Abstract
Objective
A growing body of research examining biological factors associated with suicidal behaviors highlights the role of brain-derived neurotropic factor (BDNF), involved in neurogenesis and synaptic plasticity. There is evidence suggesting that suicide attempters have lower BDNF levels than those with no history of suicide attempts. The key question addressed in the current investigation is whether differences in circulating BDNF levels persist beyond the current suicidal episode and would be observed in those with a past history of suicide attempts (SA).
Method
Plasma levels of BDNF were assessed in 73 women from the community.
Results: We found that women with a history of SA exhibited lower levels of BDNF than women with no SA history and this difference was maintained after statistically controlling for the influence of other potential psychiatric or demographic factors.
Conclusions
These findings support and extend existing research by suggesting that circulating BDNF levels are decreased among individuals with a history of SA compared to individuals with no history of SA. This relation appeared to be specific to women’s history of SA and was not explained by other potential psychiatric or demographic factors, which further highlights the role of BDNF as a promising biomarker for suicidal behavior.
Keywords: brain-derived neurotrophic factor (BDNF), suicide attempts, suicidality, biomarker
Over 800,000 individuals die by suicide yearly worldwide (“WHO | Suicide,” 2015) and, despite decades of research, suicide rates continue to rise (Curtin, Warner, & Hedegaard, 2016). An important step in developing more effective prevention and intervention programs is identifying robust indicators of risk, including biomarkers. Because a history of prior suicide attempt (SA) is the single best predictor of future suicide in the general population (Teti, Rebok, Rojas, Grendas, & Daray, 2014), this population may be ideally suited for the initial identification of these biomarkers.
Altered synaptic plasticity, which results in difficulties adapting to environmental stimuli, may play a role in the development and pathogenesis of suicidal behavior (Altshuler et al., 1990; Rajkowska, 1997; Duman et al., 2000; Garcia, 2002; Fossati et al., 2004; Torres-Platas et al., 2011; Dwivedi, 2012; Bijttebier et al., 2015; Nagy et al., 2015; Minzenberg et al., 2016). Given the critical involvement of neurotrophins, including brain-derived neurotrophic factor (BDNF), in neuronal plasticity, functioning, and neurogenesis, a number of studies have examined the association between BDNF and suicidal behavior, with the majority of studies yielding results supporting this association (for reviews, see Dwivedi, 2012; Eisen et al., 2015; Shrivastava et al., 2016). There is also evidence of decreased protein and mRNA expression of BDNF in the PFC and the hippocampus of individuals who died by suicide, regardless of depression history (Dwivedi et al., 2003; Karege, Vaudan, Schwald, Perroud, & La Harpe, 2005). Moreover, these decreases were absent in suicide completers who were receiving antidepressant treatment, which highlights the potential involvement of BDNF as a mechanism in treatment response (Karege, Bondolfi, et al., 2005). Additionally, meta-analytic findings suggest a link between a polymorphism in the BDNF gene (Val66Met) and suicide, such that carriers of the Met allele, which has been linked to lower expression of BDNF (Egan et al., 2003), were more likely to have a history of suicide attempts (Zai et al., 2012). Finally, higher methylation of CpG sites in the promoter region of the BDNF gene, which translates into lower BDNF mRNA and protein levels, was observed among individuals who died by suicide compared to individuals who died by other causes (Keller et al., 2010).
Despite the strengths of these studies, the majority of have focused on postmortem samples, currently suicidal or depressed individuals, or those with a recent suicide attempt (Dawood et al., 2007; Deveci et al., 2007; Eisen et al., 2016; Kim et al., 2007; Lee and Kim, 2009). However, to be a viable biomarker of risk, it is essential that the reductions in BDNF levels in those at risk for future suicide (e.g., those with a history of SA) be observed outside of the context of a current suicidal crisis. Thus, it remains unclear how long the changes in circulating BDNF levels persist beyond the current episode of suicidal thoughts/behavior or depression. In this project, we examined BDNF levels in individuals with and without lifetime history of SA in a community sample. We hypothesized that individuals with a history of SA would exhibit lower circulating levels of BDNF than individuals with no history of SA and that this difference would be at least partially independent of participants’ history of major depression, current depressive symptoms, current levels of suicidal ideation, and other demographic characteristics that may differ between the groups and be related to BDNF levels (e.g., income, age, ethnicity, lifetime cigarette smoking history, BMI). We also specifically examined the potential impact of the recency of the SA to determine whether any SA-related differences were due to recent suicide attempters.
METHOD
Participants and procedure
Participants were 73 women recruited from the community as part of a larger study (n = 955) focused on risk for depression and anxiety in children. Participants in this study were mothers of these children and the only inclusion criteria were that they have a child between the ages of 7 and 11 years old. From this larger sample, we selected 34 women who had a lifetime history of SA and 39 women who had no lifetime history of SA and they were matched on age, ethnicity, family income, BMI, and cigarette smoking history. The average age of the participants was 33.03 years old (SD = 5.50, Range = 24–47). The majority (60.3%) was Caucasian and the rest were African American. The median annual family income was $20,001–25,000. Table 1 contains descriptive statistics for participants with and without a lifetime history of SA. Upon arrival at the laboratory, women were asked to provide informed consent and were then administered clinical interviews. Following this, participants completed questionnaires and provided biological samples. All women were compensated $80 for their participation in the larger project, which was approved by the university’s institutional review board.
Table 1.
Group characteristics
| No SA (n = 39) |
History of SA (n = 34) |
F/χ2 | |
|---|---|---|---|
| Age (M, SD) | 32.44 (5.05) | 33.71 (5.97) | 0.97 |
| Ethnicity (% Caucasian) | 53.8 | 67.6 | 1.45 |
| BDI-II (M, SD) | 10.29 (9.27) | 15.20 (10.45) | 4.50* |
| BAI (M, SD) | 6.32 (7.52) | 9.33 (8.17) | 2.69 |
| Current suicidal ideation (% yes) | 5.1 | 11.8 | 1.06 |
| Current MDD diagnosis (% yes) | 15.4 | 23.5 | 0.78 |
| Past MDD diagnosis (% yes) | 53. | 88.2 | 2.89 |
| Annual median family income | 20,001–25,000 | 20,001–25,000 | 0.12 |
| Lifetime cigarette smoking (% who smoked ≥ 100) | 46.2 | 76.5 | 6.97* |
| BMI (M, SD) | 33.27 (9.88) | 32.69 (8.21) | 0.07 |
| Body temperature (°C) (M, SD) | 36.77 (0.40) | 36.62 (0.37) | 2.89 |
| Total protein concentration (M, SD) | 99.81 (29.74) | 93.47 (29.62) | 0.95 |
Note. BDI-II = Beck Depression Inventory-II. BAI = Beck Anxiety Inventory. BMI = Body mass index.
p < .05.
Measures
Participants’ current and past DSM-IV Axis I disorders were assessed using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First, Spitzer, Gibbon, & Williams, 2002). Of the participants, 51 (30 in the SA group) had a lifetime history of major depressive disorder (MDD), with 14 participants (8 in the SA group) meeting criteria for current MDD. In terms of other diagnoses, 30 (19 in the SA group) had a lifetime history of at least one anxiety disorder (17 current), 25 (13 in the SA group) had a lifetime history of alcohol use disorder (none current), 17 (11 in the SA group) had a history of substance use disorder (none current), 4 (2 in the SA group) had a lifetime history of bipolar disorder (2 current), and one participant, who was in the SA group, had a history of past anorexia. As part of the larger project, a subset of 20 SCID-I interviews was coded by a second interviewer to assess inter-rater reliability of diagnoses and the reliability for diagnoses of MDD was good (κ = 0.89).
Participants’ current symptoms of depression and anxiety were assessed using the Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996) and the Beck Anxiety Inventory (BAI; Beck & Steer, 1993), respectively. In this sample, this measures showed excellent internal consistency (α = .92 and .90). The average levels of depressive and anxiety symptoms in our sample were low (M = 12.57, SD = 10.07; M = 7.72, SD = 7.92). The Beck Scale for Suicide Ideation (SSI; Beck, Kovacs, & Weissman, 1979) was used to assess participants’ history of suicide attempts, which are defined as intentional self-injury with intent to die. As noted above, 34 participants had a history of at least one prior suicide attempt. Of these, 19 had only one SA and 15 had a history of multiple attempts. Among the participants with multiple attempts, the median number of attempts was 2.00 (SD = 3.36, Range = 2–15). Participants’ average age at the time of the most recent attempt was 20.85 years old (SD = 7.96), which was, on average, 13.46 years (SD = 8.25) prior to this assessment. Among those with a history of SA, BDNF levels were not significantly associated with individual differences in age at the time of the most recent attempt (r(34) = .19, p = .28) or time since the most recent attempt (r(34) = .06, p = .75). Because the SSI focused on worst-point suicidal ideation, we used item 9 from the BDI-II to index participants’ current suicidal ideation (cf. Jin et al., 2013; Woosley, Lichstein, Taylor, Riedel, & Bush, 2014). The response options for this item are “I don’t have any thoughts of killing myself,” “I have thoughts of killing myself, but I would not carry them out,” “I would like to kill myself,” and “I would kill myself if I had the chance. All but 6 participants denied any current suicidal ideation and the remainder (4 of whom had a history of SA), endorsed “I have thoughts of killing myself, but I would not carry them out.”
Whole blood was collected via BD Vacutainer Blood Collection Sets into 4.0 mL tubes, coated with Ethylenediaminetetraacetic acid (EDTA). The majority of sthe amples (53.8%) was collected between 16:00–20:00 and the rest were collected between 11:00–15:00. There was no significant difference in time of sample collection based on participants’ history of SA. Plasma was separated by centrifugation (1000 × g for 10 minutes at 4 °C) and stored at −80 °C. Quantitative multiplex enzyme immunoassay (ELISA) arrays were used to assay BDNF concentrations (Ray Biotech Inc., Norcross, GA) and each sample was run in quadruplicate. The average inter- and intra-assay coefficients of variation were 3.5% and 8.0%, respectively. The limit of detection (LOD) was 11.9 pg/mL. Values for BDNF concentrations were positively skewed (skew = 2.32, SE = 0.28) and were log transformed (after adding a constant of 2 to each value), which yielded a distribution with a skew of −0.81 (SE = 0.28). Total plasma protein concentrations were assessed via bicinchoninic acid (BCA) protein assay (Thermo Fisher Scientific, Waltham, MA).
Participants’ lifetime smoking history was assessed using the modified version of the Semi-Structured Assessment of the Genetics of Alcoholism, (SSAGA; Bucholz et al., 1994), which is a comprehensive psychiatric interview used to assess physical, psychological, social, and psychiatric manifestations of alcohol abuse/dependence and related psychiatric disorders in adults. Modifications were made to the SSAGA to collect data on maternal smoking and drinking patterns during pregnancy (Knopik et al., 2005). Body temperature was measured via an infrared thermometer (Exergen, Watertown, MA). None of the participants’ body temperatures exceeded 37.5 °C. Participants’ height and weight was measured to calculate body mass index (BMI). The majority of the samples (53.8%) were collected between 16:00–20:00 and the rest were collected between 11:00–15:00. There was no significant difference in the time of sample collection based on participants’ history of SA.
Data Analysis
We first conducted a univariate ANOVA using participants’ history of SA (yes, no) as the independent variable and circulating BDNF levels as the dependent variable to test our main hypothesis. Second, to determine the robustness of the relation, we conducted series of ANCOVAs, using participants’ SA history (yes/no) as an independent variable, circulating BDNF levels as a dependent variable, and relevant clinical and demographic variables (history of MDD [yes, no], current depressive symptoms, current levels of suicidal ideation, income, age, ethnicity [Caucasian, African American], lifetime cigarette smoking history, and BMI) entered as covariates. Separate analyses were conducted for each covariate to avoid model overfitting. Third, to examine the potential impact of single versus multiple SA, we conducted a univariate ANOVA using trichotomized SA history (none, single attempt, multiple attempts) as the independent variable and BDNF levels as a dependent variable.
RESULTS
Results of the univariate ANOVA revealed a significant association between SA history and circulating BDNF levels, F (1, 72) = 32.70, p < 0.001, η2 =0.32, such that participants with a history of SA had significantly lower levels of BDNF (M = 2.17, SEM = 1.20), than participants with no SA history (M = 3.10, SEM = 0.11) (Figure 1). In addition, using a series of ANCOVAs, we found that this group difference was maintained when we statistically controlled for the influence of participants’ (i) lifetime history of MDD (yes, no), F (1, 72) = 28.95, p < 0.001, η2 = 0.29, (ii) lifetime history of at least one anxiety disorder (yes, no), F (1, 72) = 26.65, p < 0.001, η2 = 0.28, (iii) lifetime history of bipolar disorder (yes, no), F (1, 72) = 30.88, p < 0.001, η2 = 0.31, (iv) lifetime history of alcohol use disorder (yes, no), F (1, 72) = 30.78, p < 0.001, η2 = 0.31, (v) lifetime history of substance use disorder (yes, no), F (1, 72) = 31.01, p < 0.001, η2 = 0.31, (vi) current depressive symptoms, F (1, 72) = 29.82, p < 0.001, η2 =0.30, (viii) current anxiety symptoms, F (1, 72) = 29.83, p < 0.001, η2 =0.30, and (ix) current suicidal ideation, F (1, 72) = 36.33, p < .001, η2 = 0.34. Although not a primary focus of the study, we should note that current suicidal ideation was also a significant predictor of circulating BDNF levels in this last analysis, F(1, 72) = 3.87, p = .05, η2 =0.05, suggesting that SA history and current SI are independently related to BDNF levels. In contrast, lifetime MDD history, F(1, 72) = 0.89, p = 0.77, η2 = 0.01, and current depressive symptoms, F(1, 72) = 0.23, p = 0.64, η2 = 0.003, were not significant predictors of circulating BDNF levels in their respective analyses.
Figure 1.
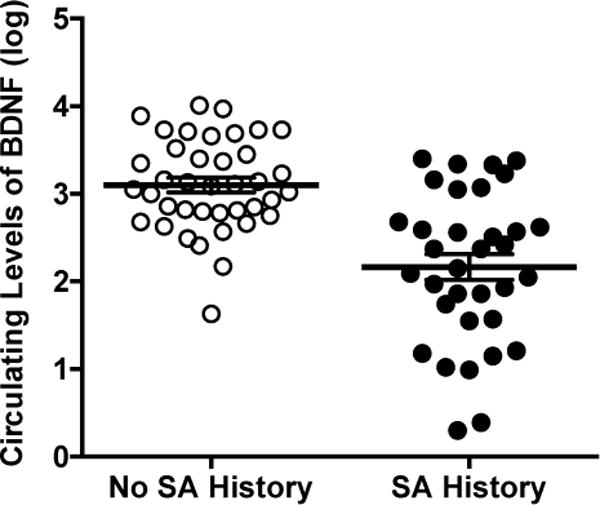
Participants with a history of suicide attempts (SA) evidenced significantly lower circulating levels of BDNF. Error bars represent standard error of the mean (SEM).
The group difference was also maintained when we statistically controlled for the influence of participants’ (iv) income, F (1, 72) = 33.73, p < 0.001, η2 = 0.33, (v) age, F (1, 72) = 35.00, p < 0.001, η2 = 0.33, (vi) ethnicity (Caucasian, African American), F (1, 72) = 32.13, p < 0.001, η2 =0.32, (vii) lifetime history of smoking cigarettes (>100 cigarettes during lifetime: yes, no), F (1, 72) = 32.59, p < 0.001, η2 =0.32, (viii) BMI, F (1, 72) = 32.14, p < 0.001, η2 = 0.32, (ix) body temperature, F (1, 72) = 37.28, p < 0.001, η2 = 0.35, and (x) time of day when the samples were collected, F (1, 72) = 32.12, p < 0.001, η2 = 0.31. Given the racial distribution of our sample, we should also note that the relation between SA history and BDNF levels was also significant when we looked separately at Caucasians, F (1, 43) = 14.73, p < 0.001, η2 = 0.26, and African Americans, F (1, 28) = 18.91, p < 0.001, η2 = 0.41. Notably, SA group difference in circulating levels of BDNF also remained significant when we included all of the clinical and demographic variables listed above as covariates in our model (lifetime history of MDD, anxiety, bipolar, alcohol, or substance use disorder, income, age, ethnicity, lifetime history of smoking cigarettes, BMI, body temperature, time of day when the samples were collected, current depressive and anxiety symptoms, current suicidal ideation), F (1, 72) = 32.70, p < 0.001, η2 = 0.37, again highlighting the robustness of the findings.
In addition, total protein concentrations in plasma did not differ between participants with versus without a history of SA (M = 99.81 mg/mL, SD = 29.74 versus M = 93.47 mg/mL, SD = 29.62), F (1, 72) = 0.95, p = 0.33, η2 = 0.13, suggesting a selective influence of SA history on circulating BDNF that was independent of differences in total protein. To ensure that our findings were not driven by participants who had a recent suicide crisis, we excluded women who had a suicide attempt within the last year from our analysis (n = 9), and the group difference was maintained, F (1, 63) = 26.25, p < 0.001, η2 =0.30. The results were also maintained when we included current antidepressant medication use (yes, no) as a covariate, F (1, 24) = 6.28, p = 0.02, η2 =0.22, though we only had data regarding the use of antidepressants for 25 participants (18 with a history of SA and 7 with no history of SA).
Finally, we examined whether the number of previous attempts (single/multiple) was linked to circulating BDNF levels. We first conducted a univariate ANOVA using trichotomized SA history (multiple SA, single SA, no SA) as the independent variable and BDNF levels as the dependent variable, and found that there was a significant main effect of SA on BDNF levels, F (1, 72) = 16.82, p < 0.001, η2 = 0.33. Bonferroni post hoc analyses revealed that the BDNF levels of participants with no SA history were significantly higher (M = 3.10, SE = 0.11) than those of participants with a history of one (M = 2.06, SE = 0.16; p <0.001) or multiple (M = 2.30, SE = 0.18; p = 0.001) suicide attempts, with the latter two groups not differing significantly (p = 0.99).
DISCUSSION
The primary goal of this study was to determine whether the previously reported association between suicidal thoughts/behavior and BDNF levels would be observed in individuals with a history of SA and whether the difference would be independent of current suicidal ideation or depression. Supporting our hypothesis, we found that women with a history of SA had lower circulating BDNF levels than those with no history of SA. Importantly, this difference remained significant when we statistically controlled for the influence of participants’ history of MDD, anxiety disorder, including panic disorder, social phobia, generalized panic disorder, or post-traumatic stress disorder, or obsessive-compulsive disorder, bipolar disorder, and alcohol or substance use disorders, their current levels of depressive and anxiety symptoms and suicidal ideation, as well as a number of demographic variables. These results suggest that reductions in BDNF are not merely a correlate of current suicidal ideation/behavior, but rather may represent a trait-like biomarker of risk. Notably, we did not find any difference in total protein levels between participants with and without a history of SA, suggesting that the decreased circulating levels of BDNF observed in participants with a history of SA were not a manifestation of a general decrease in total plasma protein. Our findings are consistent with previous studies that report decreased BDNF levels among individuals with current suicidal ideation and behavior (Dawood et al., 2007; Deveci et al., 2007; Kim et al., 2007a; Lee & Kim, 2009), but do not support the conclusions reported by Eisen et al. (2016), who found no differences in serum BDNF levels of among individuals with a recent (within 3 months) SA compared to psychiatric and community controls. Although the reason for this is unclear, methodological differences between the two studies could, at least partially, explain this discrepancy. For example, similar to previous studies examining BDNF and suicide (e.g. Kim et al., 2007b), we measure BDNF levels in plasma samples, whereas Eisen et al. (2016) focused on serum. Previous research suggests that plasma and serum BDNF levels are poorly correlated (D’Sa, Dileone, Anderson, & Sinha, 2012), likely reflecting that serum and plasma measurements represent separate pools of BDNF. Specifically, serum BDNF levels are largely reflective of the amount of BDNF secreted by platelets during coagulation, while plasma BDNF levels are derived from multiple sources, including muscular smooth muscle cells, endothelial cells, as well as monocytes and lymphocytes (D’Sa et al., 2012; Donovan et al., 1995; Lommatzsch et al., 2005; Nakahashi et al., 2000). Future research is needed to examine both plasma and serum levels of BDNF in the same sample to investigate the extent to which groups differences between individuals with and without a history of attempts are dependent on the source of measurement (plasma vs. serum). We should also note that participants’ history of MDD and current levels of depressive symptoms were not associated with circulating BDNF levels in our sample, though current levels of suicidal ideation were. Previous findings regarding the association between depression and BDNF levels are inconsistent, with some studies reporting lower BDNF levels in antidepressant-free MDD patients (Karege et al., 2002 Karege et al., 2005a; Lee and Kim, 2009), while other studies fail to find differences in BDNF levels between MDD patients and controls (Kim et al., 2007b). It is possible that we did not detect this association because the overall levels of depressive symptoms were low in our sample.
This study exhibited a number of strengths including the use of clinical interviews to assess women’s lifetime history of SA and MDD. Using a sample that was recruited with minimal exclusion criteria greatly increases the ecological validity of the findings. Additionally, our sample was approximately half Caucasian and half African American, which highlighted the stability of the findings across these groups. Despite these strengths, there are limitations that suggest important areas of future research. First, our cross-sectional design precludes any conclusions regarding the temporal precedence or causal impact of BDNF on suicidal behavior. This said, the current results provide the data necessary to support the utility of a larger longitudinal study to determine whether BDNF levels can be used to prospectively predict which individuals are at greatest risk for making a suicide attempt in the future. A second limitation is that our focus on women, although increasing the homogeneity of our sample, may also limit the generalizability of our findings to men. In addition, although we were able to rule out a number of potential third variable explanations for the observed group difference, we could not rule out all potential influences. For example, we were unable to account for the potential influence of menstrual cycle or contraception use, as this information was not collected, and previous research has suggested a link between circulating BDNF levels and ovarian function (Pluchino et al., 2009). Moreover, we did not have data for the use of medications, including antidepressants, for all participants and previous research supports the involvement of BDNF in antidepressant response (Ghosh et al., 1994; Duman et al., 1997; Monteggia et al., 2004; Duman and Monteggia, 2006; Björkholm and Monteggia, 2016). Finally, the study focused on a low-income population, which may limit the generalizability of the findings to more affluent groups.
In summary, the current results suggest that a history of SA is associated with decreased circulating levels of BDNF, a relation that appears at least partially independent of other known correlates of SA such as depression, current suicidal ideation, and demographic influences. Although conclusions must remain tentative pending longitudinal investigations, these findings support the trait-like quality of peripheral BDNF levels and support its potential as a biomarker that may allow us to determine which individuals are at greatest risk for suicide in the future.
Acknowledgments
This project was supported by National Institute of Mental Health grant MH098060 awarded to B.E. Gibb. We would like to thank Ashley Johnson, Lindsey Stone, Andrea Hanley, Katie L. Burkhouse, Mary L. Woody, Sydney Meadows, Michael Van Wie, Ariel Ravid, Devra Alper, Cope Feurer, Eric Funk, Effua Sosoo, Aliona Tsypes, and Kiera M. James for their help in conducting assessments for this project.
Footnotes
MISS ANASTACIA Y. KUDINOVA (Orcid ID: 0000-0002-3799-5924)
Conflict of Interest
The authors declare no conflict of interest.
References
- Altshuler LL, Casanova MF, Goldberg TE, Kleinman JE. The hippocampus and parahippocampus in schizophrenia, suicide, and control brains. Archives of General Psychiatry. 1990;47(11):1029–34. doi: 10.1001/archpsyc.1990.01810230045008. [DOI] [PubMed] [Google Scholar]
- Beck A, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. Journal of Consulting and Clinical Psychology. 1979;47(2):343–52. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R. In: Beck Anxiety Inventory Manual. Siegle, editor. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- Beck A, Steer R, Brown G. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bijttebier S, Caeyenberghs K, van den Ameele H, Achten E, Rujescu D, Titeca K, van Heeringen C. The vulnerability to suicidal behavior is associated with reduced connectivity strength. Frontiers in Human Neuroscience. 2015;9:632. doi: 10.3389/fnhum.2015.00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkholm C, Monteggia LM. BDNF - a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–9. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55(2):149–58. doi: 10.15288/jsa.1994.55.149. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8189735. [DOI] [PubMed] [Google Scholar]
- Curtin S, Warner M, Hedegaard H. Increase in suicide in the United States, 1999–2014. NCHS data brief, no 241. Hyattsville, MD: National Center for Health Statistics; 2016. [PubMed] [Google Scholar]
- D’Sa C, Dileone RJ, Anderson GM, Sinha R. Serum and plasma brain-derived neurotrophic factor (BDNF) in abstinent alcoholics and social drinkers. Alcohol. 2012;46(3):253–259. doi: 10.1016/j.alcohol.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood T, Anderson J, Barton D, Lambert E, Esler M, Hotchkin E, Lambert G. Reduced overflow of BDNF from the brain is linked with suicide risk in depressive illness. Molecular Psychiatry. 2007;12(11):981–3. doi: 10.1038/sj.mp.4002059. [DOI] [PubMed] [Google Scholar]
- Deveci A, Aydemir O, Taskin O, Taneli F, Esen-Danaci A. Serum BDNF levels in suicide attempters related to psychosocial stressors: a comparative study with depression. Neuropsychobiology. 2007;56(2–3):93–7. doi: 10.1159/000111539. [DOI] [PubMed] [Google Scholar]
- Donovan MJ, Miranda RC, Kraemer R, McCaffrey TA, Tessarollo L, Mahadeo D, Parada L. Neurotrophin and neurotrophin receptors in vascular smooth muscle cells. Regulation of expression in response to injury. The American Journal of Pathology. 1995;147(2):309–24. [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Archives of General Psychiatry. 1997;54(7):597–606. doi: 10.1001/archpsyc.1997.01830190015002. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9236543. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Nakagawa S, D’Sa C. Neuronal plasticity and survival in mood disorders. Biological Psychiatry. 2000;48(8):732–9. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biological Psychiatry. 2006;59(12):1116–27. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y. Brain-Derived Neurotrophic Factor in Suicide Pathophysiology. In: Dwivedi Y, editor. The Neurobiological Basis of Suicide. Boca Raton (FL): CRC Press/Taylor & Francis; 2012. [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Archives of General Psychiatry. 2003;60(8):804–15. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–69. doi: 10.1016/s0092-8674(03)00035-7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12553913. [DOI] [PubMed] [Google Scholar]
- Eisen RB, Perera S, Banfield L, Anglin R, Minuzzi L, Samaan Z. Association between BDNF levels and suicidal behaviour: a systematic review and meta-analysis. Systematic Reviews. 2015;4:187. doi: 10.1186/s13643-015-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RB, Perera S, Bawor M, Dennis BB, El-Sheikh W, DeJesus J, Samaan Z. Exploring the association between serum BDNF and attempted suicide. Scientific Reports. 2016;6(1):25229. doi: 10.1038/srep25229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati P, Radtchenko A, Boyer P. Neuroplasticity: from MRI to depressive symptoms. European Neuropsychopharmacology : The Journal of the European College of Neuropsychopharmacology. 2004;14(Suppl 5):S503–10. doi: 10.1016/j.euroneuro.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Garcia R. Stress, synaptic plasticity, and psychopathology. Reviews in the Neurosciences. 2002;13(3):195–208. doi: 10.1515/revneuro.2002.13.3.195. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Carnahan J, Greenberg ME. Science. 5153. Vol. 263. New York, N.Y: 1994. Requirement for BDNF in activity-dependent survival of cortical neurons; pp. 1618–23. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7907431. [DOI] [PubMed] [Google Scholar]
- Jin H, Atkinson JH, Duarte NA, Yu X, Shi C, Riggs PK, Heaton RK. Risks and predictors of current suicidality in HIV-infected heroin users in treatment in Yunnan, China: a controlled study. Journal of Acquired Immune Deficiency Syndromes (1999) 2013;62(3):311–6. doi: 10.1097/QAI.0b013e31827ce513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biological Psychiatry. 2005;57(9):1068–72. doi: 10.1016/j.biopsych.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Research. 2002;109(2):143–8. doi: 10.1016/s0165-1781(02)00005-7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11927139. [DOI] [PubMed] [Google Scholar]
- Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Research Molecular Brain Research. 2005;136(1–2):29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Keller S, Sarchiapone M, Zarrilli F, Videtic A, Ferraro A, Carli V, Chiariotti L. Increased BDNF promoter methylation in the Wernicke area of suicide subjects. Archives of General Psychiatry. 2010;67(3):258–67. doi: 10.1001/archgenpsychiatry.2010.9. [DOI] [PubMed] [Google Scholar]
- Kim YK, Lee HP, Won SD, Park EY, Lee HY, Lee BH, Choi SH. Low plasma BDNF is associated with suicidal behavior in major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2007a;31(1):78–85. doi: 10.1016/j.pnpbp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Kim YK, Lee HP, Won SD, Park EY, Lee HY, Lee BH, Choi SH. Low plasma BDNF is associated with suicidal behavior in major depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2007b;31(1):78–85. doi: 10.1016/j.pnpbp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Sparrow EP, Madden PAF, Bucholz KK, Hudziak JJ, Reich W, Heath AC. Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: a female twin study. Psychological Medicine. 2005;35(5):625–35. doi: 10.1017/s0033291704004155. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15918339. [DOI] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, Torpey D, Kim J, Klein DN. Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2012;53(2):207–15. doi: 10.1111/j.1469-7610.2011.02461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Kim YK. Reduced platelet BDNF level in patients with major depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2009;33(5):849–53. doi: 10.1016/j.pnpbp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, Schloetcke K, Klotz J, Schuhbaeck K, Zingler D, Zingler C, Virchow JC. Brain-derived Neurotrophic Factor in Platelets and Airflow Limitation in Asthma. American Journal of Respiratory and Critical Care Medicine. 2005;171(2):115–120. doi: 10.1164/rccm.200406-758OC. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Lesh TA, Niendam TA, Cheng Y, Carter CS. Conflict-Related Anterior Cingulate Functional Connectivity Is Associated With Past Suicidal Ideation and Behavior in Recent-Onset Psychotic Major Mood Disorders. The Journal of Neuropsychiatry and Clinical Neurosciences. 2016 doi: 10.1176/appi.neuropsych.15120422. [DOI] [PubMed]
- Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Nestler EJ. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(29):10827–32. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy C, Suderman M, Yang J, Szyf M, Mechawar N, Ernst C, Turecki G. Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Molecular Psychiatry. 2015;20(3):320–8. doi: 10.1038/mp.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, Sun B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Letters. 2000;470(2):113–7. doi: 10.1016/s0014-5793(00)01302-8. [DOI] [PubMed] [Google Scholar]
- Pluchino N, Cubeddu A, Begliuomini S, Merlini S, Giannini A, Bucci F, Genazzani AR. Daily variation of brain-derived neurotrophic factor and cortisol in women with normal menstrual cycles, undergoing oral contraception and in postmenopause. Human Reproduction (Oxford, England) 2009;24(9):2303–9. doi: 10.1093/humrep/dep119. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Morphometric methods for studying the prefrontal cortex in suicide victims and psychiatric patients. Annals of the New York Academy of Sciences. 1997;836:253–68. doi: 10.1111/j.1749-6632.1997.tb52364.x. [DOI] [PubMed] [Google Scholar]
- Shrivastava A, De Sousa A, Rao Gp. Brain-Derived neurotrophic factor and suicide in schizophrenia: Critical role of neuroprotective mechanisms as an emerging hypothesis. Indian Journal of Psychological Medicine. 2016;38(6):499. doi: 10.4103/0253-7176.194913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teti GL, Rebok F, Rojas SM, Grendas L, Daray FM. Systematic review of risk factors for suicide and suicide attempt among psychiatric patients in Latin America and Caribbean. Revista Panamericana de Salud Pública = Pan American Journal of Public Health. 2014;36(2):124–33. [PubMed] [Google Scholar]
- Torres-Platas SG, Hercher C, Davoli MA, Maussion G, Labonté B, Turecki G, Mechawar N. Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2011;36(13):2650–8. doi: 10.1038/npp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO | Suicide. 2015 Retrieved from http://www.who.int/mediacentre/factsheets/fs398/en/
- Woosley JA, Lichstein KL, Taylor DJ, Riedel BW, Bush AJ. Hopelessness mediates the relation between insomnia and suicidal ideation. Journal of Clinical Sleep Medicine : JCSM : Official Publication of the American Academy of Sleep Medicine. 2014;10(11):1223–30. doi: 10.5664/jcsm.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zai CC, Manchia M, De Luca V, Tiwari AK, Chowdhury NI, Zai GC, Kennedy JL. The brain-derived neurotrophic factor gene in suicidal behaviour: a meta-analysis. The International Journal of Neuropsychopharmacology/Official Scientific Journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2012;15(8):1037–42. doi: 10.1017/S1461145711001313. [DOI] [PubMed] [Google Scholar]


