Abstract
Objective
To synthesize research comparing post-stroke health outcomes between patients rehabilitated in skilled nursing facilities (SNFs) and inpatient rehabilitation facilities (IRFs). Secondly, to evaluate relationships between facility characteristics and outcomes.
Data Sources
PubMed and CINAHL searches spanned January 1, 1998 to October 6, 2016 and encompassed MeSH and free-text keywords for stroke, IRF/SNF, and study outcomes. Human and English limits were used.
Study Selection
Observational and experimental studies examining outcomes of adult stroke patients rehabilitated in an IRF or SNF were eligible. Studies had to provide site of care comparisons and/or analyses incorporating facility-level characteristics and had to report ≥1 primary outcome (discharge setting, functional status, readmission, quality of life, all-cause mortality). Unpublished, single-center, descriptive, and non-US studies were excluded. Articles were reviewed by one author and when uncertain, discussion with study coauthors achieved consensus. Fourteen (0.3%) titles were included.
Data Extraction
The types of data, time period, size, design, and primary outcomes were extracted. We also extracted two secondary outcomes (length of IRF/SNF stay, cost) when reported by included studies. Effect measures, modeling approaches, methods for confounding adjustment, and potential confounders were extracted. Data were abstracted by one author and the accuracy verified by a second reviewer.
Data Synthesis
Two studies evaluating community discharge, one study evaluating predicted readmission probability, and 3 studies evaluating all-cause mortality favored IRFs over SNFs. Functional status comparisons were inconsistent. No studies evaluated quality of life. Two studies confirmed increased costs in the IRF versus SNF setting. Although substantial facility variation was described, few studies characterized sources of variation.
Conclusions
The few studies comparing post-stroke outcomes indicated better outcomes (with greater costs) for patients in IRFs versus SNFs. Contemporary research on the role of the post-acute care setting and its attributes in determining health outcomes should be prioritized to inform reimbursement system reform.
Keywords: skilled nursing facilities, subacute care, stroke rehabilitation, outcome assessment (health care)
Introduction
Post-acute care costs comprise 10% of the Medicare budget.1 Among all hospital discharges in the United States in 2013, ~8 million were to post-acute care of which 7% were to inpatient rehabilitation facilities (IRFs), 40% to skilled nursing facilities (SNFs), 50% to home with the use of home health agencies (HHAs), and 2% to long-term care hospitals (LTCHs).2 IRFs, SNFs, and LTCHs are characterized by 24-hour nursing and varying intensity of physical, occupational, and speech therapy services.3,4 Clinical characteristics are similar for a large proportion of patients rehabilitated in SNFs and IRFs, and in markets with no IRFs, SNFs often substitute for IRFs.4,5 On average, IRFs are costlier owing to specific regulatory requirements affecting staffing and therapy provision.6 These requirements (e.g., a reasonable expectation for participation in and benefit from an intensive rehabilitation program) constitute selection forces which affect the choice of a post-acute care provider.
In the face of an aging population,7 post-acute care organization and delivery remains a legislative priority. To combat rising post-acute care costs, Medicare introduced a prospective payment system (PPS) for SNFs in 1998 and for IRFs in 2002.8 SNF PPS was structured to reimburse for the quantity of therapy services provided,9 while IRFs remained constrained by regulatory requirements for staffing and service provision.10 The Improving Medicare Post-Acute Care Transformation Act of 2014 (IMPACT) mandated the Secretary of the Department of Health and Human Services evaluate the feasibility of shifting from the current system with separate PPS reimbursement for LTCH, IRF, SNF, and HHA settings to a unified system.11 The overriding objective of this shift in payment is to base reimbursement on the medical complexity and the therapy needs of the patient, while mitigating longstanding incentives to provide excess therapy based in part on site of care distinctions.6
The Medicare Payment Advisory Commission (MedPAC) has recommended near-term implementation of a site-neutral payment policy for IRFs and SNFs.12 Despite extensive overlap in the populations served by SNFs and IRFs,4 minimal evidence is available to distinguish which settings of care should be preferred for an individual patient. Beyond across setting comparisons, the role of within setting variation in the type and quality of services provided to patients represents an understudied consideration for payment reform.
Developing a thorough understanding of the comparative outcomes within and between settings of post-acute care is important in the rapidly evolving policy climate. Therefore, the objectives of this systematic review were twofold. First, we sought to characterize the relationship between site of rehabilitation (IRF versus SNF) and health outcomes (i.e., discharge site, functional status, readmission, quality of life, survival, cost, length of stay). Second, we sought to evaluate the association between facility-level factors and these rehabilitation and policy relevant outcomes. Considering the extensive heterogeneity of patients’ post-acute care needs across clinical conditions, we chose to focus on stroke. Stroke is a leading contributor to the demand for post-acute care in the United States, as 250,000 all-payer discharges occur annually, with similar proportions discharged to the IRF and SNF settings.2 Since a 2009 Cochrane Review of the effects of site of care on the rehabilitation outcomes of older adults did not find any clinical trials, controlled before-and-after studies, or interrupted time-series eligible for inclusion,13 our search was expanded to include all observational study designs.
Methods
Search Strategy
We aimed to identify all relevant articles published since the SNF PPS was implemented in July, 1998. A domain-based search strategy encompassed the rehabilitation setting (e.g., IRF or SNF), disease state (stroke), and outcomes of interest. All authors provided input to develop the search term strategy. The search string was structured to include MeSH terms and free text keywords for PubMed, and a parallel search was adapted to include Major Headings and free text keywords for CINAHL. Supplemental Figure 1 details the search strategy. Searches were restricted to peer-reviewed research in humans published in English between January 1, 1998 and October 6th, 2016.
Inclusion and Exclusion Criteria
We tracked the eligibility and inclusion of articles using the guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.14 Articles were reviewed by one of the authors (MA). The eligibility of abstracts and full-text articles was determined in accordance with a review protocol that pre-specified inclusion and exclusion criteria. In the presence of uncertainty regarding an article’s eligibility, discussion with study coauthors (KL, CU) achieved consensus for all manuscripts.
Observational and experimental studies examining health outcomes of adult (age ≥18 years) stroke patients (ischemic or hemorrhagic) rehabilitated in either an IRF or SNF were eligible for inclusion. In pursuit of the dual objectives of comparing between care settings (IRF vs. SNF) and characterizing facility-level factors driving variation within and between these settings, eligible studies had to provide site of care comparisons and/or analyses incorporating facility-level characteristics of the IRF or SNF. Because patients who have suffered from a stroke may value regaining physical function and quality of life more than prolonging life, we broadened our search considerations to include health outcomes that may better capture these types of outcomes. Eligible studies had to report ≥1 of the following health outcomes: 1) Site of care after discharge from the post-acute care setting; 2) Change in physical function from baseline to a later time point during the post-acute stay or to a point after discharge from the post-acute care setting; 3) All-cause or cause-specific hospital readmission during or following the post-acute care stay; 4) Change in quality of life as measured quantitatively by one or more validated scales during or following the post-acute care stay; and 5) All-cause mortality measured during the post-acute stay or to a point after discharge from the post-acute care setting. Single-center studies, case studies/series (n<30), purely descriptive studies, unpublished research, research in non-United States populations, and studies conducted outside of the IRF and/or SNF settings were excluded.
For eligible studies, we manually reviewed the references for additional articles not identified by the database searches. No additional articles were identified from this process.
Data Abstraction
Information was extracted to describe the types of data used, how recent the data were, size of study, and study design (e.g., clinical trial, cross-sectional, cohort). Many studies used multiple linked data sources. For this reason, we listed all of the data sources used in the study and dates of data. To characterize the study size, we extracted the number of facilities and the number of patients included by site of care. Several data elements were extracted to characterize the patient population. We decided to report mean age with standard deviation in years and pre-stroke site of residence as we believed these factors would be widely available and most important to understanding the potential for confounding in research comparing IRFs to SNFs. Older patients experience worse outcomes after a stroke.15,16 Also, it may be unrealistic to believe that persons residing in a nursing home before suffering from stroke would return to the community. For each study, we extracted the specific health outcomes evaluated and the time frame during which the health outcomes were measured. While some studies reported a specific time interval for measurement (e.g., 120 days post admission), others simply reported the length of follow-up as “at the end of the post-acute stay” or “during the post-acute stay”. We also extracted two secondary outcomes: length of post-acute care stay and cost. These were included only when reported by studies which met inclusion criteria and reported a primary outcome.
To develop a list of facility-level factors to extract, we sought guidance from the general nursing home literature linking facility characteristics to quality outcomes for all nursing home residents. Disparities in resident characteristics and financial performance between nursing facilities have been linked to differences in quality measure performance.17 Facility characteristics (e.g., size, ownership, payer distribution, quality) have been found to be associated with community discharge and acute hospital readmission from nursing facilities.18,19 Originally, we sought to focus our extraction on these types of factors. However, given the few studies available evaluating the role of facility-level factors in outcomes for post-stroke patients, we decided not to restrict our extraction of this facility-level information.
Lastly, because there are different approaches to analyzing data (including choice of effect measure, adjustment variables, and modeling techniques), we extracted key information about the statistical analysis for each study. First, we extracted the type of effect measure (e.g., continuous variable measured by change in function measures, binary variable indicating death within 90 days). Second, we extracted information regarding the analytic approach (e.g., Cox model, linear model, logistic model). Third, we extracted the technique used to adjust for confounding (e.g., covariate adjustment in multivariable model, instrumental variable). Fourth, we extracted which variables were considered as confounders. We then summarized the results of the study findings. We documented the crude values of measures to facilitate comparison across studies. We also extracted a summary of adjusted findings derived from the study. Often studies presented a variety of comprehensive analyses. In these cases, we selected a few summary estimates to give a sense of the range of estimates of effect from the study, rather than to re-iterate all of the detailed estimates of effect. We did so because our end goal was to provide a synthesis of findings rather than to conduct a meta-analysis. Lastly, when standard errors were provided by the original article, we estimated 95% confidence intervals to provide consistency in reporting across the included studies.
Articles fulfilling all criteria for inclusion were initially abstracted by one author (MA) and the accuracy of abstracted data was subsequently authenticated by a second reviewer (KL). Any discrepancies detected by the second review were discussed between the authors and resolved.
Results
Search Results
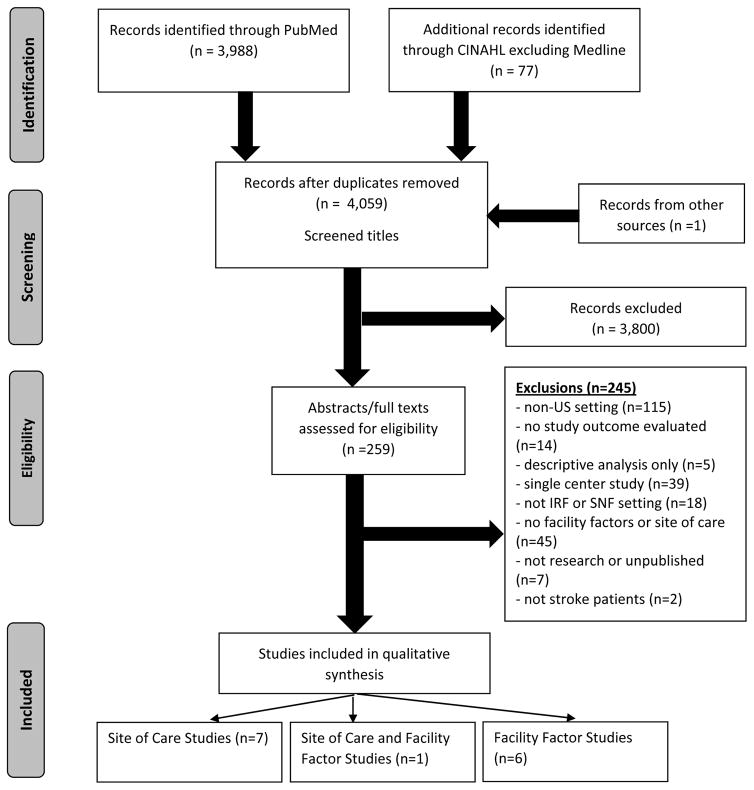
The PubMed and CINAHL searches yielded a total of 4,059 titles after duplicate removal. Upon elimination of 3,800 titles based upon title review, 259 abstracts and 32 full texts were reviewed for eligibility. Application of eligibility criteria yielded 14 full-text articles fulfilling inclusion and exclusion requirements (Figure 1), of which 8 studies compared health outcomes between patients rehabilitated in SNFs and IRFs,20–27 one of which also assessed facility characteristics,23 and 6 studies only evaluated relationships between facility characteristics and health outcomes.28–33 The most common primary reason for exclusion was for research conducted in non-US settings (n=115; 47.1%).
Figure 1.
PRISMA Flow Diagram for Selection of Eligible Studies of Skilled Nursing Facility and Inpatient Rehabilitation Hospital for Post-Stroke Care
Characteristics of Site of Care Studies
Table 1 shows the key characteristics of studies comparing outcomes between sites of care. The years of data analyzed across the 8 studies spanned 1995 to 2010, with 4 studies conducted entirely prior to the implementation of IRF and SNF PPS, 3 with intervals overlapping PPS implementation, and 1 after PPS implementation. Four studies examined populations of Medicare beneficiaries.20, 22, 24–25 Two studies used a prospective cohort design,24 one was cross-sectional,23 and the remaining studies were retrospective cohort studies.20–22, 25–26 No experimental studies were found.
Table 1.
Characteristics of Studies Comparing Outcomes Between SNF and IRF Rehabilitated Patients Post-Stroke
| Author (Year) | Data Source (Study Period) | Site(s) of Rehabilitation: # Facilities # Patients with stroke |
Mean Age in Years (SD) | Mean Baseline Function (SD) | Study Design Follow-up Duration |
Primary and Secondary Study Outcome(s) |
|---|---|---|---|---|---|---|
|
| ||||||
| Primary Exposure of Interest: Site of Care | ||||||
|
| ||||||
| Kane et al. (2000) | Medicare automated data retrieval system; patient surveys; medical record abstraction (Not recorded but before July 1997) |
SNF: # facilities not recorded; 123 patients |
SNF: 81.1 (7.3) |
SNF: Pre-stroke ADL Score (0–100): 84 |
Prospective cohort Surveys at 6 weeks, 6 months, 1 year |
Functional gain |
|
IRF: # facilities not recorded; 79 patients |
IRF: 75.5 (7.6) |
IRF: Pre-stroke ADL Score (0–100): 92 |
||||
|
| ||||||
| Hoenig et al. (2001) | Mailed survey of Veterans Health Administration Acute Care and Rehabilitation; Extant Veterans Health Administration databases (1995 to 1996) |
SNF*: 49 facilities; 1,582 patients |
SNF*: 68.7 (9.2) |
Not recorded | Cross-sectional Post-acute care duration |
Community discharge Secondary outcome: Length of stay |
|
Rehabilitation unit: 61 facilities; 3,586 patients |
Rehabilitation unit: 67.8 (9.8) |
|||||
|
Geriatric Unit: 31 facilities; 1,431 patients |
Geriatric Unit: 67.5 (9.8) |
|||||
|
| ||||||
| Deutsch et al. (2006) | Uniform Data System for Medical Rehabilitation; Medicare Provider and Analysis Review (1996 to 1997) |
SNF: 239 facilities; 3,810 patients |
SNF: 79.0 (8.4) |
SNF: Motor FIM: 39.7 (16.7) |
Retrospective cohort Post-acute care duration |
Community discharge Functional status Secondary outcome: Part A Medicare payments |
|
IRF: 631 facilities; 54,914 patients |
IRF: 76.0 (8.5) |
IRF: Motor FIM: 41.8 (14.6) |
||||
|
| ||||||
| Buntin et al. (2010) | Medicare Provider and Analysis Review; Minimum Data Set; Area Resource File; Provider of Services (2002 to 2003) |
SNF: # facilities not recorded; 87,844 patients |
SNF: 81.1 (7.4) |
Not recorded | Retrospective cohort 120 days |
All-cause mortality Secondary outcome: Part A Medicare payments |
|
IRF: # facilities not recorded; 68,906 patients |
IRF: 77.7 (7.1) |
|||||
|
| ||||||
| Kind et al. (2010) | Medicare Provider and Analysis Review; Provider of Services (1999–2000) |
SNF: # facilities not recorded; 19,434 patients |
Average age not available by site of care. White: 80 (7.4); Black: 78 (8.0); Hispanic: 79 (7.5) |
Not recorded | Retrospective cohort 30 days |
Readmission Mortality |
|
IRF: # facilities not recorded; 11,849 patients | ||||||
|
| ||||||
| Wang et al. (2011) | Kaiser Permanente California Health System Claims (1996–2005) |
SNF†: # facilities not recorded; 14,628 patients |
SNF†: 75.5 (10.7) |
Not recorded | Retrospective cohort 365 days |
Mortality |
|
IRF†: # facilities not recorded; 2,720 patients |
IRF†: 64.4 (12.5) |
|||||
|
| ||||||
| Chan et al. (2013) | Kaiser Permanente Health Care System Northern California (2008–2010) |
SNF: # facilities not recorded; 29 patients |
SNF: 79 (10.2) |
SNF: AM-PAC Motor: 40 |
Prospective cohort 6 months |
Functional status |
|
IRF: # facilities not recorded; 66 patients |
IRF: 67 (14.0) |
IRF: AM-PAC Motor: 33 |
||||
|
| ||||||
| Primary Exposure of Interest: Therapy Intensity | ||||||
|
| ||||||
| Chen et al. (2002) | Uniform Data System for Medical Rehabilitation (1996 to 1997) |
SNF‡: 10 facilities; ~216 stroke patients |
SNF§: 77.9 (9.9) |
SNF§: Transformed Median Motor FIM (0–100): 21.4 |
Retrospective cohort Post-acute care duration |
Functional gain |
|
Hospital IRF‡: 3 facilities; ~133 stroke patients |
Hospital IRF§: 73.2 (13.2) |
Hospital IRF§: Transformed Median Motor FIM (0–100): 21.4 |
||||
|
Freestanding IRF‡: 7 facilities; ~197 patients |
Freestanding IRF§: 74.6 (13.7) Stroke patients: 75 (11) |
Freestanding IRF§: Transformed Median Motor FIM (0–100): 19.5 |
||||
SNF classification included nursing home, intermediate care, or subacute care ward.
Total SNF and IRF patients summed from PAC subgroup sample sizes provided on Tables 1 and 2. Mean age estimated by taking weighted averages from data provided on Tables 1 and 2.
There were 554 patients with stroke in this study. The number of patients with stroke within each setting estimated from percentages provided on Table 3 total 546 due to rounding.
Estimates provided for all patients in the study rather than just patients with stroke.
Abbreviations: Skilled nursing facility (SNF), inpatient rehabilitation facility (IRF), Patient Assessment Instrument (PAI), functional independence measure (FIM), Activity Measure for Post Acute Care (AM-PAC)
Length of follow-up varied with the maximum length of follow-up being 1 year.26 Three studies defined duration of follow-up as admission to post-acute care until discharge and 5 studies used a set time point (e.g., range: 30 days to 1 year) (Table 1). The total study size varied with the smallest study including 202 patients,24 and the largest including 156,740 patients.20 The number of patients included ranged from 66 to 68,906 for IRFs, and the number of patients included ranged from 29 to 87,844 for SNFs. Among three studies reporting the number of facilities, the number of facilities ranged from 7 to 631 for IRFs and 10 to 239 for SNFs; other studies did not report the number but encompassed the universe of Medicare reimbursed facilities (Table 1). Among the studies reporting age by site of care, the average age of patients rehabilitated in SNFs ranged from 68.7 years to 81.1 years while the average age of patients rehabilitated in IRFs ranged from 62.9 years to 77.7 years (Table 1). Four studies reported baseline functional status by site of care (Table 1). Site of care studies often excluded nursing home residents,20, 22–24 and where reported the pre-stroke residence for most patients was in the community.21 Additional patient characteristics were summarized in Supplemental Table 1.
Characteristics of Facility Variation Studies
Studies assessing facility-level factors were published using data from 1993–2009, with 2 studies conducted entirely after both SNF and IRF PPS implementation, 1 entirely before, and 3 with intervals overlapping the dates of implementation (Table 2). The six studies only included patients rehabilitated in the IRF setting. In these studies, the number of included facilities ranged from 37 to 1,209, with one study not reporting the number of facilities (Table 2). A single study comparing IRFs and SNFs also reported facility-level factors associated with the outcomes under investigation.23
Table 2.
Characteristics of Studies Assessing Facility-Level Factors among Inpatient Rehabilitation Facilities
| Author (Year) | Data Source (Study Period) | # Facilities # Patients |
Mean Age in Years, (SD) | Primary Exposure(s) of Interest | Primary and Secondary Study Outcome(s) |
|---|---|---|---|---|---|
| Reker, O’Donnell, and Hamilton (1998) | Veterans Administration Uniform Data System (1993 to 1996) | 37 facilities 3,576 patients |
67.0 (10) | Facility Case-mix adjustment by individual-level factors |
Functional gain Secondary outcome: Length of stay |
| Strasser et al. (2005) | Veterans Administration Functional Status Outcomes Database; Team member survey (1997 to 1998) |
Inpatient and subacute rehabilitation units: 46 facilities 1,678 patients (530 rehabilitation team members) |
67.4 (11.6) | Rehabilitation team functioning, supervisor expectations, and administrative support | Functional gain Community discharge Secondary outcome: Length of stay |
| Colla et al. (2010) | Medicare Provider and Analysis Review; Provider of Services; Medicare Beneficiary Summary File; Minimum Data Set; Area Resource Files (2002 to 2003) | 911 facilities 47,434 patients |
76.3 (9.1) | Post-acute care competition | Death or institutionalization (180 days) Secondary outcomes: Length of stay Cost |
| Dobrez et al. (2010) | eRehabData (1998 to 2006) | # facilities not recorded 98,151 patients |
68.8 (10.4) | IRF Prospective Payment System payment | Functional gain Community discharge Secondary outcome: Length of stay |
| Graham et al. (2013) | Uniform Data System for Medical Rehabilitation (2006–2008) | 717 facilities 202,423 patients |
71.5 (11.9) | Volume | Functional gain Community discharge |
| Reisetter et al. (2015) | Medicare Provider and Analysis Review; IRF-Patient Assessment Instrument; Provider of Services; Medicare Beneficiary Summary File (2006 to 2009) | 1,209 facilities 145,460 patients |
78.4 (7.2) | Facility Hospital referral region |
Functional gain |
Abbreviations: inpatient rehabilitation facility (IRF), Patient Assessment Instrument (PAI), functional independence measure (FIM)
Across all 14 included studies, 7 studies did not report the site of patient residence prior to the acute hospitalization for stroke.25–27, 29–31, 33
Outcomes: Site of Care Comparisons
Primary Outcomes
Table 3 shows a summary of the studies reporting comparisons between SNFs and IRFs for the primary outcomes of interest. Results are sorted by outcome (i.e., discharge site, functional status, readmission, mortality). No studies evaluated quality of life as an outcome measure. One of the 8 studies reported results separately for freestanding and hospital-based IRFs,21 and another separately reported outcomes for inpatient geriatric and inpatient rehabilitation units.23 In the two studies directly comparing community discharge between IRFs and SNFs, patients rehabilitated in IRFs were more likely to be discharged to the community than those rehabilitated in SNFs.22, 23
Table 3.
Differences in Health Outcomes by Site of Rehabilitation (IRF and SNF) Among Patients with Stroke
| Author (Year) | Analytic Approach | Comparison | Crude Percentages or Means | Measure(s) | Summary of findings |
|---|---|---|---|---|---|
|
| |||||
| Community Discharge | |||||
|
| |||||
| Deutsch et al. (2006) | Multivariable logistic model* | IRF vs. SNF (Reference) | Stratified by disability level: Minimal motor: IRF: 98.6%; SNF: 98.6% |
aOR 95% CI |
Community discharges in IRF more common than in SNF for these patients: |
| Mild motor/mild cognitive: IRF: 96.7%; SNF: 91.7% Minimal motor/significant cognitive: IRF: 90.6%; SNF: 88.3% |
Mild motor disabilities and cognitive ratings: aOR: 2.19; 95% CI: 1.52–3.14 |
||||
| Moderate motor: IRF: 92.3%; SNF: 84.2% |
Moderate motor disabilities: aOR: 1.98; 95% CI: 1.49–2.61 |
||||
| Significant motor: IRF: 85.8%; SNF: 79.3% |
Significant motor disabilities: aOR: 1.26; 95% CI: 1.01–1.57 |
||||
| Severe motor--patients ≥ 82 years: IRF: 54.6%; SNF: 49.4% patients < 82 years: IRF: 66.4%; SNF: 52.0% |
Severe motor disabilities, patients <82 years: aOR: 1.43; 95% CI: 1.25–1.64 |
||||
|
| |||||
| Hoenig et al. (2001) | Multivariable logistic model† | Rehabilitation unit and geriatric unit vs. SNF† (Reference) |
Rehabilitation unit: 75.0% Geriatric unit: 71.8% SNF||: 66.6% |
aOR 95% CI |
Relative to those in SNFs||, patients in rehabilitation units (aOR: 1.91; 95% CI 1.47–2.50) and geriatric units (aOR:1.43; 95% CI 1.03–1.97) had increased odds of being discharged home. |
|
| |||||
| Physical Functioning | |||||
|
| |||||
| Chen et al. (2002) | Multiple linear model‡ | IRF and Acute hospital vs. SNF (Reference) | Average Rasch-transformed Mobility Gain (range 0–100): 17 | Standardized β Coefficient | Patients in SNFs made larger gains in mobility than patients in IRF (−0.20; p<0.05) or patients in acute hospitals (−0.16; p<0.05). |
|
| |||||
| Deutsch et al. (2006) | Multiple linear model* | IRF vs. SNF (Reference) | Discharge FIM motor rating stratified by disability level: Minimal motor: IRF: 86.6; SNF: 85.0 Mild motor/mild cognitive: IRF: 79.2; SNF: 78.3 Minimal motor/significant cognitive: IRF: 77.5; SNF: 77.5 Moderate motor: IRF: 73.1; SNF: 71.1 |
Adjusted β coefficient representing the mean FIM difference (IRF-SNF) 95% CI |
Clinically relevant functional gains (≥2 FIM units) in IRF more common than in SNF for these patients: |
| Significant motor: IRF: 67.1; SNF: 64.9 |
Significant motor disabilities: adjusted β: 2.40; 95% CI: 1.19–2.66 |
||||
| Severe motor-- patients ≥ 82 years: IRF: 46.1; SNF: 40.1 patients < 82 years: IRF: 49.8; SNF: 41.8 |
Severe motor disabilities— patients ≥82 years: adjusted β: 2.39; 95% CI: 1.45–3.32 patients <82 years: adjusted β: 4.24; 95% CI: 3.45–5.03 |
||||
|
| |||||
| Kane et al. (2000) | Multiple linear model§ Instrumental variable analysis |
IRF vs. SNF (Reference) | Average percentage change in the activities of daily living score at six weeks, 6 months, and 12 months. Crude average change values were not provided. IRF: 6 weeks: 23.2% improved 6 months: 13.9% improved 12 months: 7.8% improved SNF: 6 weeks: 0.7% improved 6 months: −5.9% worsened 12 months: −6.7% worsened |
Adjusted mean functional dependency scores Predicted gain in functional improvement in optimal post-acute care setting |
Relative to those in SNFs, patients in IRF settings regained more activities of daily living at six weeks. Despite some rebound loss of activities of daily living between 6 and 12 months, IRF patients fared better than SNF patients (Figure 2 of manuscript). Patients discharged to SNF would have achieved maximum functional improvement had they been discharged to home with health care. Additional gains in function by optimal post-acute care location for patients actually in SNF and IRF settings differed most at 6 weeks (IRF: 3.1%, SNF: 16.9%) and were similar at 6 months (IRF: 15.5%, SNF: 18.3%) and 12 months (IRF:15.9%, SNF: 16.2%) |
|
| |||||
| Chan et al. (2013) | Multiple linear model|| | SNF vs. IRF (reference | AMPAC score at 6 months: IRF: 52 SNF: 43 |
Adjusted β coefficient representing the mean AM-PAC difference (SNF-IRF) 95% CI | Results from two models were reported, one adjusting for hospital readmission and quantity of therapy (adjusted β: −10.1; 95% CI: −15.0 to −5.2), and the other model not adjusting for readmission and quantity of therapy (adjusted β: −6.1; 95% CI: −11.2 to −1.0). |
|
| |||||
| Hospital Readmission | |||||
|
| |||||
| Kind et al. (2010) | Unspecified statistical model with robust variables estimates to account for clustering of patients within hospitals¶ | IRF and SNF | Crude estimates not available by site of care. | Predicted probability of readmission (hospital or emergency department) 95% CI |
Predicted probabilities of readmission less for IRF than SNF in each racial/ethnic group. Blacks: IRF: 20%; 95% CI: 17.9–22.7 SNF: 26%; 95% CI: 24.2–28.6 Hispanics: IRF: 18%; 95% CI: 13.1–22.9 SNF: 28%; 95% CI: 24.0–32.6 Whites: IRF: 18%; 95% CI: 17.3–19.1 SNF: 21%; 95% CI: 20.3–21.9 |
|
| |||||
| All-cause Mortality | |||||
|
| |||||
| Buntin et al. (2010) | Generalized estimating equations (binary logit)# Instrumental variable analysis# |
IRF vs. SNF (Reference) | Mortality within 120 days IRF: 6.2% SNF: 14.7% |
Absolute difference in 120-day mortality 95% CI |
Use of IRF reduced mortality by 2.6 percentage points compared to SNFs. adjusted β: −2.58; 95% CI: 0.96–4.16 |
|
| |||||
| Kind et al. (2010) | Unspecified statistical model with robust variables estimates to account for clustering of patients within hospitals¶ | IRF and SNF | Crude estimates of 30-day mortality not available by site of care. |
Predicted probability of 30-day mortality among those with no readmissions 95% CI |
Predicted probability of death in IRF settings lower than SNF settings in each racial/ethnic group. Blacks: IRF: 2%; 95% CI: 1.6–3.3 SNF: 5%; 95% CI: 4.2–6.1 Hispanics: IRF: 1%; 95% CI: 0–1.5 SNF: 5%; 95% CI: 3.2–6.3 Whites: IRF: 2%; 95% CI: 1.9–2.5 SNF: 8%; 95% CI: 7.2–8.2 |
|
| |||||
| Wang et al. (2011) | Cox proportional hazards multivariable model** | IRF vs. SNF (Reference) | Stratified by the highest level of post-acute care within 14 and 61 days: Post-acute (14 days): IRF: 4.4% SNF: 21.4% Post-acute (61 days): IRF: 4.3% SNF: 16.2% |
Adjusted hazard rate ratio 95% CI |
Patients in IRF settings died at a rate less than half that of those in SNF settings. Post-acute (14 days): Adjusted hazard ratio: 0.33 95% CI 0.24–0.45 Post-acute (61 days): Adjusted hazard ratio: 0.42 95% CI 0.33–0.53 |
Covariates included were time from stroke onset to rehabilitation admission, admission FIM motor rating, admission FIM cognitive rating, age, hemorrhagic versus non-hemorrhagic stroke, presence of left-sided, right-sided, or bilateral paresis, presence of a tiered comorbidity, visual field deficits, living alone, median household income, race (Black, White, Other), sex, geographic region, and site of care (IRF, SNF).
SNF classification included nursing home, intermediate care, or subacute care ward. Covariates included were patient characteristics: age > 70 years, white race, Charlson comorbidity index, intubation, length of stay, second bed section an acute bed, and site of post-acute care (rehabilitation unit, geriatric unit, SNF)
Covariates included were factors measured at admission (self-care, mobility, cognition), age, sex, therapy intensity, length of stay, days since onset, interrupted stays, and site of care (general hospital, IRF, SNF).
Covariates included discharge activities of daily living score, sum of activities of daily living and instrumental activities of daily living prior to hospitalization, patient’s self-expected activities of daily living score at six weeks after hospital discharge, sex, age, race, living arrangement, cognitive status, presence of catheter, patient’s ability to exercise prudent judgement, health status prior to hospitalization, HMO membership, city, patient’s role in discharge decision making, length of hospital stay, hospital’s post-acute care facility ownership, informal support given before hospitalization, social and economic status of caregiver, acuity score at admission, comorbidity, diagnosis related group severity scores, and instability. Instruments used were predicted probabilities of specific discharge site of care.
Covariates included age, body mass index, baseline functional status, inpatient Modified-Rankin score, history of prior stroke, Charlson comorbidity index.
Covariates included age, sex, region, index hospitalization admission year, length of hospital stay, HMO membership, Medicaid indicator variable, comorbidities, measures of stroke severity (mechanical ventilation and presence of gastrostomy tube), neighborhood socioeconomic characteristics including percent over 24 years of age with a college degree and percent below the poverty line, and indicator variables for site of care (home, home with health care, IRF, SNF).
Covariates included patient demographic (age, age squared, sex, interaction of age and sex, race, urban/rural, Medicaid) and clinical characteristics (13 comorbidities and 17 complications that could influence outcome of post-acute care), indicators of type of stroke, hospital facility factors (size, teaching status, ownership, % Medicare, case-mix, and % low income), county-level HMO penetration, and indicator variables for site of care (IRF, SNF, home). Instruments used were patient-specific measures of accessibility and proximity to post-acute care providers.
Covariates included age, age squared, sex, racial/ethnic group, previous stroke, Charlson-Deyo comorbidity index, service area, acute care length of stay, and dummy variables for site of care (outpatient visits, home health care, IRF, SNF).
Abbreviations: skilled nursing facility (SNF), inpatient rehabilitation facility (IRF), adjusted odds ratio (aOR), confidence interval (CI), Functional Impairment Measure (FIM), health maintenance organization (HMO)
The functional outcome findings were informed by 4 studies comparing outcomes between IRF and SNF settings. Larger functional gains were reported in SNFs versus freestanding IRFs and IRFs within general hospitals in one study,21 while greater functional ratings at discharge22 and at 6 months27 were reported among IRF rehabilitated patients relative to SNF patients in two others; predicted functional gains and actual functional gains were larger for IRFs than SNFs in the fourth study.24
The single study reporting predicted readmission probability presented results stratified by race/ethnicity.25 The study found differences in predicted probability of “bounce back” from IRF or SNF (defined as readmission to emergency department or hospital) by racial/ethnic groups among those initially discharged to SNF, but found similar predicted probabilities of readmission for those rehabilitated in IRFs (18% for Whites and Hispanics, 20% for Blacks). Regardless of racial/ethnic group, predicted probabilities of readmission from IRFs were less than predicted probabilities of readmission from the SNFs.25
With respect to all-cause mortality, 3 studies provided comparisons between IRF and SNF settings. In each study, patients discharged to IRFs were consistently estimated to have lower adjusted mortality risk than patients discharged to SNFs (Table 3). The 2.6% lower mortality risk in IRFs versus SNFs estimated using instrumental variable methods was numerically smaller than the 3% (in black patients) to 6% (in white patients) differences in predicted probabilities of mortality estimated in another study and a 4.9% difference estimated in the same study using methods that did not account for unobserved confounders.20, 25
Secondary Outcomes
Table 4 summarizes comparative studies with respect to length of stay and cost. Two studies provided comparisons of length of stay between IRF and SNFs, with contradictory evidence. In a Medicare population, patients cared for in the IRF setting stayed on average 2 days less than in the SNF setting.22 In the Veterans Administration system, shorter lengths of stay were observed in patients rehabilitated in SNFs compared with inpatient geriatric and rehabilitation units.23 The latter study used a SNF definition that included patients cared for in nursing homes, intermediate care settings, or subacute care wards. Two studies reported the secondary outcome cost by site of care. Both reported lower costs for SNFs versus IRFs.20, 22 A $11,261 difference in 120-day post-discharge Medicare payments was reported when the estimate was adjusted for unobserved confounders using instrumental variable methods and a $10,368 difference was reported in the same study when only adjusted for observable characteristics.20 The other study reported consistently lower costs in the SNF setting across strata of baseline cognitive and functional impairment.22
Table 4.
Differences in Length of Stay and Cost by Site of Rehabilitation (IRF and SNF) Among Patients with Stroke
| Author (Year) | Analytic Approach | Comparison | Crude Percentages or Means/Medians | Measure(s) | Summary of findings |
|---|---|---|---|---|---|
| Length of Stay | |||||
| Deutsch et al. (2006) | Appeared to be descriptive. | IRF versus SNF | Graphically shown stratified by case-mix group. | Length of stay (days) Median, 10th and 90th percentiles |
Across most case-mix groups, the median length of IRF stay was significantly shorter than the median length of SNF stay. When not shorter, the median length of stay appeared similar by setting (Figure 2). |
| Hoenig et al. (2001) | Kruskall-Wallis test Mixed model ANOVA conducted on logarithm transformed length of stay adjusting for clustering of patients within hospitals* |
Rehabilitation unit, General hospital unit, versus SNF* (Reference) | Mean (standard deviation) Rehabilitation unit: 21.5 (25.5) Geriatric unit: 19.9 (24.4) SNF*: 16.6 (27.3) |
β coefficient for log-transformed of total acute and post-acute care length of stay | Relative to those in the SNFs*, the length of stay for patients in rehabilitation units was ~2.0 days greater and for those in geriatric units ~1.7 days greater. Rehabilitation unit: adjusted β: 0.30; p-value=0.0001 Geriatric unit: adjusted β: 0.24; p-value=0.001 |
| Medicare Part A Cost | |||||
| Deutsch et al. (2006) | T-tests conducted on logarithm-transformed dollars, stratified by case-mix group Details of how adjusted estimates were obtained were not included. |
IRF versus SNF |
IRF: $12,320 median SNF: $6,215 median |
Facility-specific modifications (e.g. wage, indirect medical education, rural, share of low income patients) were removed from Medicare Part A payments which were then converted to 1997 dollars. Median, 10th and 90th percentiles |
The higher IRF costs relative to SNF costs was apparent across all case-mix groups, but increased with increasing disease severity (e.g., IRF costs $2,106 more than SNF for case-mix group 101; $8,733 for the combined case-mix groups 109, 113, and 114). |
| Buntin et al. (2010) | Generalized estimating equations (linear model) † Instrumental variable analysis† |
IRF versus SNF | Mean (standard deviation) IRF: $29,160 ($23,630) SNF: $19,039 ($14,383) |
Adjusted mean difference in total post-acute care Medicare payments within 120 days of hospital discharge (real dollars 2002–2003) | IRF costs on average $11,261 more than SNF costs (95% confidence interval: $10,933 – $11,590). |
SNF classification included nursing home, intermediate care, or subacute care ward. Covariates included age > 70 years, race, Charlson comorbidity, intubated, second bed section an acute bed section, and site of care (no post-acute, geriatric, rehabilitation).
Covariates included patient demographic (age, age squared, sex, interaction of age and sex, race, urban/rural, Medicaid) and clinical characteristics (13 comorbidities and 17 complications that could influence outcome of post-acute care), indicators of type of stroke, hospital facility factors (size, teaching status, ownership, % Medicare, case-mix, and % low income), county-level HMO penetration, and indicator variables for site of care (IRF, SNF, home). Instruments used were patient-specific measures of accessibility and proximity to post-acute care providers.
Abbreviations: skilled nursing facility (SNF), inpatient rehabilitation facility (IRF), health maintenance organization (HMO)
Facility-Level Characteristics
Across all studies a total of 28 facility characteristics were reported, 26 of which were incorporated into the statistical analysis in at least one study (Table 5). Facility characteristics evaluated as primary exposures were local area competition,28 rehabilitation team efficacy,33 and volume of patients.30 Other facility characteristics were included in models as potential predictors or covariates for adjustment purposes. The volume of facility characteristics identified was heavily driven by a single study which collected primary data on personnel, coordination of care, physical facilities, and hospital characteristics.23 An exploratory backwards selection process identified a few characteristics as significantly associated with community discharge and length of stay.23 Another study collected primary data on team functioning, management practices, and physician leadership.33 While this study did report several statistically significant findings, had Bonferroni corrections been applied, it is unlikely any of the findings would have achieved statistical significance.33 One study demonstrated a small, but statistically significant association between prospective payment system payments and reductions in length of stay, discharge to home, and functional gains.29 Estimates of effect relating facility factors to study outcomes are reported in Supplemental Table 2, however the minimal overlap between characteristic-outcome pairs across studies limits summative interpretation.
Table 5.
Facility-Level Characteristics Reported by Study
| Author (Year) | Specific Facility | Hospital/Freestanding Facility | Number of Beds | Insurance Source Distribution | Ownership | Urban/Rural Location | Income Distribution | Hospital Referral Region | Geographic Location | Teaching Status | Therapy Intensity | Patient Volume | Competition | Wage Index | # of Post-Acute Care Settings | Care Team Size | Care Team Age | Organization Context* | Team Functioning* | Staff Qualifications† | Staffing Ratios‡ | Physical Environment§ | Coordination of Care|| | # of Rehab Training Programs | Availability of Home Health services | Weekend Therapy | Organizational Change | Outpatient Travel Time |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reker, O’Donnell, and Hamilton (1998) | ☑ | ✓ | ✓ | |||||||||||||||||||||||||
| Strasser et al. (2005) | ✓ | ✓ | ✓ | ☑ | ☑ | |||||||||||||||||||||||
| Colla et al. (2010) | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ||||||||||||||||||
| Dobrez et al. (2010) | ☑ | ☑ | ☑ | ☑ | ||||||||||||||||||||||||
| Graham et al. (2013) | ☑ | ☑ | ||||||||||||||||||||||||||
| Reisetter et al. (2015) | ☑ | ☑ | ||||||||||||||||||||||||||
| Site of Care Comparison Studies | ||||||||||||||||||||||||||||
| Kane et al. (2000) | ||||||||||||||||||||||||||||
| Hoenig et al. (2001) | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | |||||||||||||
| Chen et al. (2002) | ✓ | |||||||||||||||||||||||||||
| Deutsch et al. (2006) | ||||||||||||||||||||||||||||
| Buntin et al. (2010) | ||||||||||||||||||||||||||||
| Kind et al. (2010) | ||||||||||||||||||||||||||||
| Wang et al. (2011) | ||||||||||||||||||||||||||||
✓= reported descriptively; ☑ = incorporated into statistical analysis
The organizational context domain was composed of scales for administrative support and supervisor expectations. The team functioning domain was composed of scales for team organization, task orientation, innovation, interprofessional relations, communication, teamness, effectiveness, and utility of quality information.
Composed of variables: nurse specialization in stroke, PT specialization in stroke, # of different physician specialists, # of new graduates, proportion of paraprofessionals.
Nurse, allied health professional, and physician staffing ratios.
Composed of variables: diversity of rehabilitation equipment, ankle-foot orthosis, simulated home environment, adaptive kitchen, adaptive bathroom.
Composed of variables: # of different professionals at team meetings, guideline use, escort service, and rounding therapist as treating therapist.
Abbreviations: skilled nursing facility (SNF), inpatient rehabilitation facility (IRF), physical therapy (PT)
Three studies described facility variation. Facility explained 6–7% of variation in functional gains.30–32 One study estimated that 3.4% of home discharge variation was due to facility.30 Another study estimated that 7% of variation in functional gains and 13% of variation in length of stay was attributed to facility (corresponding to 23% and 38% of total explained variance in functional gain and length of stay, respectively).32 This study noted that over 60% of variance in health outcomes (length of stay and functional gain) remained unexplained by their statistical models.32
Discussion
This systematic review represents the broadest compilation to date of research comparing post-stroke outcomes between institutional post-acute care settings. A 2009 Cochrane Systematic Review was unable to find eligible studies which compared rehabilitation outcomes between post-acute care settings.13 Our synthesis of 8 eligible observational studies which compared outcomes by post-acute site of care generally aligned with the expectation that increased therapy intensity in the acute IRF setting would yield more favorable rehabilitation outcomes at a higher cost compared with the subacute SNF setting (Tables 3 & 4). Concerns that differences in care delivered between care settings other than those due to site of care limited the scope of the Cochrane review to randomized controlled trials and quasi-experimental designs.13 In contrast, we believed that understanding variation in health outcomes owing to facility-level phenomena could be useful to guide the development of regulations, best practice documents, and reimbursement models that facilitate investment in evidence-based structures and processes. Toward that goal, we identified 7 studies which described facility-level variation and evaluated facility-level factors associated with rehabilitation outcomes in patients with stroke. Our initial enthusiasm for synthesizing the role of facility characteristics driving health outcomes in post-acute stroke care was dampened by the paucity of factors studied in the SNF setting and the minimal impact such factors appeared to have on health outcomes in IRFs. One consistent finding in the current review was substantial facility-level variation in health outcomes for patients discharged to post-acute care after a stroke.
Medicare regulations require that IRFs meet thresholds for intensive therapy administration and rehabilitation physician supervision, while also stipulating that the inpatient setting should be reserved for patients with substantial potential for improvement.34 These criteria create an environment where patients are differentially selected for IRF care and larger investments in therapy would be expected to produce better rehabilitation outcomes, as was observed in all but one study comparing IRFs and SNFs.21 Previously, differences in age and comorbid disease burden between settings have been ascribed to the purposeful selection of a site with or without increased therapy intensity.5 We observed older age for SNF versus IRF patients in all 7 studies which reported age by site of care (Table 1). To address selection bias, the most commonly applied statistical method used by reviewed studies was the inclusion of a comprehensive list of sociodemographic, clinical, and complexity variables in multivariable models. Despite the expansive list of covariates, multivariable methods do not adjust for unmeasured variables. Two studies,20, 24 however, applied an instrumental variable analysis capable of adjusting for unmeasured differences between site of care groups. While evidence from these two studies suggest instrumental variable methods do remove further bias from post-acute site of care studies, the results from both studies employing the instrumental variable approach still favored IRFs.
The American Heart Association and American Stroke Association guideline for stroke rehabilitation and recovery describes the selection of the site of post-acute care as a multifactorial decision dependent upon “the severity of residual neurological deficits, resulting activity limitations, cognitive and communicative ability, psychological status, swallowing ability, premorbid functional ability, medical comorbidities, level of family/caregiver support, likelihood of returning to community living, and ability to participate in a rehabilitation program”.34 Beyond these factors, other considerations such as geography, market dynamics, and cost contribute to the selection of a setting for post-acute care.35, 36 Historically, the site of care and the quantity of therapy administered have been observed to be discordant from the medical complexity and the specific therapy needs of the patient.6 In one study included in our review, researchers estimated that in the six weeks post-stroke, only 23% of patients received post-acute care in a setting where they could achieve the maximum functional improvement.24 Given that these estimates were derived from a study conducted in the pre-PPS era for SNFs and IRFs (before 1998), the extent to which these findings extend to contemporary health care practices remains unknown.
Medicare payments surpassed post-acute provider costs by 19% in 2013, highlighting the potential for gains in post-acute care efficiency.6 Increasing the amount of therapy produces improved outcomes in both IRF and SNF settings,21, 37, 38 however limited available research suggests that in practice the amount of therapy per day received by stroke patients may have been similar historically in samples from both environments.21 In our review, three studies assessed facility-level variation in health outcomes and found extensive between-facility variation.30–32 However, most studies including specific facility factors suggested that such factors were minimally associated with health outcomes for patients with stroke (Supplemental Table 2). We believe that understanding the considerable variation in the provision of care and health outcomes across facilities warrants further investigation.
Policy Implications
If implemented, the shift to a unified PPS for post-acute care will require waiving of site of care specific regulatory requirements that contribute to the steering of patients and higher operating costs for certain settings (IRFs, LTCHs).6 By establishing a uniform reimbursement system, the goal is for the patient, caregivers, and the care team to be able to select the optimal site of care for the patient while holding important therapy-related and financial factors constant.6 From the perspective of post-acute care providers, a unified PPS is anticipated to confer the benefit of a more predictable profit margin across the spectrum of patient medical complexity.6 Materially, under a uniform PPS payments to IRFs are expected to decrease by 12% and payments to SNFs are estimated to increase by 8% as a result of the shift to medical complexity based reimbursement. Other estimated changes in payments include a 10% increase for non-profit facilities and the likely elimination of teaching and rural payment adjusters.6 The influence of such large scale and wide-ranging changes in reimbursement on the behavior of post-acute care providers remains to be determined.
The post-acute care provider response to constrained payments under PPS has been complex and variable across markets and clinical conditions.39 One reviewed study found contrasting effects of post-acute care provider competition on the costs and health outcomes of stroke and hip fracture patients.28 Increasing intensity of local competition for stroke patients yielded decrements in health outcomes and lower costs, while costs swelled and outcomes improved with rising competition for hip fracture patients.28 The present review highlights the scarcity of stroke-specific evidence regarding post-acute care provider responses to economic incentives and market conditions. As any unified PPS will still be inherently limited by the fee-for-service design, diligent monitoring and appropriately selected quality measures will remain essential to prevent stinting on care and undesired selection of patients based upon risk.6
The site of care selection process for patients, families, and providers has already been impacted during the current era of value-based purchasing and provider consolidation. The evolution of integrated and contractual business relationships exemplified by the emergence of accountable care organizations and bundled payment initiatives may concentrate hospital referrals to select post-acute care providers. Countervailing forces such as the augmentation of public reporting and consumer utilization of information on provider quality may also influence site of care selection patterns and health outcomes. Although the earliest a transition to a uniform post-acute PPS will occur is 2023, MedPAC has recommended near-term action to reform post-acute care payment through a site-neutral policy for IRFs and SNFs.12 This recommendation was informed by an earlier MedPAC analysis of Medicare patients which reported comparable risk-adjusted hospital readmission rates, mortality rates, functional changes, and expenditures in the 30 days after an IRF or SNF stay following a stroke, major joint replacement, or other hip/femur procedure.40
In the longer term, episodic reimbursement structured similar to the Bundled Payments for Care Improvement initiative has been described as a preferred payment mechanism for incentivizing coordination of care through shared accountability, while overcoming current problems posed by complex post-acute trajectories.6, 41 However, concerns remain regarding the potential for providers to engage in undesirable risk-based patient selection practices and compensatory attempts to inflate the volume of episodes in order to maintain revenues sufficient to cover high fixed costs.42 This review emphasizes the urgent need for health services research to elucidate meaningful differences between sites of post-acute care and to determine prominent factors such as competition that may influence provider behavior and patient outcomes.
Strengths and Limitations
The few eligible studies comparing post-stroke outcomes between IRFs and SNFs limit our ability to draw strong conclusions. No studies were experimental. Most studies used administrative data sources collected for other purposes and as such were susceptible to bias. Several included studies predated the IRF and SNF PPS. The applicability of findings from these studies to the current healthcare environment is unknown. Further, the most recent data available for comparisons of health outcomes between SNFs and IRFs were 7 years old. The large-scale changes in the healthcare delivery system since that time emphasize the need for continued original research. Studies evaluating facility-level factors were scant with 6 of 7 eligible studies focused solely in the IRF setting (Table 2).
This review focused on peer-reviewed research conducted in the United States, which improved the internal validity of included studies. Furthermore, our inclusion of non-experimental studies of actual care processes and health outcomes offers an advantage because it provides broader insight than what is typically observed in the controlled setting of clinical trials. We believe these decisions provide a synthesis of information more relevant to ongoing discussions in the United States regarding changes in post-acute care quality measurement and payment policies. Indeed, the large and geographically diverse populations included in the eligible studies underscore this strength.
Conclusion
Our review underscores the dearth of literature available to determine the extent to which historical differences in reimbursement under disparate IRF and SNF payment systems have productively contributed to improved health outcomes for stroke patients as opposed to inefficient resource allocation. Therefore, it will be critical to develop a deeper and contemporary understanding of the role of the post-acute care setting and its attributes (e.g., staffing, rehabilitation team composition and efficacy, volume/size, teaching status, location, and market dynamics) in determining health outcomes relevant for stroke patients, providers, and payers. This research should be prioritized to ensure reimbursement levels implemented under a unified or bundled system will be sufficient to cover high-quality care regardless of the setting.
Supplementary Material
Acknowledgments
Funding sources: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
MA is a NIH funded post-doctoral fellow (5 TL1 TR 1454-3).
Abbreviations
- HHA
Home health agency
- IMPACT
Improving Medicare Post-Acute Care Transformation Act of 2014
- IRF
inpatient rehabilitation facility
- LTCH
long term care hospital
- MedPAC
Medicare Payment Advisory Commission
- PPS
prospective payment system
- SNF
skilled nursing facility
Footnotes
Conflicts of interest: None
Previous Presentation: Poster at the Academy Health 2017 Annual Research Meeting, New Orleans, LA. June 24th, 2017
References
- 1.Medicare Payment Advisory Commission. A Data Book: Health Care Spending and the Medicare Program. 2016. Jun, [Google Scholar]
- 2.Tian W. An All-Payer View of Hospital Discharge to Postacute Care, 2013. HCUP Statistical Brief #205. 2016 May;:1–17. [PubMed] [Google Scholar]
- 3.Gage B, Ingber MJ, Morley M, et al. Post-Acute Care Payment Reform Demonstration: Final Report. 2012a;1 of 4 [Google Scholar]
- 4.Gage B, Ingber MJ, Morley M, et al. Post-Acute Care Payment Reform Demonstration: Final Report. 2012b;3 of 4 [Google Scholar]
- 5.Gage B, Smith L, Coots L, Macek J, III, Manning J, Reilly K. Analysis of the Classification Criteria for Inpatient Rehabilitation Facilities (IRFs) Report to Congress. 2009:1–51. [Google Scholar]
- 6.Medicare Payment Advisory Commission. Mandated report: developing a unified payment system for post-acure care. June 2016 Report to Congress. 2016c;Chapter 3 [Google Scholar]
- 7.Colby SL, Ortman JM. Current Population Reports. 2015. Projections of the Size and Composition of the US Population: 2014 to 2060; pp. P25–1143. [Google Scholar]
- 8.Medicare Payment Advisory Commission. Monitoring Post-Acute Care. June 2003 Report to Congress. 2003;Chapter 5 [Google Scholar]
- 9.Medicare Payment Advisory Commission. Payment Basics. 2016d. Skilled Nursing Facility Services Payment System; pp. 1–4. [Google Scholar]
- 10.Medicare Payment Advisory Commission. Inpatient Rehabilitation Facilities Payment System. Payment Basics. 2016b:1–3. [Google Scholar]
- 11.Improving Medicare Post-Acute Transformation Act of 2014, 42 USC 1395.
- 12.Medicare Payment Advisory Commission. Medicare’s post-acute care: trends and ways to rationalize payments. March 2015 Report to Congress. 2015;Chapter 7 [Google Scholar]
- 13.Ward D, Drahota A, Gal D, Severs M, Dean TP. Care Home versus Hospital and Own Home Environments for Rehabilitation of Older People (Cochrane Review) Cochrane Database of Systematic Reviews. 2008;(4) doi: 10.1002/14651858.CD003164.pub2. Art. No.: CD003164. [DOI] [PMC free article] [PubMed]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement (Reprinted from Annals of Internal Medicine) PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonarow GC, Reeves MJ, Zhao X, et al. Age-Related Differences in Characteristics, Performance Measures, Treatment Trends, and Outcomes in Patients with Ischemic Stroke. Circulation. 121(7):879–891. doi: 10.1161/CIRCULATIONAHA.109.892497. [DOI] [PubMed] [Google Scholar]
- 16.Saposnik G, Guzik AK, Reeves M, Ovbiagele B, Johnston SC. Stroke Prognostication Using Age and NIH Stroke Scale: SPAN-100. Neurology. 2013;80(1):21–28. doi: 10.1212/WNL.0b013e31827b1ace. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chisholm L, Weech-Maldonado R, Laberge A, Lin F-C, Hyer K. Nursing Home Quality and Financial Performance: Does the Racial Composition of Residents Matter? Health Services Research. 2013;48(6):2060–2080. doi: 10.1111/1475-6773.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holup AA, Gassoumis ZD, Wilber KH, Hyer K. Community Discharge of Nursing Home Residents: The Role of Facility Characteristics. Health Services Research. 2016;51(2):645–666. doi: 10.1111/1475-6773.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neuman MD, Wirtalla C, Werner RM. Association between Skilled Nursing Facility Quality Indicators and Hospital Readmissions. Journal of the American Medical Association. 2014;312(15):1542–51. doi: 10.1001/jama.2014.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buntin MB, Colla CH, Deb P, Sood N, Escarce JJ. Medicare Spending and Outcomes after Postacute Care for Stroke and Hip Fracture. Medical Care. 2010;48(9):776–84. doi: 10.1097/MLR.0b013e3181e359df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CC, Heinemann AW, Granger CV, Linn RT. Functional Gains and Therapy Intensity during Subacute Rehabilitation: A Study of 20 Facilities. Archives of Physical Medicine & Rehabilitation. 2002;83(11):1514–23. doi: 10.1053/apmr.2002.35107. [DOI] [PubMed] [Google Scholar]
- 22.Deutsch A, Granger CV, Heinemann AW, et al. Poststroke Rehabilitation: Outcomes and Reimbursement of Inpatient Rehabilitation Facilities and Subacute Rehabilitation Programs. Stroke. 2006;37(6):1477–1482. doi: 10.1161/01.STR.0000221172.99375.5a. [DOI] [PubMed] [Google Scholar]
- 23.Hoenig H, Sloane R, Horner RD, Zolkewitz M, Reker D. Differences in Rehabilitation Services and Outcomes among Stroke Patients Cared for in Veterans Hospitals. Health Services Research. 2001;35(6):1293–1318. [PMC free article] [PubMed] [Google Scholar]
- 24.Kane RL, Chen Q, Finch M, Blewett L, Burns R, Moskowitz M. The Optimal Outcomes of Post-Hospital Care under Medicare. Health Services Research. 2000;35(3):615–661. [PMC free article] [PubMed] [Google Scholar]
- 25.Kind AJ, Smith MA, Liou J, Pandhi N, Frytak JR, Finch MD. Discharge Destination’s Effect on Bounce-Back Risk in Black, White, and Hispanic Acute Ischemic Stroke Patients. Archives of Physical Medicine & Rehabilitation. 2010;91(2):189–195. doi: 10.1016/j.apmr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Sandel ME, Terdiman J, Armstrong MA, Klatsky A, Camicia M, Sidney S. Postacute Care and Ischemic Stroke Mortality: Findings from an Integrated Health Care System in Northern California. PM & R : the journal of injury, function, and rehabilitation. 2011;3(8):686–94. doi: 10.1016/j.pmrj.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 27.Chan L, Sandel ME, Jette AM, et al. Does postacute care site matter? A longitudinal study assessing functional recovery after a stroke. Arch Phys Med Rehabil. 2013;94(4):622–9. doi: 10.1016/j.apmr.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colla CH, Escarce JJ, Buntin MB, Sood N. Effects of Competition on the Cost and Quality of Inpatient Rehabilitation Care under Prospective Payment. Health Services Research. 2010;45(6 PART 2):1981–2006. doi: 10.1111/j.1475-6773.2010.01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dobrez D, Heinemann AW, Deutsch A, Manheim L, Mallinson T. Impact of Medicare’s Prospective Payment System for Inpatient Rehabilitation Facilities on Stroke Patient Outcomes. American Journal of Physical Medicine & Rehabilitation. 2010;89(3):198–204. doi: 10.1097/PHM.0b013e3181c9fb40. [DOI] [PubMed] [Google Scholar]
- 30.Graham JE, Deutsch A, O’Connell AA, Karmarkar AM, Granger CV, Ottenbacher KJ. Inpatient Rehabilitation Volume and Functional Outcomes in Stroke, Lower Extremity Fracture, and Lower Extremity Joint Replacement. Medical Care. 2013;51(5):401–12. doi: 10.1097/MLR.0b013e318286e3c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reistetter TA, Kuo YF, Karmarkar AM, Eschbach K, Teppala S, FJ, Ottenbacher KJ. Geographic and Facility Variation in Inpatient Stroke Rehabilitation: Multilevel Analysis of Functional Status. Archives of Physical Medicine & Rehabilitation. 2015;96(7):1248–54. doi: 10.1016/j.apmr.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reker DM, O’Donnell JC, Hamilton BB. Stroke Rehabilitation Outcome Variation in Veterans Affairs Rehabilitation Units: Accounting for Case-Mix. Archives of Physical Medicine & Rehabilitation. 1998;79(7):751–757. doi: 10.1016/s0003-9993(98)90351-3. [DOI] [PubMed] [Google Scholar]
- 33.Strasser DC, Falconer JA, Herrin JS, Bowen SE, Stevens AB, Uomoto J. Team Functioning and Patient Outcomes in Stroke Rehabilitation. Archives of Physical Medicine & Rehabilitation. 2005;86(3):403–409. doi: 10.1016/j.apmr.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 34.Winstein CJ, Stein J, Arena RB, et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(6):e98–e169. doi: 10.1161/STR.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 35.Buntin M, Garten A, Paddock S, Saliba D, Totten M, EJJ Impact of Levels of Service. How Much Is Postacute Care Use Affected by Its Availability? Health Services Research. 2005;40(2):413–434. doi: 10.1111/j.1475-6773.2005.00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kane RL, Lin WC, Blewett LA. Geographic Variation in the Use of Post-Acute Care. Health Services Research. 2002;37(3):667–82. doi: 10.1111/1475-6773.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jette DU, Warren RL, Wirtalla C. The Relation between Therapy Intensity and Outcomes of Rehabilitation in Skilled Nursing Facilities. Archives of Physical Medicine and Rehabilitation. 2005;86(3):373–9. doi: 10.1016/j.apmr.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Wodchis WP, Teare GF, Naglie G, et al. Skilled Nursing Facility Rehabilitation and Discharge to Home after Stroke. Archives of Physical Medicine & Rehabilitation. 2005;86(3):442–8. doi: 10.1016/j.apmr.2004.06.067. [DOI] [PubMed] [Google Scholar]
- 39.Buntin MB, Colla CH, Escarce JJ. Effects of Payment Changes on Trends in Post-Acute Care. Health Services Research. 2009;44(4):1188–210. doi: 10.1111/j.1475-6773.2009.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medicare Payment Advisory Commission. Site-neutral payments for select conditions treated in inpatient rehabilitation facilities and skilled nursing facilities. June 2014 Report to Congress. 2014;Chapter 6 [Google Scholar]
- 41.Dummit L, Marrufo G, Marshall J, et al. Final Report. Feb, The Lewin Group. 2015. “CMS Bundled Payments for Care Improvement (BPCI) Initiative Models 2–4 : Year 1 Evaluation & Monitoring Annual Report. [Google Scholar]
- 42.Weeks WB, Rauh SS, Wadsworth EB, Weinstein JN. The Unintended Consequences of Bundled Payments. Annals of Internal Medicine. 2014;(4):62–65. doi: 10.7326/0003-4819-158-1-201301010-00012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.