wnt/PCP signaling | |||
| Breast | Promotes cell migration and proliferation | [12] | ||
| PRICKLE1 | Cytoplasmic scaffold for Wnt/PCP signaling | |||
| Breast | Cell migration, proliferation and metastasis | [22], [50], [51] | ||
Acknowledgments
This work was supported by grants from the National Institutes of Health, NICHD R01 HD056034 to C.Y.C.; U54 HD029990 Project 5 to C.Y.C.; H.C. was supported by The Fai Lau Memorial Fellowship and The S.Y. Law Memorial Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Conflicts of Interest: Nothing to declare
References
- 1.Martin-Belmonte F, Perez-Moreno M. Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer. 2011;12:23–38. doi: 10.1038/nrc3169. [DOI] [PubMed] [Google Scholar]
- 2.Sokol SY. Spatial and temporal aspects of Wnt signaling and planar cell polarity during vertebrate embryonic development. Semin Cell Dev Biol. 2015;42:78–85. doi: 10.1016/j.semcdb.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.St Johnston D, Ahringer J. Cell polarity in eggs and epithelia: parallels and diversity. Cell. 2010;141:757–74. doi: 10.1016/j.cell.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Xiao X, Mruk DD, Wong CK, Cheng CY. Germ cell transport across the seminiferous epithelium during spermatogenesis. Physiology (Bethesda); 29:286–98. doi: 10.1152/physiol.00001.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karner C, Wharton KA, Jr, Carroll TJ. Planar cell polarity and vertebrate organogenesis. Semin Cell Dev Biol. 2006;17:194–203. doi: 10.1016/j.semcdb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Wada H, Okamoto H. Roles of noncanonical Wnt/PCP pathway genes in neuronal migration and neurulation in zebrafish. Zebrafish. 2009;6:3–8. doi: 10.1089/zeb.2008.0557. [DOI] [PubMed] [Google Scholar]
- 7.de Rooij DG. The spermatogonial stem cell niche. Microsc Res Tech. 2009;72:580–5. doi: 10.1002/jemt.20699. [DOI] [PubMed] [Google Scholar]
- 8.Ehmcke J, Schlatt S. A revised model for spermatogonial expansion in man: lessons from non-human primates. Reproduction. 2006;132:673–80. doi: 10.1530/rep.1.01081. [DOI] [PubMed] [Google Scholar]
- 9.Schlatt S, Ehmcke J. Regulation of spermatogenesis: an evolutionary biologist’s perspective. Semin Cell Dev Biol. 2014;29:2–16. doi: 10.1016/j.semcdb.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc Res Tech. 2010;73:241–78. doi: 10.1002/jemt.20783. [DOI] [PubMed] [Google Scholar]
- 11.Hess RA, de Franca LR. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. doi: 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- 12.de Kretser DM, Kerr JB. The cytology of the testis. In: Knobil E, Neill JB, Ewing LL, Greenwald GS, Markert CL, Pfaff DW, editors. The Physiology of Reproduction. Vol. 1. New York: Raven Press; 1988. pp. 837–932. [Google Scholar]
- 13.Xiao X, Mruk DD, Wong CK, Cheng CY. Germ cell transport across the seminiferous epithelium during spermatogenesis. Physiology (Bethesda) 2014;29:286–98. doi: 10.1152/physiol.00001.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang EI, Mruk DD, Cheng CY. Regulation of microtubule (MT)-based cytoskeleton in the seminiferous epithelium during spermatogenesis. Semin Cell Dev Biol. 2016;59:35–45. doi: 10.1016/j.semcdb.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amann RP. The cycle of the seminiferous epithelium in humans: a need to revisit? J Androl. 2008;29:469–87. doi: 10.2164/jandrol.107.004655. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Mruk DD, Xiao X, Cheng CY. Human spermatogenesis and its regulation. In: Winters SJ, Huhtaniemi IT, editors. Male Hypogonadism, Contemporary Endocrinology. New York: Springer International Publishing AG; 2017. pp. 49–72. DOI: 101007/978-3-319-53298-1_3. [Google Scholar]
- 17.Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 2: changes in spermatid organelles associated with development of spermatozoa. Microsc Res Tech. 2010;73:279–319. doi: 10.1002/jemt.20787. [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis. 2014;4:e979623. doi: 10.4161/21565562.2014.979623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng CY, Mruk DD. Biochemistry of Sertoli cell/germ cell junctions, germ cell transport, and spermiation in the seminiferous epithelium. In: Griswold MD, editor. Sertoli Cell Biology. 2nd. Amsterdam: Elsevier; 2015. pp. 333–383. [DOI] [Google Scholar]
- 20.Gaytan F, Lucena MC, Munoz E, Paniagua R. Morphometric aspects of rat testis development. J Anat. 1986;145:155–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Wing TY, Christensen AK. Morphometric studies on rat seminiferous tubules. Am J Anat. 1982;165:13–25. doi: 10.1002/aja.1001650103. [DOI] [PubMed] [Google Scholar]
- 22.Johnson L, Petty CS, Neaves WB. A comparative study of daily sperm production and testicular composition in humans and rats. Biol Reprod. 1980;22:1233–43. doi: 10.1093/biolreprod/22.5.1233. [DOI] [PubMed] [Google Scholar]
- 23.Leblond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann N Y Acad Sci. 1952;55:548–73. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- 24.Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev. 1982;3:404–17. doi: 10.1210/edrv-3-4-404. [DOI] [PubMed] [Google Scholar]
- 25.Eaton S. Cell biology of planar polarity transmission in the Drosophila wing. Mech Dev. 2003;120:1257–64. doi: 10.1016/j.mod.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Tilney LG, Saunders JC. Actin filaments, stereocilia, and hair cells of the bird cochlea. I. Length, number, width, and distribution of stereocilia of each hair cell are related to the position of the hair cell on the cochlea. J Cell Biol. 1983;96:807–21. doi: 10.1083/jcb.96.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H, Mruk DD, Lee WM, Cheng CY. Planar cell polarity (PCP) protein Vangl2 regulates ectoplasmic specialization dynamics via its effects on actin microfilaments in the testes of male rats. Endocrinology. 2016;157:2140–59. doi: 10.1210/en.2015-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gliki G, Ebnet K, Aurrand-Lions M, Imhof BA, Adams RH. Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature. 2004;431:320–4. doi: 10.1038/nature02877. [DOI] [PubMed] [Google Scholar]
- 29.Mueller S, Rosenquist TA, Takai Y, Bronson RA, Wimmer E. Loss of nectin-2 at Sertoli-spermatid junctions leads to male infertility and correlates with severe spermatozoan head and midpiece malformation, impaired binding to the zona pellucida, and oocyte penetration. Biol Reprod. 2003;69:1330–40. doi: 10.1095/biolreprod.102.014670. [DOI] [PubMed] [Google Scholar]
- 30.Tyagi Xu S, Schedl SP. Spermatid cyst polarization in Drosophila depends upon apkc and the CPEB family translational regulator orb2. PLoS Genet. 2014;10:e1004380. doi: 10.1371/journal.pgen.1004380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita E, Tanabe Y, Hirose T, Aurrand-Lions M, Kasahara T, Imhof BA, et al. Loss of partitioning-defective-3/isotype-specific interacting protein (par-3/ASIP) in the elongating spermatid of RA175 (IGSF4A/SynCAM)-deficient mice. Am J Pathol. 2007;171:1800–10. doi: 10.2353/ajpath.2007.070261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujita E, Kouroku Y, Ozeki S, Tanabe Y, Toyama Y, Maekawa M, et al. Oligo-astheno-tetratozoospermia in mice lacking RA175/TSLC1/SynCAM/IGSF4A, a cell ahesion molecule in the immunoglobulin sperfamily. Mol Cell Biol. 2006;26:718–26. doi: 10.1128/MCB.26.2.718-726.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li N, Mruk DD, Wong CK, Han D, Lee WM, Cheng CY. Formin 1 Regulates Ectoplasmic Specialization in the Rat Testis Through Its Actin Nucleation and Bundling Activity. Endocrinology. 2015;156:2969–83. doi: 10.1210/en.2015-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong EW, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci U S A. 2008;105:9657–62. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lie PP, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–67. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma D, Yang CH, McNeill H, Simon MA, Axelrod JD. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–7. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- 37.Aigouy B, Farhadifar R, Staple DB, Sagner A, Roper JC, Julicher F, et al. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 142:773–86. doi: 10.1016/j.cell.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 38.Jenny A, Mlodzik M. Planar cell polarity signaling: a common mechanism for cellular polarization. Mt Sinai J Med. 2006;73:738–50. [PubMed] [Google Scholar]
- 39.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 138:1877–92. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng Y, Axelrod JD. Asymmetric protein localization in planar cell polarity: mechanisms, puzzles, and challenges. Curr Top Dev Biol. 101:33–53. doi: 10.1016/B978-0-12-394592-1.00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matis M, Axelrod JD. Regulation of PCP by the Fat signaling pathway. Genes Dev. 27:2207–20. doi: 10.1101/gad.228098.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–33. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- 43.Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, et al. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–38. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- 44.Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28:251–5. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- 45.Torban E, Patenaude AM, Leclerc S, Rakowiecki S, Gauthier S, Andelfinger G, et al. Genetic interaction between members of the Vangl family causes neural tube defects in mice. Proc Natl Acad Sci U S A. 2008;105:3449–54. doi: 10.1073/pnas.0712126105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008;4:e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma P, Wang H, Guo R, Ma Q, Yu Z, Jiang Y, et al. Stage-dependent Dishevelled-1 expression during mouse spermatogenesis suggests a role in regulating spermatid morphological changes. Mol Reprod Dev. 2006;73:774–83. doi: 10.1002/mrd.20468. [DOI] [PubMed] [Google Scholar]
- 48.Pariante P, Dotolo R, Venditti M, Ferrara D, Donizetti A, Aniello F, et al. First Evidence of DAAM1 Localization During the Post-Natal Development of Rat Testis and in Mammalian Sperm. J Cell Physiol. 2016;231:2172–84. doi: 10.1002/jcp.25330. [DOI] [PubMed] [Google Scholar]
- 49.Aw WY, Devenport D. Planar cell polarity: global inputs establishing cellular asymmetry. Curr Opin Cell Biol. 2016;44:110–6. doi: 10.1016/j.ceb.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, Mlodzik M. Wnt-Frizzled/planar cell polarity signaling: cellular orientation by facing the wind (Wnt) Annu Rev Cell Dev Biol. 2015;31:623–46. doi: 10.1146/annurev-cellbio-100814-125315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong EW, Lee WM, Cheng CY. Secreted Frizzled-related protein 1 (sFRP1) regulates spermatid adhesion in the testis via dephosphorylation of focal adhesion kinase and the nectin-3 adhesion protein complex. FASEB J. 2012;27:464–77. doi: 10.1096/fj.12-212514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uren A, Reichsman F, Anest V, Taylor WG, Muraiso K, Bottaro DP, et al. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J Biol Chem. 2000;275:4374–82. doi: 10.1074/jbc.275.6.4374. [DOI] [PubMed] [Google Scholar]
- 53.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–34. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 54.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737–46. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- 55.Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. doi: 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- 56.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nature Rev Endocrinol. 2010;6:380–95. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lawrence PA, Casal J, Struhl G. Cell interactions and planar polarity in the abdominal epidermis of Drosophila. Development. 2004;131:4651–64. doi: 10.1242/dev.01351. [DOI] [PubMed] [Google Scholar]
- 58.Chang H, Nathans J. Responses of hair follicle-associated structures to loss of planar cell polarity signaling. Proc Natl Acad Sci U S A. 110:E908–17. doi: 10.1073/pnas.1301430110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- 60.Lu Q, Schafer DA, Adler PN. The Drosophila planar polarity gene multiple wing hairs directly regulates the actin cytoskeleton. Development. 142:2478–86. doi: 10.1242/dev.122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Babayeva S, Zilber Y, Torban E. Planar cell polarity pathway regulates actin rearrangement, cell shape, motility, and nephrin distribution in podocytes. Am J Physiol Renal Physiol. 2011;300:F549–60. doi: 10.1152/ajprenal.00566.2009. [DOI] [PubMed] [Google Scholar]
- 62.Kaucka M, Petersen J, Janovska P, Radaszkiewicz T, Smyckova L, Daulat AM, et al. Asymmetry of VANGL2 in migrating lymphocytes as a tool to monitor activity of the mammalian WNT/planar cell polarity pathway. Cell communication and signaling : CCS. 2015;13:2. doi: 10.1186/s12964-014-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winter CG, Wang B, Ballew A, Royou A, Karess R, Axelrod JD, et al. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- 64.Cui C, Chatterjee B, Lozito TP, Zhang Z, Francis RJ, Yagi H, et al. Wdpcp, a PCP protein required for ciliogenesis, regulates directional cell migration and cell polarity by direct modulation of the actin cytoskeleton. PLoS Biol. 2013;11:e1001720. doi: 10.1371/journal.pbio.1001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Drenckhahn D, Franke RP. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest. 1988;59:673–82. [PubMed] [Google Scholar]
- 66.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007;17:428–37. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 67.Babayeva S, Zilber Y, Torban E. Planar cell polarity pathway regulates actin rearrangement, cell shape, motility, and nephrin distribution in podocytes. Am J Physiol Renal Physiol. 2011;300:F549–60. doi: 10.1152/ajprenal.00566.2009. [DOI] [PubMed] [Google Scholar]
- 68.Russell LD. Further observations on tubulobulbar complexes formed by late spermatids and Sertoli cells in the rat testis. Anat Rec. 1979;194:213–32. doi: 10.1002/ar.1091940204. [DOI] [PubMed] [Google Scholar]
- 69.Russell LD. Spermatid-Sertoli tubulobulbar complexes as devices for elimination of cytoplasm from the head region in late spermatids of the rat. Anat Rec. 1979;194:233–46. doi: 10.1002/ar.1091940205. [DOI] [PubMed] [Google Scholar]
- 70.Vogl AW, Young JS, Du M. New insights into roles of tubulobulbar complexes in sperm release and turnover of blood-testis barrier. Int Rev Cell Mol Biol. 2013;303:319–55. doi: 10.1016/B978-0-12-407697-6.00008-8. [DOI] [PubMed] [Google Scholar]
- 71.Young JS, De Asis M, Guttman J, Vogl AW. Cortactin depletion results in short tubulobulbar complexes and spermiation failure in rat testes. Biol Open. 2012;1:1069–77. doi: 10.1242/bio.20122519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Young JS, Guttman JA, Vaid KS, Vogl AW. Cortactin (CTTN), N-WASP (WASL), and clathrin (CLTC) are present at podosome-like tubulobulbar complexes in the rat testis. Biol Reprod. 2009;80:153–61. doi: 10.1095/biolreprod.108.070615. [DOI] [PubMed] [Google Scholar]
- 73.Young JS, Takai YK, K L, Vogl AW. Internalization of adhesion junction proteins and their association with recycling endosome marker proteins in rat seminiferous epithelium. Reproduction. 2012;143:347–57. doi: 10.1530/REP-11-0317. [DOI] [PubMed] [Google Scholar]
- 74.Young JS, Vogl AW. Focal adhesion proteins zyxin and vinculin are co-distributed at tubulobulbar complexes. Spermatogenesis. 2012;2:63–8. doi: 10.4161/spmg.19391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA. 2010;107:11411–6. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–67. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–54. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 78.Ridley AJ. Rho GTPase signalling in cell migration. Curr Opin Cell Biol. 2015;36:103–12. doi: 10.1016/j.ceb.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sadok A, Marshall CJ. Rho GTPases: masters of cell migration. Small GTPases. 2014;5:e29710. doi: 10.4161/sgtp.29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang X, Zhu J, Yang GY, Wang QJ, Qian L, Chen YM, et al. Dishevelled promotes axon differentiation by regulating atypical protein kinase C. Nat Cell Biol. 2007;9:743–54. doi: 10.1038/ncb1603. [DOI] [PubMed] [Google Scholar]
- 81.Cha SW, Tadjuidje E, Wylie C, Heasman J. The roles of maternal Vangl2 and aPKC in Xenopus oocyte and embryo patterning. Development. 2011;138:3989–4000. doi: 10.1242/dev.068866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schlessinger K, McManus EJ, Hall A. Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J Cell Biol. 2007;178:355–61. doi: 10.1083/jcb.200701083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Etienne-Manneville S, Manneville JB, Nicholls S, Ferenczi MA, Hall A. Cdc42 and Par6-PKCzeta regulate the spatially localized association of Dlg1 and APC to control cell polarization. J Cell Biol. 2005;170:895–901. doi: 10.1083/jcb.200412172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee NPY, Mruk DD, Lee WM, Cheng CY. Is the cadherin/catenin complex a functional unit of cell-cell-actin-based adherens junctions (AJ) in the rat testis? Biol Reprod. 2003;68:489–508. doi: 10.1095/biolreprod.102.005793. [DOI] [PubMed] [Google Scholar]
- 85.O’Donnell L, Nicholls PK, O’Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis. 2011;1:14–35. doi: 10.4161/spmg.1.1.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]


