Abstract
Senescence is a leading cause of mortality, disability, and non-communicable chronic diseases in older adults. Mounting evidence indicates that the presence of cardiovascular disease and risk factors elevates the incidence of both vascular cognitive impairment and Alzheimer’s disease (AD). Age-related declines in cardiovascular function may impair cerebral blood flow (CBF) regulation, leading to the disruption of neuronal micro-environmental homeostasis. The brain is the most metabolically active organ with limited intracellular energy storage and critically depends on CBF to sustain neuronal metabolism. In patients with AD, cerebral hypoperfusion, increased CBF pulsatility, and impaired blood pressure control during orthostatic stress have been reported, indicating exaggerated, age-related decline in both cerebro- and cardiovascular function. Currently, AD lacks effective treatments; therefore, development of preventive strategy is urgently needed. Regular aerobic exercise improves cardiovascular function, which in turn may lead to a better CBF regulation, thus reducing the dementia risk. In this review, we discuss the effects of aging on cardiovascular regulation of CBF and provide new insights into the vascular mechanisms of cognitive impairment and potential effects of aerobic exercise training on CBF regulation.
Keywords: Cerebral blood flow, cerebral autoregulation, pulsatility, cerebral vasomotor reactivity, and cardiovascular function
Graphical abstract
We propose that age-related cardiovascular dysfunction profoundly alters cerebral blood flow (CBF). The stiffening and wall thickening of central elastic arteries elevate systolic (SBP) and pulse (PP) pressure which subsequently augment CBF pulsatility. The elevated mean arterial pressure (MAP), resulting from increased total peripheral resistance (TPR) and endothelial dysfunction, may induce cerebrovascular remodeling to increase the resistance (CVR) and impedance. The blunted cardiovagal baroreflex sensitivity (BRS) may lead to BP and CBF instability during extrinsic stimuli (e.g. postural change).
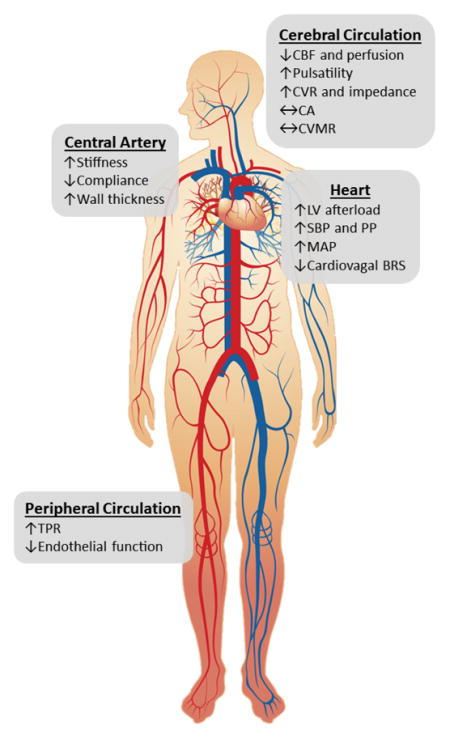
Background
We are facing the unprecedented aging of population. According to a recent report from the United Nations, a global share of older adults 60 years and over are expected to more than double from 2013 to 2050. In addition, the older population itself is aging; persons aged 80 years and over (the “oldest old”) will more than triple by 2050 (United_Nations 2013). Senescence is a leading cause of non-communicable chronic disease; therefore, population aging is expected to impose a substantial burden on our healthcare system.
Dementia represents a leading cause of death, disability, and loss of autonomy among older adults (Kochanek 2016). Alzheimer’s disease (AD) is the dominant type of dementia (Querfurth & LaFerla 2010), and its incidence doubles every 5 years after age of 65 and afflicts ~50% of adults aged >85 years (Prince et al. 2013). With the population aging, patients with AD are expected to triple from 24.3 million in 2001 to 81.1 million in 2040 (Ferri et al. 2005), if no effective therapy or preventive measures are developed in near future.
The proposed etiology of AD has been centered on the amyloid hypothesis over the last 3 decades, which states that cerebral accumulations of amyloid-β and hyper-phosphorylated tau peptides lead to neuronal dysfunction and cognitive impairment (Hardy & Selkoe 2002). However, mounting evidence indicates multifactorial nature of AD and that cerebrovascular pathology coexists in most of AD patients (Viswanathan et al. 2009). In support of vascular contributions to AD, the presence of midlife cardiovascular risk factors has been shown to accelerate age-related cognitive decline and increase the risk of AD in later life (Whitmer et al. 2005).
The brain is limited by intracellular energy substrates to sustain neuronal metabolism and critically depends on the cardiovascular supply of cerebral blood flow (CBF). Therefore, age-related impairment of cardiovascular function may impair CBF regulation and disrupt neuronal homeostasis (de la Torre 2004). In contrast, pharmaceutical and non-pharmaceutical interventions that can ameliorate age-related cardiovascular dysfunction may improve CBF supply, thereby decreasing the risk of cognitive impairment. In this regard, previous studies have demonstrated the potential benefits of aerobic exercise training on cognitive function (Smith et al. 2010). In this brief review, we will discuss 1) the effects of aging on cardiovascular regulation of CBF and 2) the association between regular aerobic exercise and CBF regulation. This review summarizes the major findings from recent studies performed in humans and discusses their clinical implications with a particular focus on cognitive impairment.
Main Determinants of CBF
The brain weighs only ~2% of the body mass while its metabolic rate accounts for ~20% of the whole body (Elia 1992). Despite a high metabolic rate, the brain contains only 3–4 umol/g of the intracellular glycogen, when compared for example to the liver that contains 200–400 umol/g (Oz et al. 2007). In addition, the rate of glycogen turnover is slow in the brain and only provides glucose under chronic hypoglycemia (Oz et al. 2009). Therefore, a stable supply of CBF is critical for normal brain function (Williams & Leggett 1989) which is regulated by both the local and systemic mechanisms.
Neurovascular coupling
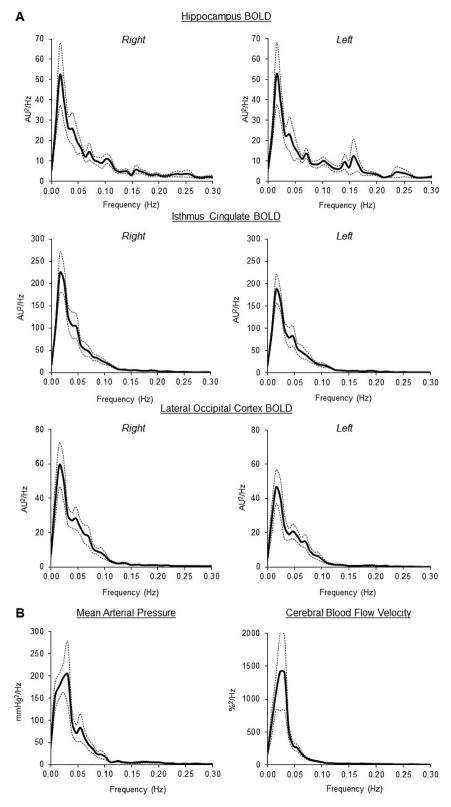
Regional CBF is tightly coupled to neuronal metabolism which is heterogeneous in space and time. During neuronal activation, synaptic release of neurotransmitters (e.g. glutamate) leads to an elevation of regional CBF (functional hyperemia) through vasodilation (Attwell et al. 2010). With advent of neuroimaging technology, functional hyperemia can be assessed by the blood-oxygen-level-dependent (BOLD) signal using functional magnetic resonance imaging (MRI) (Moseley & Glover 1995). The BOLD signal is based on T2*-weighted signal which depends on local changes in the concentration of deoxygenated hemoglobin via neurovascular coupling (Ogawa et al. 1992). In a previous study, we measured brain resting-state BOLD signal while participants quietly laid on the scanner table (Zhu et al. 2015). We found that under resting conditions, regional BOLD spontaneous fluctuations at the very low frequency (<0.05 Hz) exhibit substantial overlap with the oscillations of systemic arterial blood pressure (BP) and global CBF measured from the middle cerebral artery (Figure 1). These observations suggest that regional dynamic changes in CBF may be influenced by the upper-stream changes in cardiovascular function.
Figure 1.
(A) Power spectra of regional BOLD signals measured by functional MRI. (B) Power spectra of beat-by-beat mean arterial pressure and cerebral blood flow velocity recorded from the same study participants (n=12). Cerebral blood flow velocity was measured from the middle cerebral artery. Solid line: mean. Dotted lines: standard error. AU=arbitrary unit and BOLD=blood-oxygen-level dependent. [Adapted from Zhu DC, Tarumi T, Khan MA, Zhang R. Vascular Coupling in Resting-State fMRI: Evidence from Multiple Modalities. J Cereb Blood Flow Metab. 2015 Dec;35 (12):1910–20. Copyright © 2015 SAGE Publications]
Arterial BP
Arterial BP is the determinant of cerebral perfusion pressure (CPP), which is a pressure gradient between arterial BP and intracranial pressure and represents the driving force for CBF. While intracranial pressure is largely influenced by body posture or hydrostatic gradient between the head and heart positions (Chapman et al. 1990), arterial BP is regulated by an integrated mechanisms of autonomic, humoral, and vascular factors.
Arterial BP fluctuates spontaneously at rest and is influenced by extrinsic stimuli (e.g. postural changes) (Zhang et al. 2000, Claassen et al. 2009). In response to dynamic changes in BP, cerebrovascular bed behaves as a “high-pass filter” system, which buffers the effects of BP fluctuations on CBF via cerebral autoregulation (CA) (Lassen 1964, Rickards & Tzeng 2014). The CA is more or less a frequency dependent phenomenon that operates more efficiently at very low frequency (<0.05Hz) (Giller 1990, Aaslid et al. 1989, Zhang et al. 1998, Panerai et al. 1999) and controlled by myogenic, neurogenic, and metabolic mechanisms (McHedlishvili 1980). Recently, CA has been shown to interact with cardiovagal baroreflex function in healthy young adults for maintaining CBF and brain homeostasis (Tzeng et al. 2010). The baroreceptor reflex controls short-term changes in BP through the distortion of barosensory arteries (input) and the modulation of autonomic neural activity of the heart and vascular system (output) (Guo et al. 1982).
Pulse pressure (PP), which represents another dynamic component of arterial BP, is directly correlated with the amplitude of CBF pulsatility (Tarumi et al. 2014). In cardiovascular system, elevated PP represents a hallmark of vascular aging and results from stiffening of the central large arteries (e.g. aorta, carotid artery) (Nichols 2005). PP is likely situated outside the operating frequency range of CA and its transmission is most likely determined by the impendent property of cerebrovascular bed (Windkessel effect) (Zhu et al. 2011). With high elasticity of large cerebral arterial wall, PP may be dampened by the expansion and recoiling of cerebral arteries during each cardiac cycle.
Carbon dioxide (CO2)
The partial pressure of carbon dioxide in the arterial blood (PaCO2) has potent effects on cerebral vasomotor tone. Elevated PaCO2 (hypercapnia) dilates cerebral arteries leading to increases in CBF whereas reduced PaCO2 (hypocapnia) decreases CBF via vasoconstriction. These CBF responses to changes in PaCO2, termed cerebral vasomotor reactivity (CVMR), are likely to represent a vital homeostatic function that regulates the brain pH level and affects respiratory drive via central chemoreceptors (Chesler 2003). Cerebral vasodilation during hypercapnia increases CBF, washes out the excess CO2 from the blood, and maintains the brain pH level. The mechanism underling CVMR is not fully understood, but it is likely to involve the release of multiple vasoactive agents such as prostaglandins (Barnes et al. 2012, Fan et al. 2010) and nitric oxide (Schmetterer et al. 1997). The reduced production of prostaglandins via indomethacin ingestion has been shown to decrease basal CBF and significantly attenuate CVMR during hypo- and hypercapnia (Barnes et al. 2012, Fan et al. 2010). The inhibition of nitric oxide synthase has also shown the attenuation of hypercapnic CVMR that was reversed by administration of nitric oxide donors (Schmetterer et al. 1997). Finally, elevations of CBF during hypercapnia may be facilitated by the dilation of upper-stream extracranial arteries via shear-stress mediated release of endothelium-derived vasodilatory agents (Hoiland et al. 2017).
Cardiac output (CO)
The brain continuously receives ~15% of CO to meet the metabolic demand of neuronal activity. Although CO is a key determinant of arterial BP when coupled with total peripheral resistance, alterations in CO per se may influence CBF (Meng et al. 2015). In healthy adults, reduction of CO using lower body negative pressure and elevation of CO via albumin infusion demonstrated a linear correlation between changes in CO and CBF at rest and during exercise, independent of changes in arterial BP or PaCO2 (Ogoh et al. 2005). On the other hand, heart failure patients demonstrated a non-linear relationship between acute changes in CO and CBF while changing the posture from the supine to sitting (Fraser et al. 2015). Furthermore, heart failure patients with depressed CO showed lower CBF than normal control subjects, but heart transplantation restored their CBF to the similar level observed in the control subjects (Gruhn et al. 2001).
Autonomic neural activity
The autonomic neural activity is likely to have profound impact on dynamic CBF regulation as cerebral arteries are richly innervated by the adrenergic and cholinergic fibers (Edvinsson 1975). In healthy humans, complete autonomic blockade using trimethaphan impaired CA at the very low frequencies (Zhang et al. 2002a, Zhang et al. 2002b). Furthermore, recent studies demonstrated that sympathetic blockade using α-adrenergic antagonist (Hamner et al. 2010) and cholinergic blockade using muscarinic receptor antagonist (Hamner et al. 2012) impaired CA and enhanced the amplitude of CBF oscillations at the very low frequencies. Collectively, these findings provide strong evidence that autonomic nervous system contributes to dynamic CA in healthy adults.
Age and Steady-State CBF
The steady-state level of CBF progressively decreases in normal aging men and women while women tend to have higher levels of CBF than men (Lu et al. 2011). This age-related reduction of CBF may reflect decreased cerebral metabolic rate (Marchal et al. 1992) and cerebrovascular dysfunction (Zhu et al. 2011). Across the adult lifespan, age decreases cerebral metabolic rates for oxygen and glucose by ~5% per decade, and these reductions of metabolic rate are coupled to the concurrent decrease in CBF (Leenders et al. 1990, Petit-Taboue et al. 1998). Mechanistically, age may impair neuronal and glial mitochondrial metabolism. The in vivo MR spectroscopy study demonstrated that metabolic rates for neuronal tricarboxylic acid and glutamate-glutamine cycles are reduced in older adults (Boumezbeur et al. 2010). Aside from these aging effects, it has been shown that women have higher levels of cerebral metabolic rate for glucose (Willis et al. 2002) and oxygen (Lu et al. 2011) which may explain why women have higher levels of CBF than men.
Normal aging is associated with gradual increase in mean arterial pressure (Franklin et al. 1997). Mechanistically, heightened sympathetic neural activity and impaired peripheral vasodilatory function (e.g. endothelial dysfunction) are likely to increase total peripheral resistance and therefore mean arterial pressure in older adults (Hart et al. 2012). On the other hand, studies with direct intracranial pressure monitoring using an intra-parenchymal probe demonstrated a negative correlation between age and intracranial pressure in patients with head injury (Czosnyka et al. 2005). If this observation can be extrapolated to healthy aging adults, age may increase CPP due to the effects of both increased mean arterial pressure and decreased intracranial pressure. In the face of elevated CPP, cerebrovascular bed may undergo compensatory remodeling by increasing resistance in order to protect the delicate brain tissues from overperfusion.
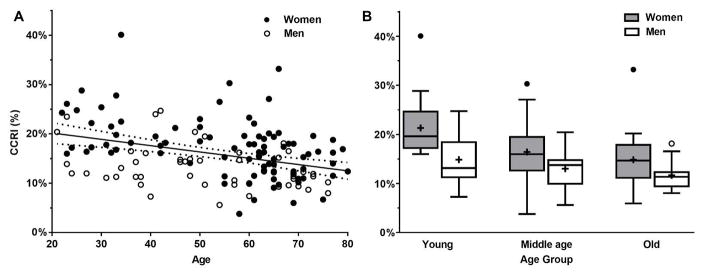
Age-related reduction of CBF may also be related to concurrent changes in CO (Brandfonbrener et al. 1955). The widely accepted dogma suggests that ~15% of CO is distributed to the brain in healthy adults (Williams & Leggett 1989); however, it is not well understood whether this proportion can change with the alteration of CO and/or CBF in aging adults. Therefore, we studied healthy aging adults (20–80 years) who do not have a history of neurological, cardio- or cerebrovascular disease. CBF was measured from the bilateral internal carotid and vertebral arteries using phase-contrast MRI and CO was measured by echocardiography. We found that advancing age is associated with the decreasing proportion of CO distributed to the brain; however, CO was maintained and only CBF was decreased in older adults (Xing et al. 2016) (Figure 2). These findings suggest that age-related reduction of CBF may not be attributed to the reduction of CO.
Figure 2.
Association between age and the proportion of cardiac output distributed to the brain (n=139). The CCRI represents the cerebral blood flow to cardiac output ratio index. Panel A shows the linear decline of CCRI with increasing age (CCRI=−0.127%×age+22.72% with R2=0.13, P<0.001). Panel B shows the association between age and CCRI in men and women separately (P<0.001 for age group, P<0.001 for sex, and P=0.26 for age and sex interaction). Young=21–45 years; middle age=45–65 years; and old=66–80 years. Error bars represent standard deviation. [Reprinted from Xing CY, Tarumi T, Liu J, Zhang Y, Turner M, Riley J, Tinajero CD, Yuan LJ, Zhang R. Distribution of Cardiac Output to the Brain across the Adult Lifespan. J Cereb Blood Flow Metab. 2016 Jan 1 (doi: 10.1177/0271678X16676826). Copyright © 2016 SAGE Publications]
With regard to clinical perspective, cerebral hypoperfusion may be linked to the pathological onset of AD. The data-driven analysis of CSF, neuroimaging, and plasma biomarkers showed that cerebral hypoperfusion, as measured by arterial spin labeling using MRI, is the earliest event of late-onset AD before the manifestation of traditional biomarker abnormalities (e.g. CSF amyloid, cerebral hypometabolism) (Iturria-Medina et al. 2016). These findings are also supported by animal studies which demonstrated that mild to moderate cerebral hypoperfusion impairs neuronal protein synthesis which can subsequently lead to learning and memory dysfunction; ischemia may further impair action potential generations which can increase cerebral glutamate concentrations and promote the accumulations of neuronal toxins such as amyloid-β proteins (Zlokovic 2011).
Age and CBF Oscillation
To understand the aging effects on cardio- and cerebrovascular variability, we measured beat-by-beat changes in heart rate, arterial BP, CBF at rest and during repeated sit-stand maneuvers in healthy adults (21–80 years) (Xing et al. 2017). The repeated sit-stand maneuvers were performed at 0.05 Hz (cycles of 10-second sit and 10-second stand) to augment BP variability at the auto-regulatory frequency. Changes in CBF were measured by transcranial Doppler (TCD) from the middle cerebral artery. To characterize cardio- and cerebrovascular hemodynamics, we used spectral and transfer function analysis that estimated gain, phase, and coherence of dynamic CA (mean arterial pressure → CBF velocity) and cardiovagal baroreflex (systolic BP → R-R interval). In short, transfer function gain reflects a magnitude relation (slope) between input and output signals while the phase represents their temporal association. The coherence function reflects a strength of their linear correlation and provides the validity of gain and phase estimation (Zhang et al. 1998).
This study demonstrated that under resting conditions, low frequency oscillations (0.07–0.20 Hz) of mean arterial pressure and CBF variability were reduced in older adults compared with young and middle-aged adults. In contrast, repeated sit-stand maneuvers (0.05 Hz) augmented their oscillations to a greater extent in older adults than in younger and middle-aged adults (Figure 3). With regard to dynamic CA, older adults showed the elevations of low and high frequency gain (0.07–0.35 Hz) under resting conditions, suggesting impaired CA. However, these differences were abolished during repeated sit-stand maneuvers. In both conditions, heart rate variability was substantially reduced and cardiovagal baroreflex sensitivity was significantly attenuated in older adults compared with young adults (Xing et al. 2017). We also observed that low frequency CA gain is inversely correlated with the baroreflex gain in young subjects, as reported from a previous study (Tzeng et al. 2010).
Figure 3.
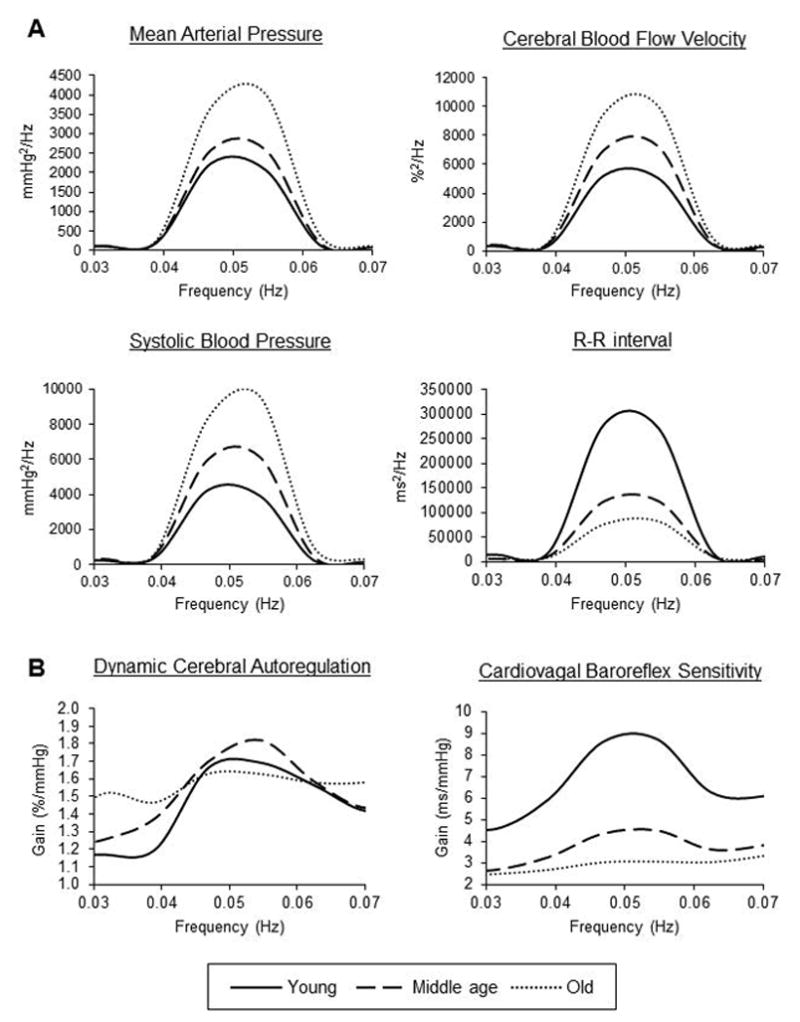
Age-related differences in (A) power spectra of cardio- and cerebrovascular hemodynamics and (B) transfer function gain of dynamic cerebral autoregulation and cardiovagal baroreflex sensitivity. All variables were recorded during repeated sit-stand maneuvers performed at 0.05 Hz. CBF velocity was recorded from the middle cerebral artery and normalized to the mean value before analysis. Young=21–44 years (n=41); middle age=45–64 years (n=50); and old=65–80 years (n=45). Group-averaged means are presented. [Adapted from Xing CY, Tarumi T, Meijers RL, Turner M, Repshas J, Xiong L, Ding K, Vongpatanasin W, Yuan LJ, Zhang R. Arterial Pressure, Heart Rate, and Cerebral Hemodynamics Across the Adult Life Span. Hypertension. 2017 Apr;69 (4):712–720. Copyright © 2017 Lippincott Williams & Wilkins. Used with permission]
In summary, these findings collectively demonstrate the presence of age-related reductions in BP, CBF, and heart rate variability in the low frequency range and impaired cardiovagal baroreflex and dynamic CA in older adults at rest. Furthermore, augmented BP and CBF variability during repeated sit-stand maneuvers indicate diminished cardiovascular regulatory capability in older adults and increased hemodynamic stress on the cerebral circulation with advanced aging (Xing et al. 2017).
Clinically, these observations suggest that postural changes may cause transient cerebral hypoperfusion and increase the risk of falls and syncope in older adults. Also, chronic intermittent cerebral hypoperfusion or ischemia is associated with white matter lesions and cognitive decline (O’Sullivan et al. 2002). In AD patients, a higher prevalence of orthostatic hypotension has been reported when compared with cognitively normal adults, and is associated with worse performance on cognitive assessment (Mehrabian et al. 2010). Mechanistically, central arterial stiffening may represent a common mechanism underling short-term BP dysregulation and cognitive decline. The stiffening of barosensory arteries, such as the aorta and carotid artery, can blunt the sensitivity of baroreceptor function (Monahan et al. 2001a, Okada et al. 2012), which in turn may contribute to the dysregulation of arterial BP, CPP, thus CBF. Consistently, our recent study also demonstrated that aortic stiffening and blunted cardiovagal baroreflex sensitivity are associated with reductions of brain neuronal fiber integrity and executive function performance in older adults (Tarumi et al. 2015). Nevertheless, the potential causal effect of arterial stiffening or blunted baroreflex sensitivity on cognitive impairment requires further research, and it should also be kept in mind that brain neurodegenerative disease per se may impair the baroreflex function.
Age and CBF Pulsatility
Advancing age progressively stiffens the proximal aorta and central large elastic arteries (e.g. carotid artery) via increasing the wall contents of collagen relative to elastin (Zieman et al. 2005). The aortic stiffening elevates left ventricular afterload, as accompanied by an earlier return of arterial pressure wave reflection (Nichols 2005). Consequently, systolic BP and PP progressively increase during adult lifespan while diastolic BP gradually decreases after middle age (Franklin et al. 1997).
Elevated PP is a strong risk factor for mortality and cerebrovascular disease (Staessen et al. 2000); however, existing data are limited as to the effect of PP on CBF in normal aging adults. Therefore, we studied healthy subjects (22–80 years) who were rigorously screened for neurological and vascular disease as well as cardiovascular risk factors, including hypertension, obesity, diabetes, and smoking. To assess steady-state and pulsatile CBF, we used phase-contrast MRI and TCD respectively while measuring aortic stiffness, carotid PP, and arterial pressure wave reflection. In addition, white matter hyperintensity volume, which reflects the severity of cerebral small vessel disease (Benjamin et al. 2016), was measured by T2-weighted fluid-attenuation-inversion-recovery imaging (Tarumi et al. 2014).
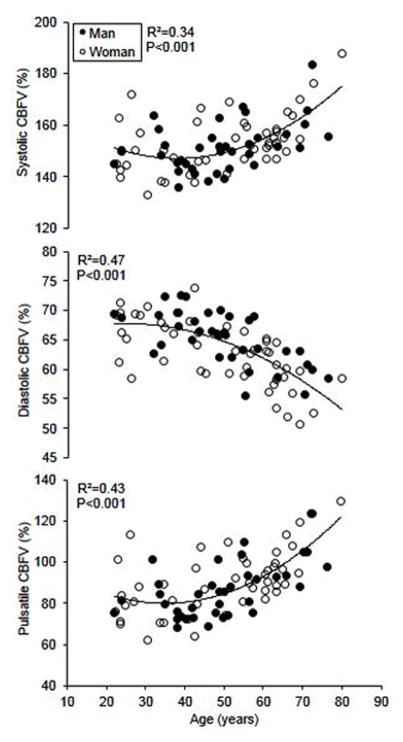
This study showed several key findings. First, advancing age is associated with an accelerated increase in CBF pulsatility after midlife (Figure 4) while steady-state CBF linearly decreases across the adult lifespan. We also observed that diastolic CBF is lower but steady-state CBF is higher in women than in men of the similar age. Second, the age- and sex-related differences in CBF pulsatility are independently associated with carotid PP. Third, higher CBF pulsatility is correlated with the greater volume of white matter hyperintensities in older adults. Collectively, these findings demonstrated a close link between cardio- and cerebrovascular hemodynamics in healthy aging adults and suggested potential clinical implications to structural brain damage (Tarumi et al. 2014).
Figure 4.
Association between age and pulsatile indices of cerebral blood flow (CBF) (n=83). The CBF pulsatile indices were calculated by normalizing the absolute systolic, diastolic, and pulsatile CBF velocity to the mean value and expressed in percentage. CBF velocity was recorded from the middle cerebral artery. [Reprinted from Tarumi T, Ayaz Khan M, Liu J, Tseng BY, Parker R, Riley J, Tinajero C, Zhang R. Cerebral Hemodynamics in Normal Aging: Central Artery Stiffness, Wave Reflection, and Pressure Pulsatility. J Cereb Blood Flow Metab. 2014 Jun;34 (6):971–8. Copyright © 2015 SAGE Publications]
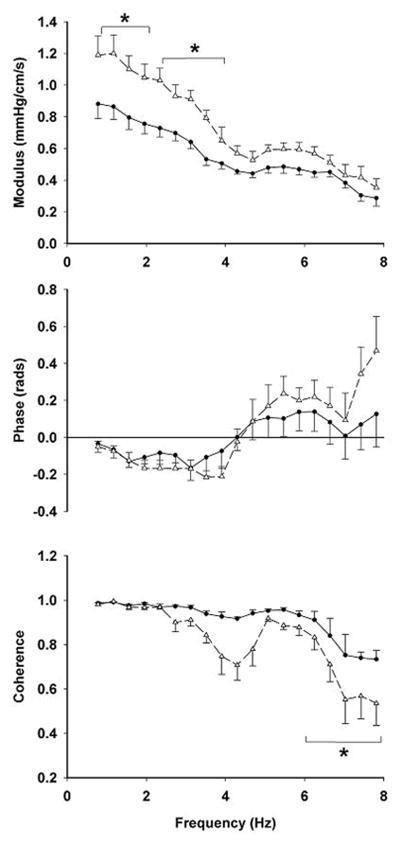
In response to elevated PP, cerebrovascular bed may undergo a compensatory remodeling to protect the underling brain tissues from hemodynamic insults. To study this hypothesis, we tested a group of young (28±4 years) and older (70±6 years) healthy adults by simultaneously recording carotid PP and CBF velocity from the middle cerebral artery using TCD. Transfer function analysis was used to quantify their magnitude (modulus) and temporal (phase) relation, which represent the cerebrovascular impedance. This study demonstrated that older adults have an increased modulus of cerebrovascular impedance compared with younger adults while the phase being similar between the groups (Figure 5). Furthermore, the elevated impedance modulus was correlated with the reduced systolic and diastolic CBF velocity, suggesting that the elevation of cerebrovascular impedance may attenuate the transmission of PP into the cerebral microcirculation (Zhu et al. 2011).
Figure 5.
Comparison of cerebrovascular impedance between young (circle with solid line, n=6) and older (triangle with dashed line, n=9) adults. The modulus, phase, and coherence of cerebrovascular impedance were calculated by transfer function analysis of carotid blood pressure and cerebral blood flow velocity that were recorded simultaneously.
cerebral blood flow velocity was recorded from the middle cerebral artery. *P<0.05 vs. young group. Error bars represent standard error. [Reprinted from Zhu YS, Tseng BY, Shibata S, Levine BD, Zhang R. Increases in cerebrovascular impedance in older adults. J Appl Physiol. 2011 Aug;111 (2):376–81. Copyright © 2011 American Physiological Society]
Clinically, aortic stiffening and augmentations of carotid PP and flow pulsatility have been shown to correlate with cerebral small vessel disease (Mitchell et al. 2011). Consistent with our observations, CBF pulsatility measured from the middle cerebral artery using TCD was positively correlated with the volume of brain whiter matter hyperintensities (Webb et al. 2012). Moreover, CBF pulsatility measured from the large intracranial arteries was elevated in AD patients and exhibited the high sensitivity and specificity for detecting AD patients compared with non-demented control subjects (Roher et al. 2011).
Age and CVMR
Previous research of the aging effects on CVMR generated inconsistent findings which may be explained by the lack of a standardized protocol for CVMR assessment. We recently studied young control and older healthy participants using 2 different CVMR protocols: 1) TCD measurement during hypoventilation and rebreathing (Zhu et al. 2013) and 2) BOLD measurement during steady-state hypercapnia (Thomas et al. 2013). In the first study, participants hyperventilated to induce a brief period of hypocapnia. Following the recovery of baseline hemodynamics, modified rebreathing method was used to induce a progressive increase in PaCO2. During the entire protocol, we measured breath-by-breath changes in end-tidal CO2 and beat-by-beat changes in arterial BP and CBF velocity.
This study demonstrated several key findings. First, older participants had lower CBF velocity and higher cerebrovascular resistance index at rest than young control participants. Second, compared with young control participants, hypocapnic CVMR (vasoconstriction) was significantly attenuated whereas hypercapnic CVMR (vasodilation) was elevated in older participants. Third, hypocapnic CVMR was inversely correlated with hypercapnic CVMR across all participants. Collectively, these observations suggest that advancing age is associated with increased cerebral vasoconstrictor tone at rest which in turn limits the hypocapnic vasoconstrictor capacity while increasing the relative hypercapnic vasodilator capacity.
In the second study, we tested a similar group of young and older participants using functional MRI during steady-state hypercapnia (Thomas et al. 2013). In this study, alternating blocks of room air (1 min) and hypercapnia (1 min) was inhaled by each participant while BOLD images were acquired continuously for 7 minutes. Hypercapnia was induced by having participants to inhale 5% CO2 balanced with 21% oxygen and 74% nitrogen. During the entire protocol, end-tidal CO2, arterial blood oxygen saturation, heart rate, and breathing rate were monitored. In contrast to the former study, we found no group difference in hypercapnic CVMR between young and older participants at both global and regional levels (Thomas et al. 2013).
The inconsistent results from these studies may be explained by different protocols used for CVMR assessment. First, neither BOLD nor TCD measures CBF per se (please read the Methodological Considerations section). Second, the steady-state and rebreathing methods may elicit different autonomic and cardiovascular responses. The rebreathing technique has been shown to elicit greater chemoreflex sensitivity and sympathetic neural response compared with the steady-state method (Mohan et al. 1999, Shoemaker et al. 2002). This greater level of sympathetic neural response may affect the vasodilatory effect of CO2 on cerebral arteries and may result in different CVMR between the protocols (Claassen et al. 2007). Third, the magnitude of hypercapnia induced by steady-state (~10 mmHg) and rebreathing (~16 mmHg) methods was different and the relationship between PaCO2 and CBF may not be linear within these ranges.
In clinical setting, CVMR assessment may help identify individuals who have elevated risks for cerebrovascular disease. According to a meta-analysis of patients with carotid arterial stenosis or occlusion, attenuation of hypercapnic CVMR was associated with the increased incidence of future stroke or transient ischemic attack (Gupta et al. 2012). Mechanistically, carotid arterial stenosis or occlusion may decrease CPP distal to the lesions and exhaust the autoregulatory vasodilatory reserve; therefore, additional stimuli such as hypercapnia may not further vasodilate the cerebral arteries (Gupta et al. 2012). In addition to stroke, attenuated hypercapnic CVMR has been shown to correlate with cognitive decline in AD patients (Silvestrini et al. 2006), microstructural damage of the cerebral white matter (Sam et al. 2016), and increased mortality (Portegies et al. 2014).
Aerobic Exercise and CBF
Cerebral perfusion
Regular aerobic exercise may attenuate age-related reductions of CBF. To test this hypothesis, we used arterial spin labeling technique to measure cerebral perfusion in Masters Athletes (MA) who have participated in lifelong aerobic exercise training and regularly competed in endurance events. Our analysis showed that compared with young control subjects, MAs and sedentary elderly adults have similar reductions of global CBF. However, when examining regional perfusion normalized against global CBF, MAs had higher perfusion in the posterior cingulate and precuneus than young control and sedentary elderly participants (Figure 6) (Thomas et al. 2013). Consistent with these observations, another study of middle-aged MAs showed the higher occipitoparietal perfusion compared with age-matched sedentary subjects (Tarumi et al. 2013).
Figure 6.
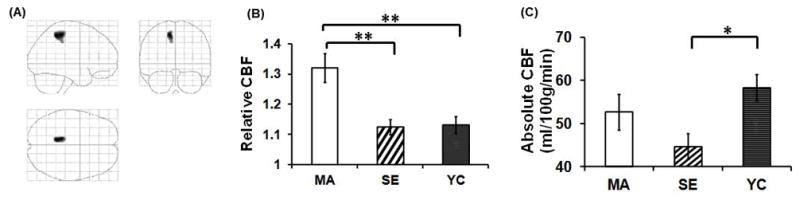
Comparison of cerebral blood flow (CBF) among endurance Masters Athletes (MA, n=10), sedentary elderly adults (SE, n=10), and young control subjects (YC, n=9). (A) Brain regions showing greater CBF in MA compared with SE (P<0.005, cluster size=250). These voxels are located in the posterior cingulate cortex and precuneus. (B) Relative CBF (normalized against whole-brain value) in the cluster highlighted in (A). (C) Absolute CBF in the cluster highlighted in (A). CBF was measured by arterial spin labeling using MRI. *P<0.05, **P<0.005. Error bars represent standard error. [Reprinted from Thomas BP, Yezhuvath US, Tseng BY, Liu P, Levine BD, Zhang R, Lu H. Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. J Magn Reson Imaging. 2013; 38(5): 1177–83. Copyright © 2013 Wiley Online Library. Used with permission.]
Furthermore, short-term aerobic exercise training may alter regional cerebral perfusion. An intervention study of 3 months aerobic exercise training reported the elevation of anterior cingulate perfusion in the previously sedentary older adults (Chapman et al. 2013). On the other hand, a 10-day cessation of aerobic exercise training in MAs decreased regional cerebral perfusion, including the hippocampus (Alfini et al. 2016). Taken together, these findings suggest that aerobic exercise training may increase regional cerebral perfusion, particularly at the area of default mode network that is known to be affected by the process of normal aging and AD (Buckner et al. 2008).
CA and cardiovagal baroreflex function
Regular aerobic exercise may not alter CA in older adults. We studied endurance MAs by measuring their beat-by-beat changes in CBF velocity from the middle cerebral artery, arterial BP, and heart rate at rest and during repeated sit-stand maneuvers. The transfer function analysis of dynamic CA showed that compared with age-matched sedentary subjects, MAs have an attenuation of the very low frequency gain under resting conditions; however, repeated sit-stand maneuvers abolished this group difference (Aengevaeren et al. 2013). Consistent with our observations, dynamic CA measured by thigh cuff technique also showed no difference between young athletes and age-matched sedentary adults (Lind-Holst et al. 2011, Ichikawa et al. 2013).
On the other hand, aerobic exercise training has been shown to increase cardiovagal baroreflex sensitivity in older adults (Aengevaeren et al. 2013, Monahan et al. 2001b, Deley et al. 2009). This may improve arterial BP regulation and decrease the contributions of CA to buffering CBF fluctuations. Mechanistically, exercise-related improvement of the baroreflex sensitivity may be linked to elevated tonic vagal activity that can decrease heart rate (Shi et al. 1995) but increase R-R interval variability (Raczak et al. 2006). Aerobic exercise training can also increase stroke volume and the elasticity of barosensory arteries, which together may improve the transduction of mechanical stimuli to the baroreceptors (Monahan et al. 2001b). Finally, endurance training may increase cardiac cholinergic responsiveness (Poller et al. 1997).
CBF pulsatility
The question of whether aerobic exercise training alters CBF pulsatility in older adults currently remains unknown. To our knowledge, there is only one single study that addressed this question in healthy young adults (Tomoto et al. 2015). In this study, collegiate tennis players underwent 16 weeks of the combined moderate-intensity continuous aerobic exercise training and high-intensity interval training. In addition to CBF pulsatility assessment using TCD, they measured carotid arterial stiffness and left ventricular systolic function. After exercise training, CBF pulsatility did not change; however, the reductions of carotid artery stiffness were individually associated with the attenuations of CBF pulsatility. Therefore, if these findings can be extrapolated to older adults, aerobic exercise training that can reduce carotid arterial stiffness may decrease CBF pulsatility in older adults.
CVMR
The previous studies investigating the effect of aerobic exercise training on CVMR showed mixed results. Using TCD during hyperventilation and modified rebreathing, we observed that both hypo- and hypercapnic CVMRs were similar between endurance MAs and age-matched sedentary older adults (Zhu et al. 2013). In a similar group of study participants, we also measured CVMR using functional MRI during steady-state hypercapnia and observed that MAs have lower CVMR than the sedentary older adults (Thomas et al. 2013). On the other hand, using TCD during steady-state hypercapnia, aerobic exercise training studies showed improvements in CVMR in healthy young and older adults (Murrell et al. 2013) as well as in stoke survivors (Ivey et al. 2011). Taken together, these studies made inconsistent observations over the effect of aerobic exercise training on CVMR and suggest that methods used to measure CBF (TCD, BOLD) or to elicit CO2 stimulus (rebreathing, steady-state hypercapnia) may alter CVMR quantifications.
Methodological Considerations
CBF is highly variable in space and time, and currently there is no single method that can measure CBF at the sufficiently high level of spatial and temporal resolutions. For example, numerous studies investigating CBF regulation have used TCD that is non-invasive, relatively inexpensive, and readily accessible. TCD has a major strength of recording CBF velocity at high temporal resolution from the large intracranial arteries, but it can only assess changes in CBF under the assumption of constant insonated arterial diameter. Although this assumption may hold true under the relatively mild stimulus (Giller et al. 1993), recent studies using high-resolution MRI revealed that moderate to severe hypo- and hypercapnia can alter the diameter of middle cerebral artery which may have significant impact on CBF quantification (Verbree et al. 2014).
Recently, color-coded duplex ultrasound imaging of the internal carotid and vertebral arteries is gaining popularity in CBF research. This technique is strengthened by the ability to measure global and regional CBF in the anterior and posterior circulations at high temporal resolution (Liu et al. 2016). This imaging method is easily accessible at bedside and can be performed on patients who have contraindications to MRI (e.g. metal implants or claustrophobia). On the other hand, technical aspect of this method can sometimes be challenging depending on the individual differences in vascular anatomy. For example, the bifurcation site of the common carotid artery may be located close to the jaw and make the placement of an ultrasound probe difficult to accurately measure the diameter and blood flow velocity in the internal carotid artery. Also, vertebral arteries are relatively small compared with the carotid arteries and located deep in the neck. In such cases, an alternative method may be phase-contrast MRI combined with the time-of-flight angiography. In our previous study, we found that color-coded duplex ultrasonography and phase-contrast MRI have the similar estimations of volumetric CBF measured from the internal and vertebral arteries (Khan et al. 2017).
Neuroimaging techniques such as positron emission tomography (PET), single-photon emission computed tomography (SPECT), and arterial spin labeling have higher spatial resolution of CBF measurement than TCD, color-coded duplex ultrasonography, or phase-contrast MRI; however, these methods are limited by lower temporal resolution. In addition, these imaging modalities are more expensive and require the infusion of radioactive tracer to blood circulation (PET and SPECT). To avoid tracer infusions, arterial spin labeling technique using MRI allows non-invasive assessment of regional cerebral perfusion, but this method is also limited by a low signal-to-noise ratio and requires multiple image acquisitions for averaging to obtain reliable results. In addition, BOLD signal acquired by functional MRI has been used to assess regional CVMR; however, this technology depends on changes in the local concentration of deoxyhemoglobin which is influenced by altered neuronal activity and/or changes in the total concentration of hemoglobin by altered CBF (Halani et al. 2015). Thus, a multi-modal approach may complement each method of CBF measurement and provides a better understanding of cerebral hemodynamics.
Summary
Age profoundly alters CBF and its regulatory mechanisms (Figure 7). Specifically, steady-state CBF progressively decreases across the adult lifespan while CBF pulsatility increases after midlife (Tarumi et al. 2014). The fluctuations of CBF during postural changes are also augmented in older adults compared with younger adults (Xing et al. 2016). These age-related changes in CBF are, at least in part, explained by the concurrent alterations of arterial BP, primarily the elevations of systolic BP and PP. In addition, although CA may not be affected by age, older adults have marked reduction of cardiovagal baroreflex sensitivity which likely augments BP and CBF fluctuations during postural changes. In patients with cerebrovascular disease and AD, these age-related alterations of CBF are exaggerated and may contribute to the disease onset and progression. In particular, cerebral hypoperfusion (Iturria-Medina et al. 2016), augmented CBF pulsatility (Roher et al. 2011), and orthostatic hypotension (Mehrabian et al. 2010) all have been reported in AD patients compared with aged-matched cognitively normal adults. In contrast, regular aerobic exercise may attenuate the age-related reduction of CBF, especially in the default mode neural network. Taken together, gaining the knowledge of normal age-related changes in CBF may help us identify the individuals at risk for cerebrovascular disease and dementia, including AD. We now understand that dementia is a multifactorial disease. Therefore, understanding of vascular mechanisms, identification of vascular biomarkers, and development of vascular-based interventions may pave promising avenues to maintain brain health in older adults.
Figure 7.
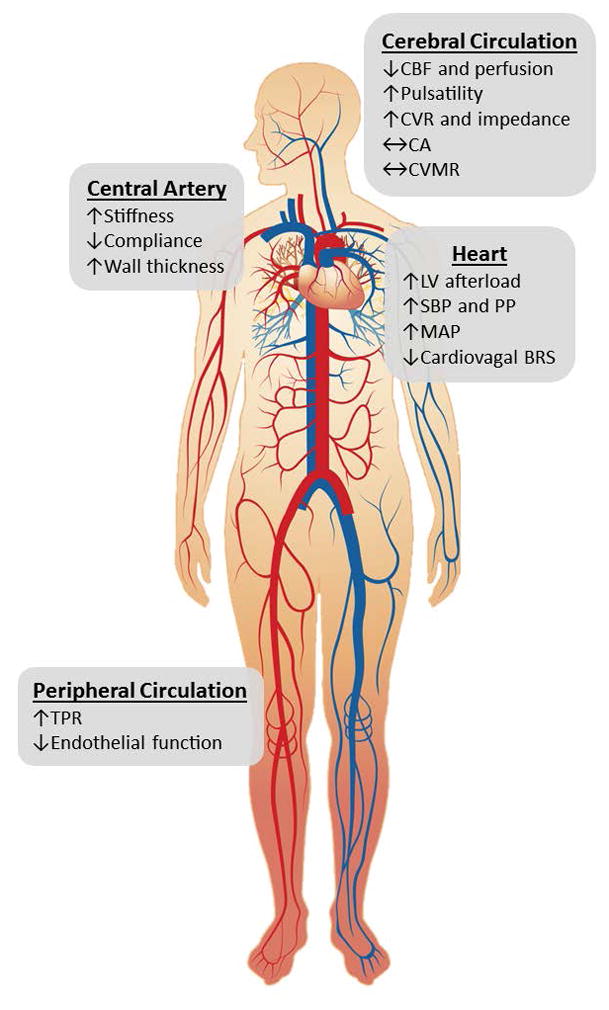
A summary schematic illustrating the effect of normal aging on cerebral and cardiovascular circulation. BRS=baroreflex sensitivity, CA=cerebral autoregulation, CBF=cerebral blood flow, CVR=cerebrovascular resistance, CVMR=cerebral vasomotor reactivity, LV=left ventricular, MAP=mean arterial pressure, PP=pulse pressure, SBP=systolic blood pressure, and TPR=total peripheral resistance
Acknowledgments
Sources of Funding: National Institutes of Health (K99HL133449 and R01AG049749)
Footnotes
Conflict of Interest/Disclosure: None
References
- 1.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- 2.Aengevaeren VL, Claassen JA, Levine BD, Zhang R. Cardiac baroreflex function and dynamic cerebral autoregulation in elderly Masters athletes. J Appl Physiol (1985) 2013;114:195–202. doi: 10.1152/japplphysiol.00402.2012. [DOI] [PubMed] [Google Scholar]
- 3.Alfini AJ, Weiss LR, Leitner BP, Smith TJ, Hagberg JM, Smith JC. Hippocampal and Cerebral Blood Flow after Exercise Cessation in Master Athletes. Front Aging Neurosci. 2016;8:184. doi: 10.3389/fnagi.2016.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes JN, Schmidt JE, Nicholson WT, Joyner MJ. Cyclooxygenase inhibition abolishes age-related differences in cerebral vasodilator responses to hypercapnia. J Appl Physiol (1985) 2012;112:1884–1890. doi: 10.1152/japplphysiol.01270.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamin P, Zeestraten E, Lambert C, et al. Progression of MRI markers in cerebral small vessel disease: Sample size considerations for clinical trials. J Cereb Blood Flow Metab. 2016;36:228–240. doi: 10.1038/jcbfm.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boumezbeur F, Mason GF, de Graaf RA, Behar KL, Cline GW, Shulman GI, Rothman DL, Petersen KF. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 2010;30:211–221. doi: 10.1038/jcbfm.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandfonbrener M, Landowne M, Shock NW. Changes in cardiac output with age. Circulation. 1955;12:557–566. doi: 10.1161/01.cir.12.4.557. [DOI] [PubMed] [Google Scholar]
- 9.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 10.Chapman PH, Cosman ER, Arnold MA. The relationship between ventricular fluid pressure and body position in normal subjects and subjects with shunts: a telemetric study. Neurosurgery. 1990;26:181–189. doi: 10.1097/00006123-199002000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Chapman SB, Aslan S, Spence JS, Defina LF, Keebler MW, Didehbani N, Lu H. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front Aging Neurosci. 2013;5:75. doi: 10.3389/fnagi.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 13.Claassen JA, Levine BD, Zhang R. Dynamic cerebral autoregulation during repeated squat-stand maneuvers. J Appl Physiol (1985) 2009;106:153–160. doi: 10.1152/japplphysiol.90822.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claassen JA, Zhang R, Fu Q, Witkowski S, Levine BD. Transcranial Doppler estimation of cerebral blood flow and cerebrovascular conductance during modified rebreathing. J Appl Physiol (1985) 2007;102:870–877. doi: 10.1152/japplphysiol.00906.2006. [DOI] [PubMed] [Google Scholar]
- 15.Czosnyka M, Balestreri M, Steiner L, Smielewski P, Hutchinson PJ, Matta B, Pickard JD. Age, intracranial pressure, autoregulation, and outcome after brain trauma. J Neurosurg. 2005;102:450–454. doi: 10.3171/jns.2005.102.3.0450. [DOI] [PubMed] [Google Scholar]
- 16.de la Torre JC. Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–190. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- 17.Deley G, Picard G, Taylor JA. Arterial baroreflex control of cardiac vagal outflow in older individuals can be enhanced by aerobic exercise training. Hypertension. 2009;53:826–832. doi: 10.1161/HYPERTENSIONAHA.109.130039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edvinsson L. Neurogenic mechanisms in the cerebrovascular bed. Autonomic nerves, amine receptors and their effects on cerebral blood flow. Acta Physiol Scand Suppl. 1975;427:1–35. [PubMed] [Google Scholar]
- 19.Elia M. Organ and tissue contribution to metabolic rate. Raven Press, LtD; New York: 1992. [Google Scholar]
- 20.Fan JL, Burgess KR, Thomas KN, Peebles KC, Lucas SJ, Lucas RA, Cotter JD, Ainslie PN. Influence of indomethacin on ventilatory and cerebrovascular responsiveness to CO2 and breathing stability: the influence of PCO2 gradients. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1648–1658. doi: 10.1152/ajpregu.00721.2009. [DOI] [PubMed] [Google Scholar]
- 21.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franklin SS, Gustin Wt, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 23.Fraser KS, Heckman GA, McKelvie RS, Harkness K, Middleton LE, Hughson RL. Cerebral hypoperfusion is exaggerated with an upright posture in heart failure: impact of depressed cardiac output. JACC Heart Fail. 2015;3:168–175. doi: 10.1016/j.jchf.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Giller CA. The frequency-dependent behavior of cerebral autoregulation. Neurosurgery. 1990;27:362–368. doi: 10.1097/00006123-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery. 1993;32:737–741. discussion 741–732. [PubMed] [Google Scholar]
- 26.Gruhn N, Larsen FS, Boesgaard S, Knudsen GM, Mortensen SA, Thomsen G, Aldershvile J. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke. 2001;32:2530–2533. doi: 10.1161/hs1101.098360. [DOI] [PubMed] [Google Scholar]
- 27.Guo GB, Thames MD, Abboud FM. Differential baroreflex control of heart rate and vascular resistance in rabbits. Relative role of carotid, aortic, and cardiopulmonary baroreceptors. Circ Res. 1982;50:554–565. doi: 10.1161/01.res.50.4.554. [DOI] [PubMed] [Google Scholar]
- 28.Gupta A, Chazen JL, Hartman M, et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta-analysis. Stroke. 2012;43:2884–2891. doi: 10.1161/STROKEAHA.112.663716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halani S, Kwinta JB, Golestani AM, Khatamian YB, Chen JJ. Comparing cerebrovascular reactivity measured using BOLD and cerebral blood flow MRI: The effect of basal vascular tension on vasodilatory and vasoconstrictive reactivity. Neuroimage. 2015;110:110–123. doi: 10.1016/j.neuroimage.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamner JW, Tan CO, Lee K, Cohen MA, Taylor JA. Sympathetic control of the cerebral vasculature in humans. Stroke. 2010;41:102–109. doi: 10.1161/STROKEAHA.109.557132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamner JW, Tan CO, Tzeng YC, Taylor JA. Cholinergic control of the cerebral vasculature in humans. J Physiol. 2012;590:6343–6352. doi: 10.1113/jphysiol.2012.245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 33.Hart EC, Joyner MJ, Wallin BG, Charkoudian N. Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol. 2012;590:2069–2079. doi: 10.1113/jphysiol.2011.224642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoiland RL, Smith KJ, Carter HH, Lewis NCS, Tymko MM, Wildfong KW, Bain AR, Green DJ, Ainslie PN. Shear-mediated dilation of the internal carotid artery occurs independent of hypercapnia. Am J Physiol Heart Circ Physiol. 2017;313:H24–H31. doi: 10.1152/ajpheart.00119.2017. [DOI] [PubMed] [Google Scholar]
- 35.Ichikawa D, Miyazawa T, Horiuchi M, Kitama T, Fisher JP, Ogoh S. Relationship between aerobic endurance training and dynamic cerebral blood flow regulation in humans. Scand J Med Sci Sports. 2013;23:e320–329. doi: 10.1111/sms.12082. [DOI] [PubMed] [Google Scholar]
- 36.Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Perez JM, Evans AC Alzheimer’s Disease Neuroimaging I. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun. 2016;7:11934. doi: 10.1038/ncomms11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ivey FM, Ryan AS, Hafer-Macko CE, Macko RF. Improved cerebral vasomotor reactivity after exercise training in hemiparetic stroke survivors. Stroke. 2011;42:1994–2000. doi: 10.1161/STROKEAHA.110.607879. [DOI] [PubMed] [Google Scholar]
- 38.Khan MA, Liu J, Tarumi T, Lawley JS, Liu P, Zhu DC, Lu H, Zhang R. Measurement of cerebral blood flow using phase contrast magnetic resonance imaging and duplex ultrasonography. J Cereb Blood Flow Metab. 2017;37:541–549. doi: 10.1177/0271678X16631149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. National Vital Statistics Reports. Vol. 65. U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES; 2016. Deaths: Final Data for 2014. [PubMed] [Google Scholar]
- 40.Lassen NA. Autoregulation of Cerebral Blood Flow. Circ Res. 1964;15(SUPPL):201–204. [PubMed] [Google Scholar]
- 41.Leenders KL, Perani D, Lammertsma AA, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain : a journal of neurology. 1990;113(Pt 1):27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- 42.Lind-Holst M, Cotter JD, Helge JW, Boushel R, Augustesen H, Van Lieshout JJ, Pott FC. Cerebral autoregulation dynamics in endurance-trained individuals. J Appl Physiol (1985) 2011;110:1327–1333. doi: 10.1152/japplphysiol.01497.2010. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Tseng BY, Khan MA, Tarumi T, Hill C, Mirshams N, Hodics TM, Hynan LS, Zhang R. Individual variability of cerebral autoregulation, posterior cerebral circulation and white matter hyperintensity. J Physiol. 2016;594:3141–3155. doi: 10.1113/JP271068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, Hebrank AC, Uh J, Park DC. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex. 2011;21:1426–1434. doi: 10.1093/cercor/bhq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marchal G, Rioux P, Petit-Taboue MC, Sette G, Travere JM, Le Poec C, Courtheoux P, Derlon JM, Baron JC. Regional cerebral oxygen consumption, blood flow, and blood volume in healthy human aging. Arch Neurol. 1992;49:1013–1020. doi: 10.1001/archneur.1992.00530340029014. [DOI] [PubMed] [Google Scholar]
- 46.McHedlishvili G. Physiological mechanisms controlling cerebral blood flow. Stroke. 1980;11:240–248. doi: 10.1161/01.str.11.3.240. [DOI] [PubMed] [Google Scholar]
- 47.Mehrabian S, Duron E, Labouree F, Rollot F, Bune A, Traykov L, Hanon O. Relationship between orthostatic hypotension and cognitive impairment in the elderly. J Neurol Sci. 2010;299:45–48. doi: 10.1016/j.jns.2010.08.056. [DOI] [PubMed] [Google Scholar]
- 48.Meng L, Hou W, Chui J, Han R, Gelb AW. Cardiac Output and Cerebral Blood Flow: The Integrated Regulation of Brain Perfusion in Adult Humans. Anesthesiology. 2015;123:1198–1208. doi: 10.1097/ALN.0000000000000872. [DOI] [PubMed] [Google Scholar]
- 49.Mitchell GF, van Buchem MA, Sigurdsson S, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility--Reykjavik study. Brain : a journal of neurology. 2011;134:3398–3407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mohan RM, Amara CE, Cunningham DA, Duffin J. Measuring central-chemoreflex sensitivity in man: rebreathing and steady-state methods compared. Respir Physiol. 1999;115:23–33. doi: 10.1016/s0034-5687(99)00003-1. [DOI] [PubMed] [Google Scholar]
- 51.Monahan KD, Dinenno FA, Seals DR, Clevenger CM, Desouza CA, Tanaka H. Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am J Physiol Heart Circ Physiol. 2001a;281:H284–289. doi: 10.1152/ajpheart.2001.281.1.H284. [DOI] [PubMed] [Google Scholar]
- 52.Monahan KD, Tanaka H, Dinenno FA, Seals DR. Central arterial compliance is associated with age- and habitual exercise-related differences in cardiovagal baroreflex sensitivity. Circulation. 2001b;104:1627–1632. doi: 10.1161/hc3901.096670. [DOI] [PubMed] [Google Scholar]
- 53.Moseley ME, Glover GH. Functional MR imaging. Capabilities and limitations. Neuroimaging Clin N Am. 1995;5:161–191. [PubMed] [Google Scholar]
- 54.Murrell CJ, Cotter JD, Thomas KN, Lucas SJ, Williams MJ, Ainslie PN. Cerebral blood flow and cerebrovascular reactivity at rest and during sub-maximal exercise: effect of age and 12-week exercise training. Age (Dordr) 2013;35:905–920. doi: 10.1007/s11357-012-9414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nichols WW, O’Rourke ME. McDonald’s Blood Flow in Arteries. Oxford University Press; New York: 2005. [Google Scholar]
- 56.O’Sullivan M, Lythgoe DJ, Pereira AC, Summers PE, Jarosz JM, Williams SC, Markus HS. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology. 2002;59:321–326. doi: 10.1212/wnl.59.3.321. [DOI] [PubMed] [Google Scholar]
- 57.Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ogoh S, Brothers RM, Barnes Q, Eubank WL, Hawkins MN, Purkayastha S, AOY, Raven PB. The effect of changes in cardiac output on middle cerebral artery mean blood velocity at rest and during exercise. J Physiol. 2005;569:697–704. doi: 10.1113/jphysiol.2005.095836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okada Y, Galbreath MM, Shibata S, Jarvis SS, VanGundy TB, Meier RL, Vongpatanasin W, Levine BD, Fu Q. Relationship between sympathetic baroreflex sensitivity and arterial stiffness in elderly men and women. Hypertension. 2012;59:98–104. doi: 10.1161/HYPERTENSIONAHA.111.176560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oz G, Kumar A, Rao JP, Kodl CT, Chow L, Eberly LE, Seaquist ER. Human brain glycogen metabolism during and after hypoglycemia. Diabetes. 2009;58:1978–1985. doi: 10.2337/db09-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oz G, Seaquist ER, Kumar A, Criego AB, Benedict LE, Rao JP, Henry PG, Van De Moortele PF, Gruetter R. Human brain glycogen content and metabolism: implications on its role in brain energy metabolism. Am J Physiol Endocrinol Metab. 2007;292:E946–951. doi: 10.1152/ajpendo.00424.2006. [DOI] [PubMed] [Google Scholar]
- 62.Panerai RB, Dawson SL, Potter JF. Linear and nonlinear analysis of human dynamic cerebral autoregulation. Am J Physiol. 1999;277:H1089–1099. doi: 10.1152/ajpheart.1999.277.3.H1089. [DOI] [PubMed] [Google Scholar]
- 63.Petit-Taboue MC, Landeau B, Desson JF, Desgranges B, Baron JC. Effects of healthy aging on the regional cerebral metabolic rate of glucose assessed with statistical parametric mapping. Neuroimage. 1998;7:176–184. doi: 10.1006/nimg.1997.0318. [DOI] [PubMed] [Google Scholar]
- 64.Poller U, Nedelka G, Radke J, Ponicke K, Brodde OE. Age-dependent changes in cardiac muscarinic receptor function in healthy volunteers. J Am Coll Cardiol. 1997;29:187–193. doi: 10.1016/s0735-1097(96)00437-8. [DOI] [PubMed] [Google Scholar]
- 65.Portegies ML, de Bruijn RF, Hofman A, Koudstaal PJ, Ikram MA. Cerebral vasomotor reactivity and risk of mortality: the Rotterdam Study. Stroke. 2014;45:42–47. doi: 10.1161/STROKEAHA.113.002348. [DOI] [PubMed] [Google Scholar]
- 66.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75. e62. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 67.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 68.Raczak G, Danilowicz-Szymanowicz L, Kobuszewska-Chwirot M, Ratkowski W, Figura-Chmielewska M, Szwoch M. Long-term exercise training improves autonomic nervous system profile in professional runners. Kardiol Pol. 2006;64:135–140. discussion 141–132. [PubMed] [Google Scholar]
- 69.Rickards CA, Tzeng YC. Arterial pressure and cerebral blood flow variability: friend or foe? A review. Front Physiol. 2014;5:120. doi: 10.3389/fphys.2014.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roher AE, Garami Z, Tyas SL, et al. Transcranial doppler ultrasound blood flow velocity and pulsatility index as systemic indicators for Alzheimer’s disease. Alzheimers Dement. 2011;7:445–455. doi: 10.1016/j.jalz.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sam K, Peltenburg B, Conklin J, et al. Cerebrovascular reactivity and white matter integrity. Neurology. 2016;87:2333–2339. doi: 10.1212/WNL.0000000000003373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmetterer L, Findl O, Strenn K, Graselli U, Kastner J, Eichler HG, Wolzt M. Role of NO in the O2 and CO2 responsiveness of cerebral and ocular circulation in humans. Am J Physiol. 1997;273:R2005–2012. doi: 10.1152/ajpregu.1997.273.6.R2005. [DOI] [PubMed] [Google Scholar]
- 73.Shi X, Stevens GH, Foresman BH, Stern SA, Raven PB. Autonomic nervous system control of the heart: endurance exercise training. Med Sci Sports Exerc. 1995;27:1406–1413. [PubMed] [Google Scholar]
- 74.Shoemaker JK, Vovk A, Cunningham DA. Peripheral chemoreceptor contributions to sympathetic and cardiovascular responses during hypercapnia. Can J Physiol Pharmacol. 2002;80:1136–1144. doi: 10.1139/y02-148. [DOI] [PubMed] [Google Scholar]
- 75.Silvestrini M, Pasqualetti P, Baruffaldi R, Bartolini M, Handouk Y, Matteis M, Moffa F, Provinciali L, Vernieri F. Cerebrovascular reactivity and cognitive decline in patients with Alzheimer disease. Stroke. 2006;37:1010–1015. doi: 10.1161/01.STR.0000206439.62025.97. [DOI] [PubMed] [Google Scholar]
- 76.Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet. 2000;355:865–872. doi: 10.1016/s0140-6736(99)07330-4. [DOI] [PubMed] [Google Scholar]
- 78.Tarumi T, Ayaz Khan M, Liu J, Tseng BY, Parker R, Riley J, Tinajero C, Zhang R. Cerebral hemodynamics in normal aging: central artery stiffness, wave reflection, and pressure pulsatility. J Cereb Blood Flow Metab. 2014;34:971–978. doi: 10.1038/jcbfm.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tarumi T, de Jong DL, Zhu DC, et al. Central artery stiffness, baroreflex sensitivity, and brain white matter neuronal fiber integrity in older adults. Neuroimage. 2015;110:162–170. doi: 10.1016/j.neuroimage.2015.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tarumi T, Gonzales MM, Fallow B, Nualnim N, Pyron M, Tanaka H, Haley AP. Central artery stiffness, neuropsychological function, and cerebral perfusion in sedentary and endurance-trained middle-aged adults. J Hypertens. 2013;31:2400–2409. doi: 10.1097/HJH.0b013e328364decc. [DOI] [PubMed] [Google Scholar]
- 81.Thomas BP, Yezhuvath US, Tseng BY, Liu P, Levine BD, Zhang R, Lu H. Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. J Magn Reson Imaging. 2013;38:1177–1183. doi: 10.1002/jmri.24090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tomoto T, Sugawara J, Nogami Y, Aonuma K, Maeda S. The influence of central arterial compliance on cerebrovascular hemodynamics: insights from endurance training intervention. J Appl Physiol (1985) 2015;119:445–451. doi: 10.1152/japplphysiol.00129.2015. [DOI] [PubMed] [Google Scholar]
- 83.Tzeng YC, Lucas SJ, Atkinson G, Willie CK, Ainslie PN. Fundamental relationships between arterial baroreflex sensitivity and dynamic cerebral autoregulation in humans. J Appl Physiol (1985) 2010;108:1162–1168. doi: 10.1152/japplphysiol.01390.2009. [DOI] [PubMed] [Google Scholar]
- 84.United_Nations. World population ageing 2013. Department of Economic and Social Affairs PD; 2013. [Google Scholar]
- 85.Verbree J, Bronzwaer AS, Ghariq E, Versluis MJ, Daemen MJ, van Buchem MA, Dahan A, van Lieshout JJ, van Osch MJ. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol (1985) 2014;117:1084–1089. doi: 10.1152/japplphysiol.00651.2014. [DOI] [PubMed] [Google Scholar]
- 86.Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia: how to move forward? Neurology. 2009;72:368–374. doi: 10.1212/01.wnl.0000341271.90478.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Webb AJ, Simoni M, Mazzucco S, Kuker W, Schulz U, Rothwell PM. Increased cerebral arterial pulsatility in patients with leukoaraiosis: arterial stiffness enhances transmission of aortic pulsatility. Stroke. 2012;43:2631–2636. doi: 10.1161/STROKEAHA.112.655837. [DOI] [PubMed] [Google Scholar]
- 88.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 89.Williams LR, Leggett RW. Reference values for resting blood flow to organs of man. Clin Phys Physiol Meas. 1989;10:187–217. doi: 10.1088/0143-0815/10/3/001. [DOI] [PubMed] [Google Scholar]
- 90.Willis MW, Ketter TA, Kimbrell TA, George MS, Herscovitch P, Danielson AL, Benson BE, Post RM. Age, sex and laterality effects on cerebral glucose metabolism in healthy adults. Psychiatry Res. 2002;114:23–37. doi: 10.1016/s0925-4927(01)00126-3. [DOI] [PubMed] [Google Scholar]
- 91.Xing CY, Tarumi T, Liu J, Zhang Y, Turner M, Riley J, Tinajero CD, Yuan LJ, Zhang R. Distribution of cardiac output to the brain across the adult lifespan. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16676826. 271678X16676826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xing CY, Tarumi T, Meijers RL, et al. Arterial Pressure, Heart Rate, and Cerebral Hemodynamics Across the Adult Life Span. Hypertension. 2017;69:712–720. doi: 10.1161/HYPERTENSIONAHA.116.08986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang R, Iwasaki K, Zuckerman JH, Behbehani K, Crandall CG, Levine BD. Mechanism of blood pressure and R-R variability: insights from ganglion blockade in humans. J Physiol. 2002a;543:337–348. doi: 10.1113/jphysiol.2001.013398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol. 1998;274:H233–241. doi: 10.1152/ajpheart.1998.274.1.h233. [DOI] [PubMed] [Google Scholar]
- 95.Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation. 2002b;106:1814–1820. doi: 10.1161/01.cir.0000031798.07790.fe. [DOI] [PubMed] [Google Scholar]
- 96.Zhang R, Zuckerman JH, Levine BD. Spontaneous fluctuations in cerebral blood flow: insights from extended-duration recordings in humans. Am J Physiol Heart Circ Physiol. 2000;278:H1848–1855. doi: 10.1152/ajpheart.2000.278.6.H1848. [DOI] [PubMed] [Google Scholar]
- 97.Zhu DC, Tarumi T, Khan MA, Zhang R. Vascular coupling in resting-state fMRI: evidence from multiple modalities. J Cereb Blood Flow Metab. 2015;35:1910–1920. doi: 10.1038/jcbfm.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu YS, Tarumi T, Tseng BY, Palmer DM, Levine BD, Zhang R. Cerebral vasomotor reactivity during hypo- and hypercapnia in sedentary elderly and Masters athletes. J Cereb Blood Flow Metab. 2013;33:1190–1196. doi: 10.1038/jcbfm.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu YS, Tseng BY, Shibata S, Levine BD, Zhang R. Increases in cerebrovascular impedance in older adults. J Appl Physiol (1985) 2011;111:376–381. doi: 10.1152/japplphysiol.01418.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 101.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]