Summary
Obstructive sleep apnea (OSA) is common, and many cross-sectional and longitudinal studies have established OSA as an independent risk factor for the development of a variety of adverse metabolic disease states, including hypertension, insulin resistance, type 2 diabetes, nonalcoholic fatty liver disease, dyslipidemia, and atherosclerosis. Nasal continuous positive airway pressure (CPAP) has long been the mainstay of therapy for OSA, but definitive studies demonstrating the efficacy of CPAP in improving metabolic outcomes, or in reducing incident disease burden, are lacking; moreover, CPAP has variable rates of adherence. Therefore, the future of OSA management, particularly with respect to limiting OSA-related metabolic dysfunction, likely lies in a coming wave of alternative approaches to endophenotyping OSA patients, personalized care, and defining and targeting mechanisms of OSA-induced adverse health outcomes.
Introduction
Obstructive sleep apnea (OSA) is a common disorder, with major neurocognitive and cardiometabolic sequelae [1, 2]. Conservative estimates suggest that at least 10% of the U.S. population is afflicted with OSA, and yet the majority of disease remains undiagnosed and untreated [3]. Lack of awareness on the part of patients and clinicians is largely to blame, but in addition, existing diagnostic and therapeutic strategies are imperfect. Nasal continuous positive airway pressure (CPAP) has good efficacy for treatment of OSA, but adherence is quite variable [4]. Alternative therapies exist, but outcome data are mixed and it is difficult to predict response to therapy [5, 6].
Obesity, Type 2 Diabetes, and Sleep Apnea
OSA has been associated with a number of metabolic and cardiovascular consequences, although the causal pathways are still debated [7–9]. Obesity is a strong risk factor for OSA, and thus epidemiological studies can be complicated by confounding variables and interactive effects [10]. With respect to OSA and diabetes, a number of observations are worth emphasizing:
Obesity is a risk factor for both OSA and type 2 diabetes mellitus (DM). Thus, with the obesity epidemic, it is likely that the prevalence of both OSA and DM will continue to rise [11, 12]. To complicate matters, some diabetes therapies (e.g. insulin, sulfonylureas) may promote weight gain, and in theory worsen OSA.
OSA may be an independent risk factor for hyperglycemia in type 2 DM. Repetitive apnea leads to the release of counter-regulatory hormones, which can contribute to elevated plasma glucose concentrations. Indeed some data suggest that improvements in glycemic control can occur with OSA therapy, although these results are inconsistent across studies [9, 13–15]. As one example, Pamidi et al. observed improved glucose response and insulin sensitivity with OSA treatment compared to placebo, when CPAP adherence was strictly monitored and optimized [9].
OSA may be an independent risk factor for microvascular complications in type 2 DM as well, including diabetic retinopathy [16], neuropathy and foot ulcers [17], and nephropathy [18], though much remains to be confirmed in large-scale studies.
Type 2 DM may be a risk factor for OSA. Neuromyopathy can affect the upper airway and has potential to impair pharyngeal protective reflexes. The data regarding this assertion are somewhat controversial, but some studies do suggest worsening of apnea over time in those with diabetes [19, 20].
Both type 2 DM and OSA may be independent vascular risk factors, perhaps working through different mechanisms. Studies have shown that type 2 DM affects the microcirculation and vascular smooth muscle, whereas OSA primarily affects the endothelium [21–23]. Thus, one might predict that treatment of one disease process without attention to the other may leave residual vascular risk, and that in theory maximum vascular risk reduction occurs when both type 2 DM and OSA are treated in concert.
At present, although roughly 86% of obese type 2 DM patients have evidence of clinically important OSA [12], the vast majority of disease remains undiagnosed and untreated. Indeed, one year after the diagnosis of OSA is given to both the patient and the practitioner, fewer than 5% of such patients are receiving therapy [20]. This finding is particularly worrying when one considers how urgently new modalities of treatment are needed, since existing treatment strategies in diabetes (glycemic control, as well as control of blood pressure and lipids) are inadequate to return mortality to baseline. As tight glycemic control might even increase the risk of death [24], new approaches to care are clearly warranted. Thus, we would advocate for OSA as a major potential therapeutic target in type 2 DM.
The relationship between OSA and obesity may be just as complex. OSA is commonly thought to be a disorder strictly related to obesity. Approximately 70% of OSA patients are obese [25], and the majority of morbidly obese patients have OSA [26]. A single standard deviation increase in any measure of body habitus is associated with a threefold increased risk of having an abnormal AHI [27]. There is clear evidence that weight reduction in obese patients with OSA can both improve AHI and upper airway collapsibility [28, 29]. Marked weight loss, such as with bariatric surgery, is associated with a dramatic improvement in OSA, but it is important to note that such patients usually do not demonstrate resolution of OSA to an AHI ≤5/h standard [30]. Interestingly, although weight loss results in an improvement in OSA, there is considerable variability in individual responses. Some patients who lose weight and are initially cured of OSA redevelop the disorder despite maintenance of weight loss [31]. CPAP therapy has been infrequently compared to weight loss in randomized, controlled trials, but one such study which looked at a variety of metabolic and inflammatory markers as outcomes found that CPAP plus weight loss, or weight loss alone, were each more effective than CPAP alone at improving the metabolic profile [32]. Other studies evaluating these interventions are currently ongoing. Clearly, predicting individual responses to weight reduction therapy in obese patients with OSA, and defining the additional benefit afforded by CPAP, is of tantamount importance in the field. Indeed, considerable effort has been applied toward identifying patients who may be at highest risk of obesity related complications including OSA. The “fingerprint” of the gut microbiome [33] or microRNAs [34, 35] may hold promise toward identifying patients who might particularly benefit from aggressive OSA treatment.
Given the tight association between obesity and OSA, one might consider whether treating OSA may itself lead to weight loss, with the rationale that CPAP therapy makes patients less sleepy. In our experience, this is a common perception held by patients who present to our Sleep Center. Indeed, an early study seemed to suggest this might be the case: Loube et al. described a small series in which patients who were adherent to CPAP for their OSA were more likely to experience significant weight loss on follow-up [36]. However, this finding has not been replicated in subsequent studies [37], and a meta-analysis has shown that CPAP likely leads to a small but statistically significant weight gain [38]. In our practice, we advise patients who may be eager to start CPAP so that they can be more active and therefore shed pounds, that there are no convincing data to suggest that commencing OSA treatment of any kind, aside from surgical or lifestyle modification specifically targeting weight, will lead to weight reduction.
Atherosclerosis, NAFLD, and Sleep Apnea
In the case of non-alcoholic fatty liver disease (NAFLD), several epidemiological studies have suggested that OSA may be an important risk factor, independent of obesity [39–43]. The causal pathways are being investigated but involve insulin resistance and the hypoxic burden of sleep apnea [44, 45]. In addition, liver fibrosis, a major clinical consequence of NAFLD, is thought to be enhanced in OSA patients compared to matched controls. Corey et al. showed that absence of OSA is protective of fibrosis in patients with NAFLD in multivariate analyses [46]. Limited data suggest that treatment of OSA may improve liver enzymes [47, 48], although further clinical trials are clearly needed. Some data have implicated Hypoxia Inducible Factors-1 and -2 (HIF-1 and HIF-2) as key mechanistic determinants of OSA-induced liver damage [45, 49, 50]. Studies remain pre-clinical and require further corroboration. Pharmacological studies to manipulate the pathways from OSA to liver fibrosis are clearly lacking, but may be another potential therapeutic approach in the future [51].
Because there has long been evidence of the association between OSA and cardiovascular complications such as myocardial infarction and stroke, researchers have recently examined whether OSA may increase the risk for atherosclerosis. The majority of such studies over the last several years suggest that OSA is independently associated with atherosclerosis [52, 53]. When one considers that atherosclerosis is not just a disorder of lipid metabolism but also involves chronic inflammation [54], and that OSA induces vascular inflammation independent of obesity [55], this paradigm of OSA as a risk factor for atherosclerosis is biologically plausible. Although separating OSA from obesity effects can be challenging, Drager et al. examined a group of otherwise healthy young men with OSA, compared to matched controls, and found that OSA severity correlated with vascular abnormalities, including increased arterial stiffness and early signs of atherosclerosis [56]. Additionally, Savransky et al. demonstrated that chronic intermittent hypoxia (IH) in a mouse model of OSA induced aortic atherosclerosis [57], a finding which has been subsequently reproduced. Thus, in theory, OSA may cause atherosclerosis, via intermittent hypoxia. This knowledge may help guide future diagnostic and therapeutic strategies in OSA.
Novel Diagnostic and Therapeutic Approaches
One might argue that, in the past, access to polysomnography has limited testing for sleep apnea in patients with, or at risk of, metabolic disease. New testing methods are emerging that allow cheap and accessible diagnosis of OSA in the home (in addition to other sleep disorders), obviating the need for polysomnography in many cases. Technologies such as mobile device platforms, and wearable actigraphy and encephalographic monitors, are on the cusp of widespread use, and may not only be used to expand diagnostic options, but also to monitor sleep quality and quantity so as to ensure treatment efficacy. Wearable technologies may even be viable in patients with low pretest probability for OSA (which has not been supported by previous guidelines), or could be used to capture night to night variability in OSA occurrence, e.g. in those with intermittent alcohol intake. We and others have been using novel portable diagnostic approaches to optimize accuracy of home-based OSA testing, and to determine mechanisms underlying apnea: for instance, to derive surrogate measures of arousal threshold and loop gain (both properties of ventilatory control).
The future of OSA therapy is likely predicated on identifying mechanisms underlying apnea in an individualized manner [5, 58]. Considerable evidence suggests that OSA is a highly heterogeneous disease state, and that likewise the mechanisms underlying OSA are variable. Therefore, OSA may also be a disease amenable to personalized therapy [59, 60]. Some patients with OSA have primarily anatomical abnormalities and may respond to upper airway surgical procedures [61]. For patients who have primarily upper airway muscle dysfunction, hypoglossal nerve stimulation or muscle training exercises may be useful [62]. In cases with unstable ventilatory control (high loop gain), strategies to stabilize breathing, such as oxygen or acetazolamide, may be effective [63, 64]. Finally, in patients with a low arousal threshold (a propensity to wake up easily), some data support the use of sedative/hypnotics to raise the arousal threshold and thus stabilize breathing [65, 66]. Many patients have multiple underlying pathophysiological abnormalities; such individuals could require combinations of therapies to eliminate OSA [67]. Thus, an improved understanding of the pathophysiology of OSA may allow for an individualized approach to therapy.
Although CPAP is sometimes regarded as difficult to tolerate, we believe that many patients can achieve excellent results using this approach. Adherence to CPAP therapy is comparable to that of other chronic medical therapies, such as inhaler use in asthma, anticonvulsant use in epilepsy, and optimal glycemic control in type 2 DM. Room for improvement exists with adherence to medical therapy in general, and CPAP is no exception. Along these lines, a number of approaches can be used to facilitate CPAP compliance. First, intensive support and education have clear benefits to CPAP adherence [68]. Education of the patient as well as the bed partner can increase long term use. Second, subtle adjustments in type of mask, mask fitting, and humidification, can improve patient tolerance. Similarly, medications such as nasal sprays for rhinitis and occasionally short term use of hypnotics can facilitate adherence to PAP therapy [69]. Third, modern technology currently allows physicians to monitor patient CPAP use in their homes, and even permits remote adjustment to pressure settings. Moreover, some devices now give real time patient feedback to adjust the mask, in order to minimize residual apnea and air leaks. Early data suggest that excellent adherence can be achieved using such new at-home technologies [70]. Thus, although CPAP treatment first emerged in the early 1980s, it is nonetheless likely to remain an important component of OSA therapy even as new pathophysiological insights emerge.
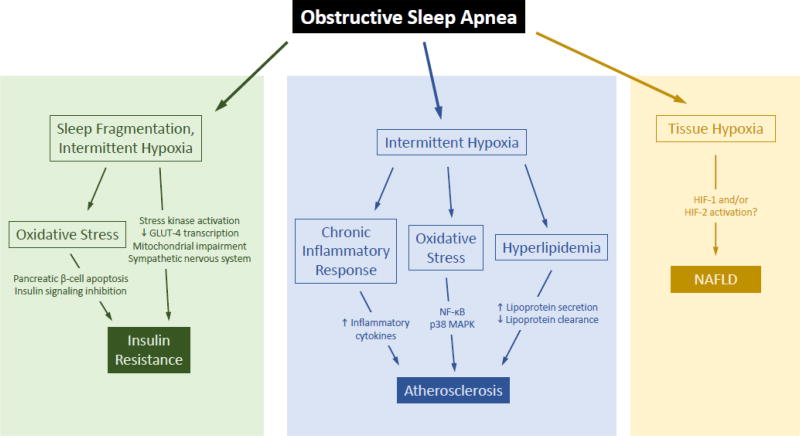
Another therapeutic approach is to minimize apnea consequences by blocking end organ effects of the disease (Figure 1). For example, OSA can lead to varying degrees of hypoxemia, hypercapnia, catecholamine surges with associated sleep fragmentation, oxidative stress, and sympathoexcitation. Some have suggested that anti-oxidant therapy may be a means to minimize downstream effects of sleep apnea [71], though at present, clinical trials on the use of antioxidants have yielded inconsistent results [72]. Similarly, catecholamines are counter-regulatory hormones which can cause hyperglycemia and associated consequences, and may be a target. Exposure to IH in experimental animal models of OSA recapitulates many of the metabolic effects of OSA seen in humans [73–76]. In such models, adrenal medullectomy improves IH-induced glucose intolerance [77]. Similarly, Jun et al. showed that IH resulted in fasting hyperglycemia, glucose intolerance, and insulin resistance, and that either adrenal medullectomy or administration of phentolamine, an α-adrenergic antagonist, reversed most of these changes [78]. Thus, blocking sympathetic output may be a feasible approach to attenuating various metabolic consequences of OSA.
Figure 1. Potential mechanisms by which obstructive sleep apnea may lead to adverse metabolic outcomes.
OSA causes sleep fragmentation and intermittent hypoxia, each of which has been linked to insulin resistance via sympathetic nervous system over-activation, mitochondrial dysfunction, and reduced muscle expression of GLUT-4. Oxidative stress may result in pancreatic β-cell apoptosis and inhibition of insulin signaling pathways. In experimental models of OSA, intermittent hypoxia causes hyperlipidemia and chronic inflammation, each of which may promote atherosclerosis. The mechanisms by which OSA worsens nonalcoholic fatty liver disease are less well developed, but may stem from tissue hypoxia and HIF-1 or HIF-2 activation.
Conclusions
In summary, the prevalence and consequences of sleep disordered breathing, specifically OSA, have been largely under-appreciated in patients with metabolic disease. We believe further mechanistic research, and a more complete understanding of OSA pathogenesis, may define therapy in the future, and lead to a more personalized approach to treatment. In addition, studying mechanisms underlying OSA consequences will likely result in new therapeutic strategies to minimize the impact of apnea, should it occur. Until such targeted therapies are available, the diagnosis and treatment of OSA should be pursued using existing modalities. Excellent clinical outcomes can be achieved in most patients with good adherence using modern technology in a supportive environment.
Acknowledgments
Funding sources
Dr. Malhotra is PI on NIH R01 HL085188, K24 HL132105, T32 HL134632 and co-investigator on R21 HL121794, R01 HL 119201, R01 HL081823. As an Officer of the American Thoracic Society, Dr. Malhotra has relinquished all outside personal income since 2012. ResMed, Inc. provided a philanthropic donation to UC San Diego in support of a sleep center. Dr. Mesarwi is supported by an award from the American Sleep Medicine Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no financial conflicts of interest to disclose with respect to authorship of this manuscript.
References
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–8. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 4.Weaver TE, Mancini C, Maislin G, Cater J, Staley B, Landis JR, et al. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial. Am J Respir Crit Care Med. 2012;186:677–83. doi: 10.1164/rccm.201202-0200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malhotra A. Hypoglossal-nerve stimulation for obstructive sleep apnea. N Engl J Med. 2014;370:170–1. doi: 10.1056/NEJMe1314084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayas NT, Owens RL, Kheirandish-Gozal L. Update in Sleep Medicine 2014. Am J Respir Crit Care Med. 2015;192:415–20. doi: 10.1164/rccm.201503-0647UP. [DOI] [PubMed] [Google Scholar]
- 7.Punjabi NM, Beamer BA. Alterations in Glucose Disposal in Sleep-disordered Breathing. Am J Respir Crit Care Med. 2009;179:235–40. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pamidi S, Tasali E. Continuous Positive Airway Pressure for Improving Glycemic Control in Type 2 Diabetes: Where Do We Stand? Am J Respir Crit Care Med. 2016;194:397–400. doi: 10.1164/rccm.201604-0698ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pamidi S, Wroblewski K, Stepien M, Sharif-Sidi K, Kilkus J, Whitmore H, et al. Eight Hours of Nightly Continuous Positive Airway Pressure Treatment of Obstructive Sleep Apnea Improves Glucose Metabolism in Patients with Prediabetes. A Randomized Controlled Trial. Am J Respir Crit Care Med. 2015;192:96–105. doi: 10.1164/rccm.201408-1564OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kendzerska T, Leung RS, Gershon AS, Tomlinson G, Ayas N. The Interaction of Obesity and Nocturnal Hypoxemia on Cardiovascular Consequences in Adults with Suspected Obstructive Sleep Apnea. A Historical Observational Study. Ann Am Thorac Soc. 2016;13:2234–41. doi: 10.1513/AnnalsATS.201604-263OC. [DOI] [PubMed] [Google Scholar]
- 11.McTigue K, Larson JC, Valoski A, Burke G, Kotchen J, Lewis CE, et al. Mortality and cardiac and vascular outcomes in extremely obese women. JAMA. 2006;296:79–86. doi: 10.1001/jama.296.1.79. [DOI] [PubMed] [Google Scholar]
- 12.Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB, et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32:1017–9. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pallayova M. Diabetes, sleep apnea, and glucose control. Am J Respir Crit Care Med. 2010;182:287. doi: 10.1164/ajrccm.182.2.287. [DOI] [PubMed] [Google Scholar]
- 14.Chami HA, Gottlieb DJ, Redline S, Punjabi NM. Association between Glucose Metabolism and Sleep-disordered Breathing during REM Sleep. Am J Respir Crit Care Med. 2015;192:1118–26. doi: 10.1164/rccm.201501-0046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakker JP, Weng J, Wang R, Redline S, Punjabi NM, Patel SR. Associations between Obstructive Sleep Apnea, Sleep Duration, and Abnormal Fasting Glucose. The Multi-Ethnic Study of Atherosclerosis. Am J Respir Crit Care Med. 2015;192:745–53. doi: 10.1164/rccm.201502-0366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altaf QA, Dodson P, Ali A, Raymond NT, Wharton H, Fellows H, et al. Obstructive Sleep Apnoea and Retinopathy in Patients with Type 2 Diabetes: A Longitudinal Study. Am J Respir Crit Care Med. 2017 doi: 10.1164/rccm.201701-0175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altaf QA, Ali A, Piya MK, Raymond NT, Tahrani AA. The relationship between obstructive sleep apnea and intra-epidermal nerve fiber density, PARP activation and foot ulceration in patients with type 2 diabetes. J Diabetes Complications. 2016;30:1315–20. doi: 10.1016/j.jdiacomp.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 18.Tahrani AA, Ali A, Raymond NT, Begum S, Dubb K, Altaf QA, et al. Obstructive sleep apnea and diabetic nephropathy: a cohort study. Diabetes Care. 2013;36:3718–25. doi: 10.2337/dc13-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saboisky JP, Butler JE, Luu BL, Gandevia SC. Neurogenic changes in the upper airway of obstructive sleep apnoea. Curr Neurol Neurosci Rep. 2015;15:12. doi: 10.1007/s11910-015-0537-1. [DOI] [PubMed] [Google Scholar]
- 20.Foster GD, Borradaile KE, Sanders MH, Millman R, Zammit G, Newman AB, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169:1619–26. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yim-Yeh S, Rahangdale S, Nguyen AT, Jordan AS, Novack V, Veves A, et al. Obstructive sleep apnea and aging effects on macrovascular and microcirculatory function. Sleep. 2010;33:1177–83. doi: 10.1093/sleep/33.9.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yim-Yeh S, Rahangdale S, Nguyen AT, Stevenson KE, Novack V, Veves A, et al. Vascular dysfunction in obstructive sleep apnea and type 2 diabetes mellitus. Obesity (Silver Spring) 2011;19:17–22. doi: 10.1038/oby.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doupis J, Rahangdale S, Gnardellis C, Pena SE, Malhotra A, Veves A. Effects of diabetes and obesity on vascular reactivity, inflammatory cytokines, and growth factors. Obesity (Silver Spring) 2011;19:729–35. doi: 10.1038/oby.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohli P, Sarmiento K, Malhotra A. Update in sleep medicine 2012. Am J Respir Crit Care Med. 2013;187:1056–60. doi: 10.1164/rccm.201302-0315UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–45. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 26.Valencia-Flores M, Orea A, Castano VA, Resendiz M, Rosales M, Rebollar V, et al. Prevalence of sleep apnea and electrocardiographic disturbances in morbidly obese patients. Obes Res. 2000;8:262–9. doi: 10.1038/oby.2000.31. [DOI] [PubMed] [Google Scholar]
- 27.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 28.Smith PL, Gold AR, Meyers DA, Haponik EF, Bleecker ER. Weight loss in mildly to moderately obese patients with obstructive sleep apnea. Ann Intern Med. 1985;103:850–5. doi: 10.7326/0003-4819-103-6-850. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz AR, Gold AR, Schubert N, Stryzak A, Wise RA, Permutt S, et al. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1991;144:494–8. doi: 10.1164/ajrccm/144.3_Pt_1.494. [DOI] [PubMed] [Google Scholar]
- 30.Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535–42. doi: 10.1016/j.amjmed.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 31.Sampol G, Munoz X, Sagales MT, Marti S, Roca A, Dolors de la Calzada M, et al. Long-term efficacy of dietary weight loss in sleep apnoea/hypopnoea syndrome. Eur Respir J. 1998;12:1156–9. doi: 10.1183/09031936.98.12051156. [DOI] [PubMed] [Google Scholar]
- 32.Chirinos JA, Gurubhagavatula I, Teff K, Rader DJ, Wadden TA, Townsend R, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370:2265–75. doi: 10.1056/NEJMoa1306187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue J, Zhou D, Poulsen O, Imamura T, Hsiao YH, Smith TH, et al. Intermittent Hypoxia and Hypercapnia Accelerate Atherosclerosis, Partially via Trimethylamine-Oxide. Am J Respir Cell Mol Biol. 2017 doi: 10.1165/rcmb.2017-0086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li K, Wei P, Qin Y, Wei Y. MicroRNA expression profiling and bioinformatics analysis of dysregulated microRNAs in obstructive sleep apnea patients. Medicine (Baltimore) 2017;96:e7917. doi: 10.1097/MD.0000000000007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McEvoy RD, Michael MZ. Measuring Blood microRNAs to Provide Personalized Advice to Sleep Apnea Patients With Resistant Hypertension: Dreaming the Future. J Am Coll Cardiol. 2015;66:1033–5. doi: 10.1016/j.jacc.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Loube DI, Loube AA, Erman MK. Continuous positive airway pressure treatment results in weight less in obese and overweight patients with obstructive sleep apnea. J Am Diet Assoc. 1997;97:896–7. doi: 10.1016/s0002-8223(97)00220-4. [DOI] [PubMed] [Google Scholar]
- 37.Redenius R, Murphy C, O'Neill E, Al-Hamwi M, Zallek SN. Does CPAP lead to change in BMI? J Clin Sleep Med. 2008;4:205–9. [PMC free article] [PubMed] [Google Scholar]
- 38.Drager LF, Brunoni AR, Jenner R, Lorenzi-Filho G, Bensenor IM, Lotufo PA. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258–64. doi: 10.1136/thoraxjnl-2014-205361. [DOI] [PubMed] [Google Scholar]
- 39.Aron-Wisnewsky J, Minville C, Tordjman J, Levy P, Bouillot JL, Basdevant A, et al. Chronic intermittent hypoxia is a major trigger for non-alcoholic fatty liver disease in morbid obese. J Hepatol. 2012;56:225–33. doi: 10.1016/j.jhep.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 40.Tanne F, Gagnadoux F, Chazouilleres O, Fleury B, Wendum D, Lasnier E, et al. Chronic liver injury during obstructive sleep apnea. Hepatology. 2005;41:1290–6. doi: 10.1002/hep.20725. [DOI] [PubMed] [Google Scholar]
- 41.Kallwitz ER, Herdegen J, Madura J, Jakate S, Cotler SJ. Liver enzymes and histology in obese patients with obstructive sleep apnea. J Clin Gastroenterol. 2007;41:918–21. doi: 10.1097/01.mcg.0000225692.62121.55. [DOI] [PubMed] [Google Scholar]
- 42.Polotsky VY, Patil SP, Savransky V, Laffan A, Fonti S, Frame LA, et al. Obstructive sleep apnea, insulin resistance, and steatohepatitis in severe obesity. Am J Respir Crit Care Med. 2009;179:228–34. doi: 10.1164/rccm.200804-608OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benotti P, Wood GC, Argyropoulos G, Pack A, Keenan BT, Gao X, et al. The impact of obstructive sleep apnea on nonalcoholic fatty liver disease in patients with severe obesity. Obesity (Silver Spring) 2016;24:871–7. doi: 10.1002/oby.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mesarwi OA, Sharma EV, Jun JC, Polotsky VY. Metabolic dysfunction in obstructive sleep apnea: A critical examination of underlying mechanisms. Sleep Biol Rhythms. 2015;13:2–17. doi: 10.1111/sbr.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mesarwi OA, Shin MK, Bevans-Fonti S, Schlesinger C, Shaw J, Polotsky VY. Hepatocyte Hypoxia Inducible Factor-1 Mediates the Development of Liver Fibrosis in a Mouse Model of Nonalcoholic Fatty Liver Disease. PLoS One. 2016;11:e0168572. doi: 10.1371/journal.pone.0168572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corey KE, Misdraji J, Gelrud L, King LY, Zheng H, Malhotra A, et al. Obstructive Sleep Apnea Is Associated with Nonalcoholic Steatohepatitis and Advanced Liver Histology. Dig Dis Sci. 2015;60:2523–8. doi: 10.1007/s10620-015-3650-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hang LW, Chen CF, Wang CB, Wu TN, Liang WM, Chou TC. The association between continuous positive airway pressure therapy and liver disease development in obstructive sleep apnea/hypopnea syndrome patients: a nationwide population-based cohort study in Taiwan. Sleep Breath. 2016 doi: 10.1007/s11325-016-1439-4. [DOI] [PubMed] [Google Scholar]
- 48.Trzepizur W, Boursier J, Mansour Y, Le Vaillant M, Chollet S, Pigeanne T, et al. Association Between Severity of Obstructive Sleep Apnea and Blood Markers of Liver Injury. Clin Gastroenterol Hepatol. 2016;14:1657–61. doi: 10.1016/j.cgh.2016.04.037. [DOI] [PubMed] [Google Scholar]
- 49.Copple BL, Bustamante JJ, Welch TP, Kim ND, Moon JO. Hypoxia-inducible factor-dependent production of profibrotic mediators by hypoxic hepatocytes. Liver Int. 2009;29:1010–21. doi: 10.1111/j.1478-3231.2009.02015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roth KJ, Copple BL. Role of Hypoxia-Inducible Factors in the Development of Liver Fibrosis. Cell Mol Gastroenterol Hepatol. 2015;1:589–97. doi: 10.1016/j.jcmgh.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mesarwi OA, Shin MK, Drager LF, Bevans-Fonti S, Jun JC, Putcha N, et al. Lysyl Oxidase as a Serum Biomarker of Liver Fibrosis in Patients with Severe Obesity and Obstructive Sleep Apnea. Sleep. 2015;38:1583–91. doi: 10.5665/sleep.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma L, Zhang J, Liu Y. Roles and Mechanisms of Obstructive Sleep Apnea-Hypopnea Syndrome and Chronic Intermittent Hypoxia in Atherosclerosis: Evidence and Prospective. Oxid Med Cell Longev. 2016;2016:8215082. doi: 10.1155/2016/8215082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drager LF, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: an emerging risk factor for atherosclerosis. Chest. 2011;140:534–42. doi: 10.1378/chest.10-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–51. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jelic S, Lederer DJ, Adams T, Padeletti M, Colombo PC, Factor PH, et al. Vascular inflammation in obesity and sleep apnea. Circulation. 2010;121:1014–21. doi: 10.1161/CIRCULATIONAHA.109.900357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:613–8. doi: 10.1164/rccm.200503-340OC. [DOI] [PubMed] [Google Scholar]
- 57.Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, et al. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med. 2007;175:1290–7. doi: 10.1164/rccm.200612-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383:736–47. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jen R, Grandner MA, Malhotra A. Future of Sleep-Disordered Breathing Therapy Using a Mechanistic Approach. Can J Cardiol. 2015;31:880–8. doi: 10.1016/j.cjca.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pack AI. Application of Personalized, Predictive, Preventative, and Participatory (P4) Medicine to Obstructive Sleep Apnea. A Roadmap for Improving Care? Ann Am Thorac Soc. 2016;13:1456–67. doi: 10.1513/AnnalsATS.201604-235PS. [DOI] [PubMed] [Google Scholar]
- 61.Schwab RJ, Kim C, Bagchi S, Keenan BT, Comyn FL, Wang S, et al. Understanding the anatomic basis for obstructive sleep apnea syndrome in adolescents. Am J Respir Crit Care Med. 2015;191:1295–309. doi: 10.1164/rccm.201501-0169OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sands SA, Eckert DJ, Jordan AS, Edwards BA, Owens RL, Butler JP, et al. Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea. Am J Respir Crit Care Med. 2014;190:930–7. doi: 10.1164/rccm.201404-0783OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edwards BA, Sands SA, Eckert DJ, White DP, Butler JP, Owens RL, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol. 2012;590:1199–211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sands SA, Mebrate Y, Edwards BA, Nemati S, Manisty CH, Desai AS, et al. Resonance as the Mechanism of Daytime Periodic Breathing in Patients with Heart Failure. Am J Respir Crit Care Med. 2017;195:237–46. doi: 10.1164/rccm.201604-0761OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smales ET, Edwards BA, Deyoung PN, McSharry DG, Wellman A, Velasquez A, et al. Trazodone Effects on Obstructive Sleep Apnea and Non-REM Arousal Threshold. Ann Am Thorac Soc. 2015;12:758–64. doi: 10.1513/AnnalsATS.201408-399OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heinzer RC, White DP, Jordan AS, Lo YL, Dover L, Stevenson K, et al. Trazodone increases arousal threshold in obstructive sleep apnoea. Eur Respir J. 2008;31:1308–12. doi: 10.1183/09031936.00067607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Edwards BA, Sands SA, Owens RL, Eckert DJ, Landry S, White DP, et al. The Combination of Supplemental Oxygen and a Hypnotic Markedly Improves Obstructive Sleep Apnea in Patients with a Mild to Moderate Upper Airway Collapsibility. Sleep. 2016;39:1973–83. doi: 10.5665/sleep.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoy CJ, Vennelle M, Kingshott RN, Engleman HM, Douglas NJ. Can intensive support improve continuous positive airway pressure use in patients with the sleep apnea/hypopnea syndrome? Am J Respir Crit Care Med. 1999;159:1096–100. doi: 10.1164/ajrccm.159.4.9808008. [DOI] [PubMed] [Google Scholar]
- 69.Lettieri CJ, Shah AA, Holley AB, Kelly WF, Chang AS, Roop SA, et al. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Ann Intern Med. 2009;151:696–702. doi: 10.7326/0003-4819-151-10-200911170-00006. [DOI] [PubMed] [Google Scholar]
- 70.Parsons EC, Mattox EA, Beste LA, Au DH, Young BA, Chang MF, et al. Development of a Sleep Telementorship Program for Rural Department of Veterans Affairs Primary Care Providers: Sleep Veterans Affairs Extension for Community Healthcare Outcomes. Ann Am Thorac Soc. 2017;14:267–74. doi: 10.1513/AnnalsATS.201605-361BC. [DOI] [PubMed] [Google Scholar]
- 71.Nanduri J, Vaddi DR, Khan SA, Wang N, Makerenko V, Prabhakar NR. Xanthine oxidase mediates hypoxia-inducible factor-2alpha degradation by intermittent hypoxia. PLoS One. 2013;8:e75838. doi: 10.1371/journal.pone.0075838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.El Solh AA, Saliba R, Bosinski T, Grant BJ, Berbary E, Miller N. Allopurinol improves endothelial function in sleep apnoea: a randomised controlled study. Eur Respir J. 2006;27:997–1002. doi: 10.1183/09031936.06.00101005. [DOI] [PubMed] [Google Scholar]
- 73.Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, et al. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol. 2003;552:253–64. doi: 10.1113/jphysiol.2003.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O'Doherty RM, et al. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med. 2007;175:851–7. doi: 10.1164/rccm.200610-1527OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O'Donnell CP. Metabolic consequences of intermittent hypoxia. Adv Exp Med Biol. 2007;618:41–9. doi: 10.1007/978-0-387-75434-5_4. [DOI] [PubMed] [Google Scholar]
- 76.Drager LF, Li J, Reinke C, Bevans-Fonti S, Jun JC, Polotsky VY. Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity. Obesity (Silver Spring) 2011;19:2167–74. doi: 10.1038/oby.2011.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shin MK, Han W, Bevans-Fonti S, Jun JC, Punjabi NM, Polotsky VY. The effect of adrenal medullectomy on metabolic responses to chronic intermittent hypoxia. Respir Physiol Neurobiol. 2014;203:60–7. doi: 10.1016/j.resp.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jun JC, Shin MK, Devera R, Yao Q, Mesarwi O, Bevans-Fonti S, et al. Intermittent hypoxia-induced glucose intolerance is abolished by alpha-adrenergic blockade or adrenal medullectomy. Am J Physiol Endocrinol Metab. 2014;307:E1073–83. doi: 10.1152/ajpendo.00373.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]