Abstract
Evidence on the associations of birth weight and prepubertal nutritional status with menarcheal age for low- and middle-income countries is limited. We investigated these relations using the Young Lives younger cohort for 2001 Indian, Peruvian, and Vietnamese girls born in 2001–2002. Girls were followed at approximately ages 1, 5, 8, and 12 years. Weibull survival models estimated hazards of earlier menarche on the basis of birth weight Z-scores (BWZ), and age-8 BMI-for-age Z-scores (BMIZ) and height-for-age Z-scores (HAZ). Estimates controlled for potential individual-, mother-, and household-level confounders and for changes in anthropometry between 1 and 8 years. In adjusted models, BWZ predicted later age at menarche (HR = 0.90, 95% CI: 0.83–0.97). Conversely, HAZ (HR = 1.66, 95% CI 1.5–1.83) and BMIZ at 8 years (HR = 1.28, 95% CI: 1.18–1.38) predicted earlier menarche. Changes in HAZ and BMIZ between 1 and 8 years were not associated with earlier menarche. Associations were consistent across countries, though with variation in estimated magnitudes. Maternal height and age were associated with later menarche. This evidence points to consistently robust and opposite associations of birthweight versus prepubertal attained height and BMI with menarcheal age in three diverse settings in terms of nutrition, ethnicity, and socioeconomic status.
Keywords: menarche, birthweight, childhood height/BMI, low- and middle-income countries, adolescents
Introduction
Menarcheal age, a key indicator of girls’ reproductive maturity, is an important marker of health and social trajectories, and as such it is relevant to public health.1,2 Early menarche is associated with lower attained adult height and higher body mass index (BMI),3–7 some cancers,8 cardiovascular disease,9 psychosocial disorders,10,11 and risky health behaviors,11 with life course implications for health and socioeconomic outcomes for women and the next generation.2,12,13
While the secular fall in menarcheal age in advanced economies has decelerated,3,14,15 evidence points to steep declines in age at first menstruation in countries undergoing rapid health, economic, and social development.16,17 Genetics contribute 50–80% of the variation in menarcheal age,2,14 but modifiable factors, such as child anthropometrics, diets, socioeconomic conditions, endocrine disruptors, and chemical exposures also have been investigated.2,5,15,18–20 A broad cross-sectional and longitudinal literature focuses on birth weight and postnatal nutritional status in predicting timing of menses.18,19,21–27 Studies highlighted inverse relations between prepubertal BMI and/or height and menarcheal age,5,18,26 as well as positive associations between birth weight and menarcheal age,19,21,24,25,28 though with a few exceptions.22,23 Specifically, two studies, one using data from a British cohort and the other data from a cohort in the United States (New York, NY), reported that birth weight was negatively associated with age at menarche once infant growth (as measured by changes in percentile ranks) was controlled for. However, the associations between menarcheal age and both birth weight and infant growth disappeared when controlling for prepubertal body size and composition in the UK cohort and were never statistically significant in the U.S. study.22,23 Inconsistency in these studies may be attributable to methodological differences,2,24 such as varying types of data employed (cohorts vs. clinical studies); the periods of child development considered (e.g., infancy or early or mid-childhood); how nutritional status and its changes were measured (e.g., height and/or weight/BMI vs. gender and age-standardized scores; changes in ranks vs. differences in height and/or weight/BMI; or Z-scores from one period to another); and the relatively small sample sizes of some studies. Another limitation is the focus of most literature on Caucasian samples from high-income countries,2,19,27 with only two studies investigating these relations in multiethnic samples in the United States.23,29
For low- and middle-income countries (LMICs), evidence has suggested that stunting delays puberty,14,30–32 though few studies have investigated birth weight as a marker for prenatal influences.18,33 The mechanisms for the influence of birth weight and postnatal nutrition on menarche are complex but possibly mediated by interactions between environmental exposures and genetics.15,19 Limited research on LMICs hampers generalization of the existing literature to contexts characterized by maternal and child malnutrition (as opposed to overnutrition, which constitutes a key focus of the literature in high-income countries) and rapid shifts in environmental predictors like diets and urbanization.34,35 We addressed this gap by examining longitudinal associations of birth weight and prepubertal nutritional status on earlier menarche in India, Peru, and Vietnam. This is the first study to assess these relations across such economically, socially, and ethnically diverse cohorts.
Materials and methods
Data came from the Indian (Andhra Pradesh, Telangana), Peruvian, and Vietnamese Young Lives (YL) samples. Within each country, YL randomly recruited ~ 2000 children (approximately half girls) born in 2001–2002 from 20 sentinel sites. The first data collection occurred shortly after recruitment (at age ~ 1 year), and subsequent survey waves followed children prospectively in 2006, 2009, and 2013 at approximate ages 5, 8, and 12 years, respectively. In each household, only one child of the target age was recruited at baseline. Sites reflected the variety of each country’s socioeconomic and agroclimatic contexts. In each round, trained field personnel collected detailed data on girls and their households. Parents gave informed consent at enrollment and each subsequent round, and older children gave their assent. Consent, sampling, and related information is reported elsewhere.36 This research was approved by the University of Pennsylvania institutional review board and conformed to the principles embodied by the Declaration of Helsinki. Initially, the ethics committees of the Instituto de Investigación Nutricional and London School of Tropical Hygiene and Medicine approved the YL study in Peru and globally, respectively. Later, the IRB boards from the University of Oxford and the other countries’ lead institutions approved the project (for further information on ethics: http://www.younglives.org.uk/content/research-ethics).
Study measures
Our main outcome was menarcheal age (in years) as assessed in 2013 (round 4, ~ 12 years). Adolescents were asked to report whether menarche had occurred and at what age in years if applicable. No further information about menarche (e.g., exact date of menarche or month/season) was elicited. Birth weight was copied from birth certificates, if available. If the birth certificate was not available, information from other documents, such as vaccination or other health records, was copied, as long as it was originally recorded within 1 week of birth (although the exact date at which the weight was taken, and hence age in days of the baby at the time, was not known). In these cases, the birth weight was recorded as “documented,” but the type of birth weight documentation was not specified. If there was no documentation, then the mother’s report of birth weight was recorded and coded as “not documented.” Birth weight Z-scores (BWZ) were calculated for all girls, and height-for-age Z-scores (HAZ) and BMI-for-age Z-scores (BMIZ) at about age 8 years (in round 3) were calculated using World Health Organization (WHO) international reference standards and the girls’ ages in months.37,38
Analyses
A conceptual model based on the reviewed literature, linking birth weight and postnatal nutrition with age at menarche, underlies our analysis (Fig.1). Weibull survival models estimated hazard rates of menarche by ~ 12 years in relation to BWZ, HAZ, and BMIZ at 8 years. Premenarcheal girls were censored. Hazard ratios (HR) for earlier menarche are reported. Weibull multivariate models included, as potential confounders: dichotomous variables for first-born children and for urban residence at ~ 1 year; maternal height, maternal age at girl’s birth, and maternal schooling; binary indicators of girls’ previous-day consumption of fruits and vegetables, meat and fish, eggs, legumes, and milk and dairy at 8 years; and household socioeconomic status (SES) at 8 years, a composite wealth index (range 0–100) including service access, housing quality, and asset ownership. In fully adjusted models, changes in HAZ and BMIZ between 1 and 8 years were included. HAZ at 1 year was adjusted for age.39 Pooled-sample estimates included country indicator variables. We conducted a range of checks to assess the robustness of our findings. We used Stata 13.1 for all analyses.
Figure 1.
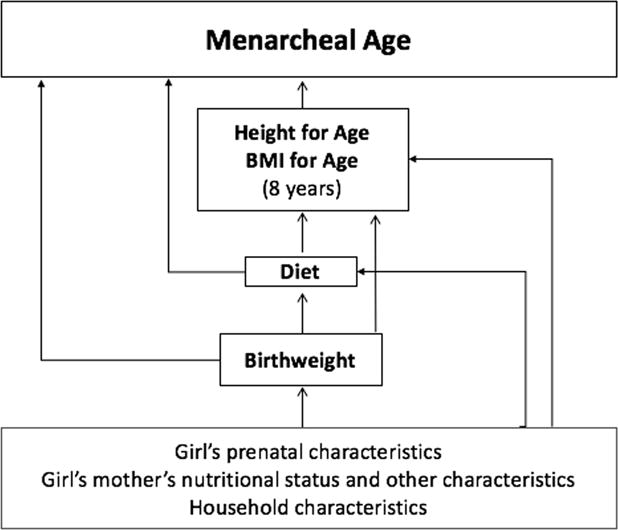
Theoretical framework: birth weight and postnatal associations of nutritional status and menarcheal age.
Results
Of 2931 girls (India: 934; Peru: 1025; Vietnam: 972), 2120 girls had birth weight data (India: 402; Peru: 878; Vietnam: 840). Girls with birth weight data tended to be urban, to be from higher SES families, to have better baseline nutritional status (P < 0.001), and to be more likely to have had menarche by ~ 12 years (P < 0.001). Birth weight was obtained from birth or other health record documentation for 44% of the pooled sample (India: ~ 52%: Peru: ~ 66%; Vietnam: ~ 18%). Of the girls with birth weight data, 119 (5.2%) were lost to follow-up by round 4, so our analytical sample was 2001 girls (India: 379; Peru: 814; Vietnam: 808). Lost-to-follow-up girls were more likely to be urban (P < 0.01) at age ~ 1 year, with no differences by wealth index, birth weight, or maternal education (available upon request).
Menarche information was available for almost all girls interviewed at round 4, with only five exceptions (India: 1; Peru: 1; Vietnam: 3). By age 12 years, 32% (n = 638) had experienced menarche (Table 1), but menarcheal age was missing for 13 of these girls (India: 3; Vietnam: 10).
Table 1.
Description of study sample, stratified by menarche
| Full sample | India | Peru | Vietnam | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre- menaracheal (n = 1358) |
Menarcheal (n = 638) |
Pre- menaracheal (n = 284) |
Menarcheal (n = 94) |
Pre- menaracheal (n = 544) |
Menarcheal (n = 269) |
Pre- menaracheal (n = 540) |
Menarcheal (n = 275) |
|||||
| Age in months (Round 4, 2013) | 144.08 | 145.30 | P < 0.001 | 143.55 | 144.93 | P = 0.003 | 142.42 | 143.93 | P < 0.001 | 146.07 | 146.81 | P = 0.006 |
| (4.00) | (3.82) | (3.91) | (3.63) | (3.51) | (3.78) | (3.65) | (3.35) | |||||
| First child | 0.43 | 0.46 | P = 0.205 | 0.45 | 0.57 | P = 0.032 | 0.39 | 0.42 | P = 0.405 | 0.48 | 0.47 | P = 0.941 |
| (0.50) | (0.50) | (0.50) | (0.50) | (0.49) | (0.49) | (0.50) | (0.50) | |||||
| Maternal height (cm) | 151.43 | 152.10 | P = 0.02 | 151.17 | 152.24 | P = 0.173 | 150.56 | 150.75 | P = 0.657 | 152.43 | 153.35 | P = 0.033 |
| (5.94) | (5.88) | (6.86) | (5.33) | (5.50) | (5.71) | (5.69) | (5.95) | |||||
| Maternal age at girl’s birth | 25.14 | 25.57 | P = 0.144 | 22.48 | 22.16 | P = 0.495 | 25.86 | 25.82 | P = 0.936 | 25.87 | 26.55 | P = 0.116 |
| (5.96) | (6.12) | (3.97) | (4.05) | (6.65) | (6.70) | (5.73) | (5.73) | |||||
| Maternal education (years) | 6.90 | 8.58 | P < 0.001 | 5.02 | 7.18 | P < 0.001 | 7.84 | 9.21 | P < 0.001 | 6.96 | 8.45 | P < 0.001 |
| (4.26) | (4.07) | (4.66) | (4.62) | (4.48) | (4.32) | (3.39) | (3.44) | |||||
| Urban location at 1 year | 0.40 | 0.57 | P < 0.001 | 0.38 | 0.47 | P = 0.117 | 0.65 | 0.79 | P < 0.001 | 0.15 | 0.39 | P < 0.001 |
| (0.49) | (0.50) | (0.49) | (0.50) | (0.48) | (0.41) | (0.37) | (0.49) | |||||
| Birth weight Z-scores | −0.51 | –0.43 | P = 0.126 | –1.18 | –1.21 | P = 0.815 | –0.18 | –0.21 | P = 0.758 | −0.49 | –0.37 | P = 0.108 |
| (1.16) | (1.12) | (1.29) | (1.15) | (1.10) | (1.12) | (0.98) | (0.99) | |||||
| BMI-for-age Z-scores at 1 year | −0.08 | 0.13 | P < 0.001 | −0.99 | −0.64 | P < 0.001 | 0.78 | 0.88 | P =0.242 | –0.47 | –0.33 | P = 0.031 |
| (1.28) | (1.21) | (1.07) | (1.00) | (1.20) | (1.13) | (0.88) | (0.94) | |||||
| Height-for-age Z-scores at 1 year | –1.11 | –0.79 | P < 0.001 | –1.06 | –0.72 | P = 0.028 | –1.18 | –0.92 | P < 0.001 | –1.06 | –0.70 | P < 0.001 |
| (1.20) | (1.19) | (1.32) | (1.28) | (1.18) | (1.28) | (1.16) | (1.04) | |||||
| Variables at 8 years (Round 3, 2009) | ||||||||||||
| BMI-for-age Z-scores | −0.54 | 0.04 | P < 0.001 | −1.29 | −0.84 | P < 0.001 | 0.29 | 0.65 | P < 0.001 | −1.00 | −0.27 | P < 0.001 |
| (1.23) | (1.26) | (0.99) | (1.23) | (1.02) | (1.00) | (1.03) | (1.20) | |||||
| Height-for-age Z-scores | –1.24 | –0.63 | P < 0.001 | –1.33 | –0.77 | P < 0.001 | –1.25 | –0.61 | P < 0.001 | –1.18 | –0.60 | P < 0.001 |
| (0.93) | (0.98) | (0.97) | (0.93) | (0.95) | (1.02) | (0.89) | (0.96) | |||||
| Household wealth index (SES) | 53.44 | 61.73 | P < 0.001 | 52.24 | 59.75 | P < 0.001 | 53.62 | 62.65 | P < 0.001 | 53.89 | 61.51 | P < 0.001 |
| (18.38) | (18.42) | (17.02) | (14.65) | (20.49) | (18.73) | (16.73) | (19.28) | |||||
| Consumed fruits and vegetables the previous day | 0.93 | 0.96 | P = 0.007 | 0.96 | 0.97 | P = 0.762 | 0.89 | 0.95 | P < 0.001 | 0.96 | 0.97 | P = 0.315 |
| (0.25) | (0.19) | (0.19) | (0.18) | (0.32) | (0.22) | (0.20) | (0.16) | |||||
| Consumed meat and fish the previous day | 0.59 | 0.66 | P = 0.002 | 0.10 | 0.12 | P = 0.59 | 0.82 | 0.87 | P = 0.01 | 0.62 | 0.65 | P = 0.342 |
| (0.49) | (0.47) | (0.30) | (0.32) | (0.38) | (0.34) | (0.49) | (0.48) | |||||
| Consumed eggs the previous day | 0.39 | 0.44 | P = 0.075 | 0.13 | 0.15 | P = 0.713 | 0.61 | 0.62 | P = 0.65 | 0.32 | 0.35 | P = 0.339 |
| (0.49) | (0.50) | (0.34) | (0.36) | (0.49) | (0.49) | (0.47) | (0.48) | |||||
| Consumed legumes the previous day | 0.29 | 0.29 | P = 0.95 | 0.19 | 0.17 | P = 0.645 | 0.46 | 0.46 | P = 0.887 | 0.18 | 0.16 | P = 0.595 |
| (0.45) | (0.45) | (0.40) | (0.38) | (0.50) | (0.50) | (0.38) | (0.37) | |||||
| Consumed milk and dairy products the previous day | 0.58 | 0.65 | P = 0.002 | 0.57 | 0.69 | P = 0.378 | 0.77 | 0.79 | P = 0.398 | 0.39 | 0.49 | P = 0.004 |
| (0.49) | (0.48) | (0.50) | (0.46) | (0.42) | (0.41) | (0.49) | (0.50) | |||||
NOTE: Differences in means of continuous variables were assessed using t-tests, while differences in proportion in binary variables were assessed with χ2 tests.
Table 1 presents descriptive statistics by occurrence of menarche by age ~ 12 years. With some cross-country variation, girls who had menarche were generally slightly older, lived in urban areas and higher-SES households, and were more likely to have consumed animal-source protein and fruits and vegetables the previous day. While there were no significant differences in BWZ, postmenarche girls appeared to be on higher HAZ and BMIZ trajectories from an early age. Correlations among HAZ, BMIZ, and BWZ were in the 0.23–0.27 range (P < 0.001). Although our key measures were correlated, implying some degree of overlap in their predictive power, the fact that they were only moderately correlated pointed to some degree of independent predictive power for HAZ, BMIZ, and BWZ in their associations with menarcheal age.
We estimated models sequentially to test the predictive powers of BWZ, HAZ, and BMIZ at 8years and childhood gains in HAZ and BMIZ. Higher BWZ was associated with lower hazard of early menarche only when HAZ and BMIZ at 8 years were included (HR = 0.90, 95% CI: 0.83–0.97) (Table 2, column 3). Greater HAZ (HR = 1.68, 95% CI: 1.54–1.83) and BMIZ (HR = 1.32, 95% CI: 1.23–1.42) at 8 years increased hazards of earlier menarche. Covariate adjustments (column 4) and changes in HAZ and BMIZ between 1 year and 8 years did not affect these findings. Changes in HAZ and BMIZ were not associated with menarcheal age (column 5).
Table 2.
Weibull survival model, pooled sample, hazard ratios, and 95% confidence intervals (in parentheses).
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| Birth weight Z-scores | 1.05 | 0.90*** | 0.88*** | 0.88*** | |
|
|
|||||
| (0.973–1.127) | (0.830–0.967) | (0.813–0.951) | (0.811–0.953) | ||
|
|
|||||
| Height-for-age Z-scores (HAZ) at age 8 years | 1.64*** | 1.68*** | 1.66*** | 1.68*** | |
|
|
|||||
| (1.507–1.788) | (1.540–1.832) | (1.501–1.832) | (1.512–1.871) | ||
|
|
|||||
| BMI-for-age Z-scores (BMIZ) at age 8 years | 1.31*** | 1.32*** | 1.28*** | 1.23*** | |
|
|
|||||
| (1.215–1.402) | (1.231–1.422) | (1.183–1.377) | (1.111–1.354) | ||
|
|
|||||
| First child | 0.90 | 0.89 | |||
|
|
|||||
| (0.739–1.096) | (0.728–1.082) | ||||
|
|
|||||
| Maternal height (cm) | 0.98*** | 0.98*** | |||
|
|
|||||
| (0.964–0.995) | (0.965–0.995) | ||||
|
|
|||||
| Maternal age at girl’s birth | 0.98* | 0.98* | |||
|
|
|||||
| (0.968–1.002) | (0.968–1.001) | ||||
|
|
|||||
| Maternal education (years) | 1.01 | 1.01 | |||
|
|
|||||
| (0.982–1.034) | (0.981–1.033) | ||||
|
|
|||||
| Urban location at age 1 year | 1.26** | 1.28** | |||
|
|
|||||
| (1.008–1.565) | (1.024–1.592) | ||||
|
|
|||||
| SES at age 8 years | 1.01*** | 1.01*** | |||
|
|
|||||
| (1.004–1.016) | (1.003–1.016) | ||||
|
|
|||||
| India (reference country) | |||||
|
|
|||||
| (0.909–1.339) | (0.899–1.324) | ||||
|
|
|||||
| Peru (Reference: India) | 1.69*** | 1.15 | 1.24 | 1.37* | 1.53** |
|
|
|||||
| (1.318–2.155) | (0.885–1.491) | (0.952–1.625) | (0.978–1.916) | (1.070–2.177) | |
|
|
|||||
| Vietnam (Reference: India) | 1.18 | 1.01 | 1.08 | 1.33* | 1.39** |
|
|
|||||
| (0.931–1.504) | (0.794–1.284) | (0.845–1.381) | (0.995–1.789) | (1.034–1.874) | |
|
|
|||||
| Difference in BMIZ between 8 years and 1 years | 0.97 | ||||
|
|
|||||
| (0.878–1.080) | |||||
|
|
|||||
| Difference in HAZ between 8 years and 1 years | 1.07 | ||||
|
|
|||||
| (0.969–1.172) | |||||
|
|
|||||
| Observations | 1987 | 1889 | 1887 | 1806 | 1796 |
Note:
P < 0.01,
P < 0.05,
P < 0.1.
Model 1 includes birth weight Z-scores; model 2 includes prepubertal HAZ and BMIZ; model 3 includes birthweight Z-scores, HAZ, and BMIZ; model 4 includes birthweight Z-scores, HAZ, and BMIZ and adjusts for the following covariates: girl is first child; maternal height in cm; maternal age at girl’s birth; maternal education in years; urban location at age 1 year; socioeconomic status at 8 years; binary indicators of girls’ previous-day consumption of fruits and vegetables, meat and fish, eggs, legumes, and milk and dairy at 8 years. Model 5 builds on model 4 and includes the differences in BMIZ and HAZ between 1 and 8 years. All models include country dummies, with India as the reference country.
Earlier menarche was predicted by household SES (HR = 1.01, 95% CI: 1–1.02) and urban residence (HR = 1.26, 95% CI: 1.01–1.57). No dietary factors were associated with menarcheal age. Girls with shorter and younger mothers had higher earlier-menarche hazards, as did Peruvian and Vietnamese girls (compared with Indians). Girls from higher-SES households and who were living in urban areas at young ages were also more likely to have their first menstrual period earlier. Results were unchanged if birth weight, height, and BMI (and changes in height and BMI) were included instead of Z-scores (Table S1, online only); similarly, they were robust to alternative model specifications based on logistic regression for odds of menarche (available upon request). Estimates were also robust to inclusion of changes in height and BMI between 1 and 5 years and between 5 and 8 years, with no differences noted by age period (available upon request). Separate-country models yielded findings consistent with pooled-sample estimates, though with some cross-country variation (Table S2). As an additional robustness check, we investigated whether having birth weight documentation (available for 44% of the sample), as opposed to reported birth weight, might have affected our estimates. Table S3 reports the results of Weibull survival models for the pooled sample stratified by the availability of birth weight documentation. Models 1–3 present the results of the survival models where birth weight is the only predictive factor, whereas models 4–6 report the estimation results for the adjusted models in which birth weight is considered alongside prepubertal body size (BMI and HAZ) and child, maternal, and household covariates. While the magnitude of the birth weight coefficient did not vary significantly in the stratified models, its estimation did lose precision in the subsample without documented birth weight. Nonetheless, the coefficients for HAZ and BMIZ remained robust (P < 0.01) in these models. Log-rank tests failed to reject the hypothesis of equality of the survival functions between the undocumented and documented subsamples (P = 0.447). Model 7 tested whether the predictive role of birth weight documentation availability varied by country (as there was considerable variation across the three countries in the share of girls that had birth weight documentation); we did not find evidence of significant differences by country.
Finally, to investigate further issues of potential collinearity between BMIZ (that takes height into account) and HAZ beyond bivariate correlations, we ran additional models in which BMIZ and HAZ were separately included as predictors and where BMIZ was substituted with weight-for-age Z-scores (WAZ), which do not take height into account. We did not find evidence of collinearity between BMIZ and HAZ affecting the coefficient estimates of either when examining them separately. Similarly, the coefficient for WAZ was very similar to that of BMIZ (available upon request).
Discussion
Birth weight and postnatal nutritional status may affect the onset of menses in multifaceted and context-specific ways.19,25 We explored these relationships with data from contemporaneous Indian, Peruvian, and Vietnamese cohorts. Our findings highlighted the opposite associations of birth weight versus prepubertal HAZ and BMIZ with earlier menarche hazards. Girls heavier at birth experienced later menarche, while girls with greater prepubertal height and BMI had earlier menarche, consistent with previous studies, which are primarily from high-income contexts.18,19,21,24,25,28,29 Biological causes for these findings are still uncertain.2,19,27 Menarche, a milestone event that marks the transition from childhood to sexual maturation and adolescence, is, together with previous stages of pubertal development, regulated by the hypothalamic–pituitary–gonadal axis.2 A recent review points to activation of the hypothalamic–pituitary–gonadal axis that occurs in utero until the first months after birth and its reactivitation at the start of puberty after a quiescent period during childhood.2 At present, understanding of the exact mechanisms that lead to the reactivation of the hypothalamic–pituitary–gonadal function is incomplete, but the available evidence points to a complex and dynamic interplay of genetic, nutritional, metabolic, and environmental factors.2,14,20 Leptin, insulin, and other hormones have also been hypothesized to play roles in accelerating pubertal onset, leading to earlier menarche, as their concentrations are higher in overweight/obese girls.2,5 Consistent with this literature, we found an association between prepubertal adiposity, for which BMI is a surrogate marker, and earlier menarche.2,5,15,40,41 The literature has also highlighted the role of prepubertal skeletal maturity, in addition to adiposity, for menarcheal timing.5,29,42 We proxied skeletal maturity with HAZ and found that HAZ at ~ 8 years had strong and independent predictive power, pointing to the importance of both HAZ and BMIZ for menarcheal timing. Menarcheal age was not associated with changes in height and BMI between ages 1 and 8 years, suggesting that attained prepubertal size (height and weight), rather than prior growth, was the key predictor of timing of menarche in our sample.
This study was the first to comparatively assess predictive roles of birth weight and postnatal nutrition on menarcheal age using large cross-country and multiethnic cohorts, previously a gap in the literature.2,19,27 Our evidence was consistent across these ethnically diverse samples living in distinct socioeconomic and agroclimatic communities, though with variation in estimated magnitudes. Similar variation in magnitude was observed in multiethnic cohorts from the United States.23,29 Differences in magnitude in our case may be partly attributable to cross-country disparities in genetics and environment.
This study also contributed to the understanding of the influence of environmental and intergenerational factors on timing of menarche in LMICs, which are currently not well characterized.43 Prior evidence is scarce, with studies from a (mostly urban) Filipino sample18 and a low-income, black metropolitan South African population.33 We expanded this literature by examining variation in menarcheal age across urban/rural areas, SES, maternal characteristics, girls’ diets, and other potential predictors in three countries. Consistent with previous literature, we found that urban residence and higher SES were associated with earlier onset,17,18,22,23 although the small magnitude of the household SES coefficient dismisses a strong association with menarche once other child, maternal, and household variables were taken into account. The limited size of the estimated SES coefficient may also reflect the scaling of the variable we used in this analysis (a 100-point scale, ranging from 1 to 100). Additional estimates that we conducted suggested that a one–standard deviation increase in SES would increase the risk of earlier menarche by 20%.
Higher maternal height, a proxy in part for genetic endowments and maternal early-life nutrition status, and older age at childbirth were associated with later menarche, highlighting roles of maternal factors.18,33,43 Previous research has suggested that earlier age at menarche represents an intergenerational marker of a faster growth tempo, characterized by rapid gains in weight and height during infancy and childhood, leading to taller childhood stature, but also increasing the chances of earlier menarche and, consequently, of shorter adult stature.44 Unfortunately, we did not have data on maternal menarche or parental timing of pubertal maturation, which constitute strong determinants of age of pubertal development for the next generation.45 Without information on maternal menarcheal age, we could not verify maternal age at first menstruation as an independent risk factor for daughters’ age at menarche, as a number of studies had previously reported.18,44–46
The associations between diet and the onset of menses was evaluated but were not shown to be confounders or mediators, in contrast to some previous evidence.15 Higher animal protein intakes in mid-childhood were associated with earlier menarche in two contemporaneous cohorts in Germany,47 the United Kingdom,48 Colombia,49 and Iran,50 while vegetable protein intakes were positively associated with menarcheal age in the same German cohort.47 A study from a smaller sample in Boston, Massachusetts reported similar results for (energy-adjusted) animal and vegetable protein.51 In contrast to these studies, which included detailed dietary assessments, we did not have individual food frequencies over a period or quantitative dietary intakes,35 and this may have weakened our capacity to evaluate the relationship between diets and age at first menstruation in our sample.
This study also broadened the literature on roles of BMI in accelerating menarche by examining this relation in high-undernutrition contexts characterized by a contemporaneous trend towards increased overweight and obesity rates.34 Overweight/obesity (BMIZ≥1SD) prevalence at age 8 was 15% in our sample (India: 3%, Vietnam: 10%, Peru: 25%), comparable to the South African cohort52 but lower than the prevalence in advanced economies.53 Although the roles of minimum weight and adiposity thresholds have been established in the literature as critical preconditions for the start of puberty and accelerating the onset of menarche,15,54–56 we used BMIZ rather than WAZ as our key marker for adiposity. Nonetheless, the robustness checks we conducted did not point to strong differences in the predictive power of the two variables or to strong collinearity between BMIZ (which takes height into account) and HAZ. We did not have data for all the study countries on abdominal circumferences, which may have served as an additional measure of prepubertal adiposity,41 or for weight-for-height Z-scores, as the international reference standards are not available for school-age children for this specific measure.57 In the future, we aim to study the relationship between abdominal circumference in childhood and menarche in the case of Peru, which is the only YL country in which this information was collected. A further limitation of this study is lack of birth length data, which precluded assessment of relations between different birth weight/length combinations and menarcheal age18,24 and in-depth examination of infant growth and weight gains.22,23,33 Similarly, lack of gestational age data precluded examining prematurity as a marker for impaired prenatal environment.18,33,58 Another limitation is a reduced sample size due to missing birth weight data.
Furthermore, as there may be systematic differences between birth weights reported by mothers and verified by birth or health cards,59,60 we conducted sensitivity checks to assess whether the availability of birth weight documentation for only 44% of the sample may have affected our estimates. We found that the estimated survival functions between the documented and undocumented subsamples were statistically not different, and the predictive role of birth weight documentation did not vary by country. However, the estimates of the birth weight coefficients were less precise for girls that lacked birth weight documentation, which may be due to the measurement errors in the reported measures of birth weight. The potential biases stemming from maternal reported birth weight as compared with health documentation have been highlighted in recent research conducted in a sample of LMICs.59 Specifically, memory-recalled birth weights tended to overrepresent specific values and the extremes of the weight distribution as compared with birth weights from health cards, although, for some of the countries analyzed (including India), there were no strong differences between recalled birth weight and health cards. Another limitation that we could address with the available data pertains to whether the birth weight recorded from documentation was the weight at birth or the weight within the first week. Differing time points of measurement could introduce measurement error to our estimates, as babies tend to lose their birth weight in the first days after their birth before gaining it back, with some variation in the period of time it takes to regain birth weight.61,62 Although we believe our measure of proxy birth weight is probably more accurate than many other assessments conducted in LMICs (e.g., until recently, most global estimates of birth weight based on household surveys relied on maternal reported size of the baby as compared with other children rather than a continuous measure of weight63), these issues reflect the challenges of measuring birth weight in contexts where a large share of births occur outside health facilities and without the supervision of professional health personnel.59,63
Although our rates of censored observations were similar to previous studies,25,58 future rounds of data will allow investigation that avoids censoring. Furthermore, they will allow inclusion of the Ethiopian sample (also part of the YL study) where, at round 4, almost no girl had experienced menarche. An additional limitation to the present study is that age at menarche was only reported in years, as in several previous studies,22,23,41,48–50 and no other information, such as date or month of menarche, was elicited, in contrast to longitudinal cohort studies from the Philippines18 and Australia.23 Information about the month and year of the first menstrual period would have allowed for the estimation of menarcheal age in months, which in turn would have enhanced the accuracy of our estimates. Furthermore, we could not investigate any seasonality in menarche in these very diverse contexts. In the Northern hemisphere, previous research suggested that menarche peaks in winter or summer as compared with autumn and spring.64–66 We leave the investigation on the relationship between month and season at birth and age at menarche, which was also studied in the context of the Northern hemisphere,66,67 to future research. On the other hand, compared with other studies that asked adult women to retrospectively recall their age at menarche, often many years later,23,41 we anticipate that recall bias for menarcheal age in our data should be relatively lower, given the relatively short time span between the occurrence of menarche and the interview date. Hence, we conclude that the age in years reported should be relatively reliable.41,68,66
Advantages of our data include low attrition rates and related bias through extensive tracking.36 To reduce measurement error, birth weight/vaccination documentation was used when possible,33 and body size measures were carefully assessed through double measurement. Care was devoted to ensure girls could answer potential culturally sensitive questions comfortably.
In LMIC contexts, where child malnutrition is widespread and adult height is associated with key educational, health, and economic outcomes12 as well as with offspring health,13 early menarche may be a concern. Studies from Europe have shown that constrained prenatal growth accelerates progression to menarche and shortens growth spurt durations, resulting in shorter final attained height.27 The findings presented herein suggested that birth weight is predictive of later menarcheal age and thus increased exposure time to linear growth that results in higher adult height. This evidence highlighted the roles of both maternal and early-life factors, as captured by maternal height and growth in utero, as well as prepubertal physiological conditions, in predicting the menarcheal timing. Height and weight growth between birth and age 8 years appeared to be largely irrelevant––what mattered more (in addition to birth weight) was prepubertal body size. Thus, improving nutrition for pregnant women to ensure a healthy birth weight is important; interventions for postnatal infant and child nutrition should be geared toward healthy eating habits and lifestyles to avoid childhood overweight/obesity and to ensure that menarche does not occur prematurely.
Supplementary Material
Table S1. Weibull model with raw birth weight, height and BMI, pooled sample, hazard ratios, and 95% confidence intervals.
Table S2. Weibull survival model stratified by country, hazard ratios, and 95% confidence intervals.
Table S3. Robustness check for availability of birth weight documentation. Weibull survival models, hazard ratios, and 95% confidence intervals.
Acknowledgments
This study was supported by the Sackler Institute Collaborative Initiative Aimed at Using Existing Datasets to Conduct Research on Adolescent Women’s Nutritional Status (Grant Number 567850), the Eunice Shriver Kennedy National Institute of Child Health and Development (NICHD, Grant R01 HD070993), and Grand Challenges Canada (Grant 0072-03). We thank attendees at the Sackler grantee meeting on November 3–4, 2016 at the Sackler Insitute in New York and at the Young Lives Conference on Adolescence, Youth and Gender: Building Knowledge for Change, September 8–9, 2016 at the University of Oxford for valuable comments on our research findings. The authors would like to acknowledge Young Lives, an international study of childhood poverty, for providing them with preferential access to the data. Young Lives has been funded from 2001 to 2017 by UK Aid from the Department for International Development (DFID) and was co-funded by Irish Aid from 2014 to 2015. None of the funders had a role in the design, analysis, or writing of this article. The views expressed are those of the authors. They are not necessarily those of or endorsed by Young Lives, the University of Oxford, or DFID. E.A., W.S., M.E.P., and J.R.B. contributed to the conceptualization, data interpretation, writing, and revision of this paper. E.A. conducted the data analysis.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Bellis MA, Downing J, Ashton JR. Adults at 12? Trends in puberty and their public health consequences. J Epidemiol Community Health. 2006;60(11):910–911. doi: 10.1136/jech.2006.049379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abreu AP, Kaiser UB. Pubertal development and regulation. Lancet Diabetes Endocrinol. 2016;4(3):254–264. doi: 10.1016/S2213-8587(15)00418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onland-Moret NC, Peeters PHM, van Gils CH, et al. Age at menarche in relation to adult height: the EPIC study. Am J Epidemiol. 2005;162(7):623–632. doi: 10.1093/aje/kwi260. [DOI] [PubMed] [Google Scholar]
- 4.Pierce MB, Leon DA. Age at menarche and adult BMI in the Aberdeen Children of the 1950s Cohort Study. Am J Clin Nutr. 2005;82(4):733–739. doi: 10.1093/ajcn/82.4.733. [DOI] [PubMed] [Google Scholar]
- 5.Marcovecchio ML, Chiarelli F. Obesity and growth during childhood and puberty. World Rev Nutr Diet. 2013;106:135–141. doi: 10.1159/000342545. [DOI] [PubMed] [Google Scholar]
- 6.Stein AD, Lundeen EA, Martorell R, et al. Pubertal Development and Prepubertal Height and Weight Jointly Predict Young Adult Height and Body Mass Index in a Prospective Study in South Africa. J Nutr. 2016;146(7):1394–1401. doi: 10.3945/jn.116.231076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yousefi M, Karmaus W, Zhang H, et al. Relationships between age of puberty onset and height at age 18 years in girls and boys. World J Pediatr. 2013;9(3):230–238. doi: 10.1007/s12519-013-0399-z. [DOI] [PubMed] [Google Scholar]
- 8.Parkin D. 15. Cancers attributable to reproductive factors in the UK in 2010. Br J Cancer. 2011;105(10):73–76. doi: 10.1038/bjc.2011.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charalampopoulos D, Mcloughlin A, Elks CE, Ong KK. Systematic Reviews and Meta- and Pooled Analyses Age at Menarche and Risks of All-Cause and Cardiovascular Death : A Systematic Review and Meta-Analysis. 2014;180(Ci):29–40. doi: 10.1093/aje/kwu113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendle J, Turkheimer E, Emery RE. Detrimental Psychological Outcomes Associated with Early Pubertal Timing in Adolescent Girls. Dev Rev. 2007;27(2):151–171. doi: 10.1016/j.dr.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaudineau A, Ehlinger V, Vayssiere C, et al. Factors associated with early menarche: results from the French Health Behaviour in School-aged Children (HBSC) study. BMC Public Health. 2010;10(1):175. doi: 10.1186/1471-2458-10-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adair LS, Fall CH, Osmond C, et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382(9891):525–534. doi: 10.1016/S0140-6736(13)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaw Addo O, Stein AD, Fall CH, et al. Maternal Height and Child Growth Patterns, on behalf of the Consortium on Health Orientated Research in Transitional Societies (COHORTS) Group*. J Pediatr. 2013;163:549–554.e1. doi: 10.1016/j.jpeds.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole TJ. Secular trends in growth. Proc Nutr Soc. 2000;59(2):317–324. doi: 10.1017/s0029665100000355. [DOI] [PubMed] [Google Scholar]
- 15.Biro FM, Kiess W. Contemporary Trends in Onset and Completion of Puberty, Gain in Height and Adiposity. Endocrine Development. 2016;29:122–133. doi: 10.1159/000438881. [DOI] [PubMed] [Google Scholar]
- 16.Prentice S, Fulford AJ, Jarjou LMA, Goldberg GR, Prentice A. Evidence for a downward secular trend in age of menarche in a rural Gambian population. Ann Hum Biol. 2010;37(5):717–721. doi: 10.3109/03014461003727606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pathak PK, Tripathi N, Subramanian SV. Secular trends in menarcheal age in india-evidence from the Indian Human Development Surveypa. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0111027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adair LS. Size at birth predicts age at menarche. Pediatrics. 2001;107(4):E59. doi: 10.1542/peds.107.4.e59. [DOI] [PubMed] [Google Scholar]
- 19.Hui LL, Leung GM, Wong M, Lam TH, Schooling CM. Small for gestational age and age at puberty: evidence from Hong Kong’s “Children of 1997” birth cohort. Am J Epidemiol. 2012;176(9):785–793. doi: 10.1093/aje/kws159. [DOI] [PubMed] [Google Scholar]
- 20.Roth CL, DiVall S. Consequences of Early Life Programing by Genetic and Environmental Influences: A Synthesis Regarding Pubertal Timing. Endocrine Development. 2016;29:134–152. doi: 10.1159/000438883. [DOI] [PubMed] [Google Scholar]
- 21.Cooper C. Childhood growth and age at menarche. Br J Obs Gynaecol. 1996 Aug;103:814–817. doi: 10.1111/j.1471-0528.1996.tb09879.x. [DOI] [PubMed] [Google Scholar]
- 22.dos Santos Silva I, De Stavola BL, Mann V, Kuh D, Hardy R, Wadsworth MEJ. Prenatal factors, childhood growth trajectories and age at menarche. Int J Epidemiol. 2002;31(2):405–412. doi: 10.1093/ije/31.2.405. [DOI] [PubMed] [Google Scholar]
- 23.Terry MB, Ferris JS, Tehranifar P, Wei Y, Flom JD. Birth weight, postnatal growth, and age at menarche. Am J Epidemiol. 2009;170(1):72–79. doi: 10.1093/aje/kwp095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tam CS, De Zegher F, Garnett SP, Baur LA, Cowell CT. Opposing influences of prenatal and postnatal growth on the timing of menarche. J Clin Endocrinol Metab. 2006;91(11):4369–4373. doi: 10.1210/jc.2006-0953. [DOI] [PubMed] [Google Scholar]
- 25.Sloboda DM, Hart R, Doherty DA, Pennell CE, Hickey M. Age at menarche: Influences of prenatal and postnatal growth. J Clin Endocrinol Metab. 2007;92(1):46–50. doi: 10.1210/jc.2006-1378. [DOI] [PubMed] [Google Scholar]
- 26.Blell M, Pollard TM, Pearce MS. Predictors of age at menarche in the newcastle thousand families study. J Biosoc Sci. 2008;40(4):563–575. doi: 10.1017/S0021932007002696. [DOI] [PubMed] [Google Scholar]
- 27.Ibanez L, Ferrer A, Marcos MV, Hierro FR, de Zegher F. Early Puberty: Rapid Progression and Reduced Final Height in Girls With Low Birth Weight. Pediatrics. 2000;106(5):e72–e72. doi: 10.1542/peds.106.5.e72. [DOI] [PubMed] [Google Scholar]
- 28.Persson I, Ahlsson F, Ewald U, et al. Influence of perinatal factors on the onset of puberty in boys and girls: implications for interpretation of link with risk of long term diseases. Am J Epidemiol. 1999;150(7):747–755. doi: 10.1093/oxfordjournals.aje.a010077. [DOI] [PubMed] [Google Scholar]
- 29.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Relation of age at menarche to race, time period, and anthropometric dimensions: the Bogalusa Heart Study. Pediatrics. 2002;110(4):e43. doi: 10.1542/peds.110.4.e43. [DOI] [PubMed] [Google Scholar]
- 30.Simondon KB, Simondon F, Simon I, et al. Preschool stunting, age at menarche and adolescent height: a longitudinal study in rural Senegal. Eur J Clin Nutr. 1998;52:412–418. doi: 10.1038/sj.ejcn.1600577. [DOI] [PubMed] [Google Scholar]
- 31.Khan AD, Schroeder DG, Martorell R, Haas JD. Early Childhood Determinants of Age at Menarche in Rural Guatemala. Am J Hum Biol. 1996;723(8):717–723. doi: 10.1002/(SICI)1520-6300(1996)8:6<717::AID-AJHB3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 32.Friedman JF, Phillips-Howard PA, Mirel LB, et al. Progression of stunting and its predictors among school-aged children in western Kenya. Eur J Clin Nutr. 2005;59(8):914–922. doi: 10.1038/sj.ejcn.1602161. [DOI] [PubMed] [Google Scholar]
- 33.Salgin B, Norris SA, Prentice P, et al. Even transient rapid infancy weight gain is associated with higher BMI in young adults and earlier menarche. Int J Obes (Lond) 2015;39(6):939–944. doi: 10.1038/ijo.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70(1):3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aurino E, Fernandes M, Penny ME. The nutrition transition and adolescents’ diets in low- and middle-income countries: a cross-cohort comparison. Public Health Nutr. 2017;20(1):72–81. doi: 10.1017/S1368980016001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnett I, Ariana P, Petrou S, et al. Cohort profile: The young lives study. Int J Epidemiol. 2013;42(3):701–708. doi: 10.1093/ije/dys082. [DOI] [PubMed] [Google Scholar]
- 37.de Onis M, Garza C, Victora CG, Bhan MK, Norum KR. The WHO Multicentre Growth Reference Study (MGRS): Rationale, planning, and implementation. Food Nutr Bull. 2004;25(1 (supplement 1)):S3–S89. [Google Scholar]
- 38.de Onis M, et al. development of a WHO growth reference for school-aged children and adolescents children and adolescents. Bull World Health Organ. 2007;85(9):649–732. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crookston BT, Schott W, Cueto S, et al. Postinfancy growth, schooling, and cognitive achievement: Young lives 1–4. Am J Clin Nutr. 2013;98(6):1555–1563. doi: 10.3945/ajcn.113.067561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121(Suppl):S208–S217. doi: 10.1542/peds.2007-1813F. [DOI] [PubMed] [Google Scholar]
- 41.Bubach S, Menezes AMB, Barros FC, et al. Impact of the age at menarche on body composition in adulthood: results from two birth cohort studies. BMC Public Health. 2016;16(1):1007. doi: 10.1186/s12889-016-3649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellison PT. Prediction of age at menarche from annual height increments. Am J Phys Anthropol. 1981;56(1):71–75. doi: 10.1002/ajpa.1330560108. [DOI] [PubMed] [Google Scholar]
- 43.Hui LL, Leung GM, Lam TH, Schooling CM. Inter-generational influences on age at onset of puberty: Hong Kong’s “children of 1997” birth cohort. Int J Epidemiol. 2012;41(1):292–300. doi: 10.1093/ije/dyr163. [DOI] [PubMed] [Google Scholar]
- 44.Ong KK, Northstone K, Wells JCK, et al. Earlier mother’s age at menarche predicts rapid infancy growth and childhood obesity. PLoS Med. 2007;4(4):e132. doi: 10.1371/journal.pmed.0040132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wohlfahrt-Veje C, Mouritsen A, Hagen CP, et al. Pubertal Onset in Boys and Girls Is Influenced by Pubertal Timing of Both Parents. J Clin Endocrinol Metab. 2016;101:2667–2674. doi: 10.1210/jc.2016-1073. [DOI] [PubMed] [Google Scholar]
- 46.Ersoy B, Balkan C, Gunay T, Egemen A. The factors affecting the relation between the menarcheal age of mother and daughter. Child Care Health Dev. 2005;31(3):303–308. doi: 10.1111/j.1365-2214.2005.00501.x. [DOI] [PubMed] [Google Scholar]
- 47.Gunther, Karaolis-danckert N, Kroke A, Remer T, Buyken AE. Dietary Protein Intake throughout Childhood Is Associated with the Timing of Puberty 1–3. 2010;(17):565–571. doi: 10.3945/jn.109.114934. [DOI] [PubMed] [Google Scholar]
- 48.Rogers IS, Northstone K, Dunger DB, Cooper AR, Ness AR, Emmett PM. Diet throughout childhood and age at menarche in a contemporary cohort of British girls. Public Health Nutr. 2010;13(12):2052–2063. doi: 10.1017/S1368980010001461. [DOI] [PubMed] [Google Scholar]
- 49.Jansen EC, Marin C, Mora-Plazas M, Villamor E. Higher Childhood Red Meat Intake Frequency Is Associated with Earlier Age at Menarche. J Nutr. 2016;146(4):792–798. doi: 10.3945/jn.115.226456. [DOI] [PubMed] [Google Scholar]
- 50.Ramezani Tehrani F, Mirmiran P, Gholami R, Moslehi N, Azizi F. Factors influencing menarcheal age: results from the cohort of tehran lipid and glucose study. Int J Endocrinol Metab. 2014;12(3):e16130. doi: 10.5812/ijem.16130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berkey CS, Gardner JD, Lindsay Frazier A, Colditz GA. Relation of childhood diet and body size to menarche and adolescent growth in girls. Am J Epidemiol. 2000;152(5):446–452. doi: 10.1093/aje/152.5.446. [DOI] [PubMed] [Google Scholar]
- 52.Lundeen EA, Norris SA, Martorell R, et al. Early Life Growth Predicts Pubertal Development in South African Adolescents. J Nutr. 2016;146(3):622–629. doi: 10.3945/jn.115.222000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nicklas TA, Baranowski T, Cullen KW, et al. Eating Patterns, Dietary Quality and Obesity. J Am Coll Nutr. 2001;20(6):599–608. doi: 10.1080/07315724.2001.10719064. [DOI] [PubMed] [Google Scholar]
- 54.Abreu AP, Kaiser UB, Terasawa E, et al. Pubertal development and regulation. Lancet Diabetes Endocrinol. 2016;4(3):254–264. doi: 10.1016/S2213-8587(15)00418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frisch RE, Revelle R. Height and weight at menarche and a hypothesis of menarche. Arch Dis Child. 1971;46(249):695–701. doi: 10.1136/adc.46.249.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frisch RE, McArthur JW. Menstrual cycles: fatness as a determinant of minimum weight for height necessary for their maintenance or onset. Science. 1974;185(4155):949–951. doi: 10.1126/science.185.4155.949. [DOI] [PubMed] [Google Scholar]
- 57.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koziel S, Jankowska EA. Effect of low versus normal birthweight on menarche in 14-year-old Polish girls. J Paediatr Child Health. 2002;38(3):268–271. doi: 10.1046/j.1440-1754.2002.00793.x. [DOI] [PubMed] [Google Scholar]
- 59.Channon AAR, Padmadas SS, McDonald JW. Measuring Birth Weight in Developing Countries: Does the Method of Reporting in Retrospective Surveys Matter? Matern Child Health J. 2011;15(1):12–18. doi: 10.1007/s10995-009-0553-3. [DOI] [PubMed] [Google Scholar]
- 60.Subramanyam MA, Ackerson LK, Subramanian SV. Patterning in Birthweight in India : Analysis of Maternal Recall and Health Card Data. 2010;5(7):1–9. doi: 10.1371/journal.pone.0011424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wright CM, Parkinson KN. Postnatal weight loss in term infants: what is normal and do growth charts allow for it? Arch Dis Child Fetal Neonatal Ed. 2004;89(3):F254–7. doi: 10.1136/adc.2003.026906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noel-Weiss J, Courant G, Woodend AK. Physiological weight loss in the breastfed neonate: a systematic review. Open Med. 2008;2(4):e99–e110. [PMC free article] [PubMed] [Google Scholar]
- 63.United Nations Children’s Fund and World Health Organization. Low Birthweight Country, Regional and Global Estimates. New York: UNICEF; 2004. [Google Scholar]
- 64.Rodgers JL, Buster M. Seasonally of Menarche among U.S. Females: Correlates and Linkages. Ann N Y Acad Sci. 1994;709(1):196–196. doi: 10.1111/j.1749-6632.1994.tb30398.x. [DOI] [PubMed] [Google Scholar]
- 65.Matchock RL, Susman EJ, Brown FM. Seasonal rhythms of menarche in the United States: Correlates to menarcheal age, birth age, and birth month. Women’s Heal Issues. 2004;14(6):184–192. doi: 10.1016/j.whi.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 66.Boldsen JL. Season of birth and recalled age at menarche. J Biosoc Sci. 1992;24(2):167–173. doi: 10.1017/s0021932000019702. [DOI] [PubMed] [Google Scholar]
- 67.Kliś K, Jarzebak K, Borowska-Strugińska B, Mulawa A, Zurawiecka M, Wronka I. Season of birth influences the timing of first menstruation. Am J Hum Biol. 2016;28(2):226–232. doi: 10.1002/ajhb.22783. [DOI] [PubMed] [Google Scholar]
- 68.Koo MM, Rohan TE. Accuracy of short-term recall of age at menarche. Ann Hum Biol. 1997;24(1):61–64. doi: 10.1080/03014469700004782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Weibull model with raw birth weight, height and BMI, pooled sample, hazard ratios, and 95% confidence intervals.
Table S2. Weibull survival model stratified by country, hazard ratios, and 95% confidence intervals.
Table S3. Robustness check for availability of birth weight documentation. Weibull survival models, hazard ratios, and 95% confidence intervals.


