Abstract
This study aimed to provide insight into the development of postural control abilities in youth. A total of 276 typically developing adolescents (155 males, 121 females) with a mean age of 13.23 years (range of 7.11 – 18.80) were recruited for participation. Subjects performed two-minute quiet standing trials in bipedal stance on a force plate. Center of pressure (COP) trajectories were quantified using Sample Entropy (SampEn) in the anterior-posterior direction (SampEn-AP), SampEn in the medial-lateral direction (SampEn-ML), and Path Length (PL) measures. Three separate linear regression analyses were conducted to predict the relationship between age and each of the response variables after adjusting for individuals’ physical characteristics. Linear regression models showed an inverse relationship between age and entropy measures after adjusting for body mass index. Results indicated that chronological age was predictive of entropy and path length patterns. Specifically, older adolescents exhibited center of pressure displacement (smaller path length) and less complex, more regular center of pressure displacement patterns (lower SampEn-AP and SampEn-ML values) compared to the younger children. These findings support prior studies suggesting that developmental changes in postural control abilities may continue throughout adolescence and into adulthood.
Keywords: postural control, postural sway, center of pressure displacement, adolescence, development, spatial and temporal analysis
1. Introduction
Good postural control is necessary for purposeful and safe movement while performing functional activities. Even a seemingly simple task such as sitting quietly requires intricate coordination of multiple forms of sensory information into appropriate motor outputs. The refinement of effective and efficient postural management is a developmental process that unfolds slowly.(Shumway-Cook & Woollacott, 1985) Postural control continues to develop throughout childhood, but conflicting accounts surround the age at which children achieve postural control abilities comparable to that of adults.(Barozzi et al., 2014; Quatman-Yates, Quatman, Meszaros, Paterno, & Hewett, 2011) Some studies indicate that adult-like abilities are reached by 10 years,(Shumway-Cook & Woollacott, 1985; Wolff et al., 1998) while others suggest that maturation of postural control abilities continues beyond 15 years.(Barozzi et al., 2014; Cumberworth, Patel, Rogers, & Kenyon, 2007; Ferber-Viart, Ionescu, Morlet, Froehlich, & Dubreuil, 2007; Gouleme, Ezane, Wiener-Vacher, & Bucci, 2014; Hirabayashi & Iwasaki, 1995; Steindl, Kunz, Schrott-Fischer, & Scholtz, 2006) The reason for this discrepancy is not entirely clear but is likely related, at least in part, to varying measurement approaches and study designs.(Steindl et al., 2006) Methods to evaluate postural control have become more sophisticated, and our capacity to understand the maturation of postural control has progressed.(Riley & Turvey, 2002) Exploration of postural control evolution throughout childhood and adolescence could provide new insights into typical and atypical development and healthy versus impaired states.(Barozzi et al., 2014; Quatman-Yates et al., 2011)
The evaluation of the temporal order and structure of the variability in center of pressure (COP) displacement patterns has progressed considerably methodologically and clinically over the last decade.(Riley, Balasubramaniam, & Turvey, 1999; Riley & Turvey, 2002; Stergiou, Harbourne, & Cavanaugh, 2006) The emergence of complexity and systems science approaches in the biomedical sciences has led to increasing recognition that the temporal order and structure of the variability in physiologic and behavioral signals (e.g., heart rate variability and COP displacement variability) may provide powerful insights into the underlying health and physiological state of the body.(Goldberger, 1997; Goldberger et al., 2002; Lipsitz & Goldberger, 1992; Stergiou et al., 2006) Specifically, the temporal order and structure of the variability in these signals are theorized to reveal the degree of integrated and coordinative activity of the systems involved in producing the signal. Consequently, there has been a surging interest in attempts to integrate temporal and structural variability analyses of physiological and behavioral signals into healthcare practices to evaluate for impairments and monitor recovery.(Cavanaugh et al., 2006; Chesnokov, 2008) Prevalent examples include the use of entropy and fractal measures to evaluate postural control impairments in individuals with concussions,(Cavanaugh et al., 2006) developmental delay,(Deffeyes, Harbourne, Stuberg, & Stergiou, 2010) and Parkinson’s disease.(Schlenstedt et al., 2016; Schmit et al., 2006)
Mechanistically the temporal order and structure of variability differs in cases of injury and disease. However, clinical interpretation of these signals can be complicated, particularly for younger and older individuals, as postural control abilities are expected to improve with maturation and then decline later in life.(Hytonen, Pyykko, Aalto, & Starck, 1993; Shumway-Cook & Woollacott, 1985) In order to better evaluate the validity of the diagnostic and clinical utility of these signals, it is necessary to expand our understanding of the way these signals can be affected by natural growth and development processes. The purpose of this study was to investigate the impact of adolescent development on COP displacement dynamics in quiet stance for a large cohort of healthy children. It was hypothesized that developmental changes in COP displacement dynamics would be observed and indicative of maturational improvements in postural control.
2. Methods
2.1 Participants
All study methods were completed in accordance with an Institutional Review Board approved protocol. Participants were recruited from physical education classes and extracurricular activities (e.g., chess team, debate team, and sports teams) from one middle school and one high school in a large metropolitan area. The overall recruitment strategy was designed to capture children with a wide range of physical and cognitive abilities. To avoid self-selection bias, we invited all students who were members of these classes and programs to enroll as participants. We then excluded cases for analysis based on the following reasons: 1) the consenting guardian or child reported any pre-existing health conditions with the potential to significantly alter COP displacement (e.g., developmental delay, cerebral palsy, documented central nervous system abnormalities or significant musculoskeletal conditions/injuries); 2) the child had a current acute lower extremity injury leading to the use of an assistive device, brace, or immobilizer; or 3) a study administrator reported doubts about concentration, effort, or compliance with the assessment instructions for the child.
Legal guardians and students were notified by school administrators, teachers, and the study staff about potential participation for the study via newsletters, announcements sent home to families, and word-of-mouth. Each school was compensated with $25 per child enrolled. Study packets with details regarding study procedures, a physical activity questionnaire, a medical history questionnaire, and parental permission forms were sent home to legal guardians. Legal guardians were first asked to read the study materials and indicate their decision to allow or not allow their child to participate. If permission was granted, the guardian was asked to complete the enclosed activity and medical history forms for the child. If permission was not granted, the guardian was asked to return the packet with those forms blank. School-designated administrators collected all of the folders and only provided study staff the folders of children whose parents granted permission for participation. Assent by the child was obtained at the time of the first in person visit.
2.2 Procedures
A quiet room at each school was selected for the study procedures. Participants’ height and body mass were assessed using a tape measure and a Tanita digital scale. Force plate assessments were administered using one of two portable AccuSway+ force plates (AMTI, Boston, Massachusetts) and Balance Clinic software. Center of pressure (COP) trajectories were collected at 50 Hz for two 2-minute trials (1 trial with eyes open, 1 trial with eyes closed) for each participant. This protocol aligned with a previously published protocol which demonstrated high test-retest reliability in a small sample of high-school students.(Quatman-Yates et al., 2015; Quatman-Yates et al., 2013) The force platform used and the order of the eyes open versus eyes closed conditions were selected in a counterbalanced order for each participant. This resulted in time series consisting of 6,000 data samples per time trial. To test for potential differences between the two force platforms, we compared the underlying signal noise inherent to each platform for each outcome measure by placing a 60 kg static weight on each platform and recording the COP for two minutes.
Study team members helped position each child in the center of the force plate with the child’s shoeless feet together and arms resting naturally at the child’s side. The following script was read to participants: “Everyone naturally has a little postural sway. This is a test to look at your body’s natural sway. Don’t try to create any extra sway but don’t try to stop it either. We want you to be as natural as possible. We’re going to do two trials, one eyes open and the other eyes closed. You’re going to stand on the platform facing the “x” with feet together so they’re touching with your hands naturally resting at your sides. You’ll be standing for two minutes, which feels like a really long time. Do your best to try not to fidget. Just try to remain as natural as possible throughout the 2 minute trial.”
2.3 Data Processing and Analysis
Participants’ ages at the time of the assessment were used for analysis. Each Each participants’ body mass index (BMI) was calculated using their body mass and height recorded on the day of the postural control assessments by dividing the body mass by the square of the body height (kg/m2). COP position data (each consisting of 6,000 data point samples) were quantified using Sample Entropy (SampEn) in the anterior-posterior direction (SampEn-AP), SampEn in the medial-lateral direction (SampEn-ML), and Path Length (PL) measures. SampEn captures the structure of COP variability in terms of the repeatability of small sub-sets of data strings within the time series. A high degree of repeatability (lower SampEn) is indicative of a time series that is more structured (i.e., more deterministic, and in that sense, less complex), while lower repeatability (higher SampEn) indicates a less structured (i.e., more irregular and thus more complex) time series.
A data value is considered to repeat if the values of the two data points are separated by less than a specified tolerance (r). This procedure of determining repeating (i.e. recurrent) data points is commonly performed with consecutive data points (termed a “template”) of a chosen length m. Values of r = 0.10 and m = 3 were chosen for the present analysis based on methods discussed in Ramdani et al.(Ramdani, Seigle, Lagarde, Bouchara, & Bernard, 2009) Path length is the summed Euclidean distance traveled by the COP over the course of a trial. Quatman-Yates et al. provided a detailed description of the procedures and interpretations of SampEn and PL analyses specific to the protocol used in this study.(Quatman-Yates et al., 2015)
2.4 Statistical Analysis
Descriptive statistics were computed for each variable to describe the basic features of the data. Inspection of quantile–quantile plots determined that SampEn-AP, SampEn-ML, and PL were normally distributed and amenable to analysis with traditional parametric techniques. Two different modeling strategies were used to estimate the relationship between age and the aforementioned response variables (SampEn-AP, SampEn-ML, and PL). First, linear regression models were used to estimate the relationship between age and response variables considering body mass independently and after adjusting for BMI. Gender was initially included as a covariate in linear regression models but was dropped later due to lack of statistical significance. Next, quantile regression models were conducted to assess the relation between age and the distributions of the response variables.
The equation for the quantile regression for path length as an example, can be written as:
Where i is individual tested, β0(q) is the intercept of the qth quantile, β1(q) (age)i is the slope coefficient of age for the qth quantile, and ωi is the random error term. Different sets of curvatures (linear, quadratic, and cubic) were tested for quantile regression models. The final model was selected using the Wald test statistic. Based on the Wald test statistics, age was included as the linear effect in the quantile regression models for all response variables. The statistical significance level was set at the nominal (α = 0.05) level. All analyses were conducted with SAS software version 9.4 (SAS Institute Inc., Cary, NC).
3. Results
A total of 276 subjects (155 males, 121 females) with a mean (± SD) age of 13.23 ± 3.07 years, weight of 51.88 kg ± 19.60 kg, height of 1.57 meters ± 0.16 meters, and BMI of 20.24 ± 4.60 were included in the final analyses. Descriptive details for the final pool of participant data used in the analyses are reported in Table 1. No statistically significant differences in the inherent noise of the platforms were detected.
Table 1.
Descriptive variables used in analysis
| Variable | Mean (Stddev) | Range |
|---|---|---|
| Age | 13.23 (3.07) | 7.11 – 18.80 |
| Weight (kg) | 51.88 (19.60) | 19.40 – 114.20 |
| Height (meter) | 1.57 (0.16) | 1.18 – 1.90 |
| BMI | 20.24 (4.60) | 11.87 – 38.80 |
| EO SampEn-AP | 0.36 (0.10) | 0.13 – 0.88 |
| EO SampEn-ML | 0.37 (0.15) | 0.09 – 0.92 |
| EO Path Length | 97.90 (27.15) | 51.06 – 244.36 |
| EC SampEn-AP | 0.39 (0.10) | 0.18 – 0.84 |
| EC SampEn-ML | 0.39 (0.13) | 0.07 – 0. 89 |
| EC PL | 124.53 (37.29) | 62.38 – 453.61 |
Stddev: Standard deviation; EO: Eyes open condition; EC: Eyes closed condition; SampEn-AP: Sample entropy in anterior-posterior direction; SampEn-ML: Sample entropy in medial-lateral direction; PL: Path length
Linear regression analyses showed an inverse relationship between age and entropy measures for both the eyes open (EO) and eyes closed (EC) conditions. The estimates were the same for body mass independently and BMI adjusted. Specifically, older age was associated with lower values of entropy and path length (e.g., βage(EO SampEn-AP) = −0.006, p <.01; βage(EO SampEn-Ml) = −0.01, p <.01; βage(EO PL) = −4.56, p <.01). The quantile regression plots (Figures 1–3) illustrate how entropy and path length changed with age when moving along the distribution of the former for the EO condition. For example, the slope of the 90th percentile line (Figure 1) indicated how the value of EO SampEn-AP changed with age as we moved along the 90th percentile of the distribution values of EO SampEn-AP. The slope of the 90% quantile regression lines were steeper than the 10% line (Table 2), indicating that the distribution of entropy measures and path length were less spread out (or variance was lower) for older as compared to younger children. The 50th and 90th quantiles of entropy measures (EO SampEn-AP, EO SampEn-ML) and EO PL showed higher negative relationships with age compared to the 10th quantile of the entropy and path length.
Figure 1.
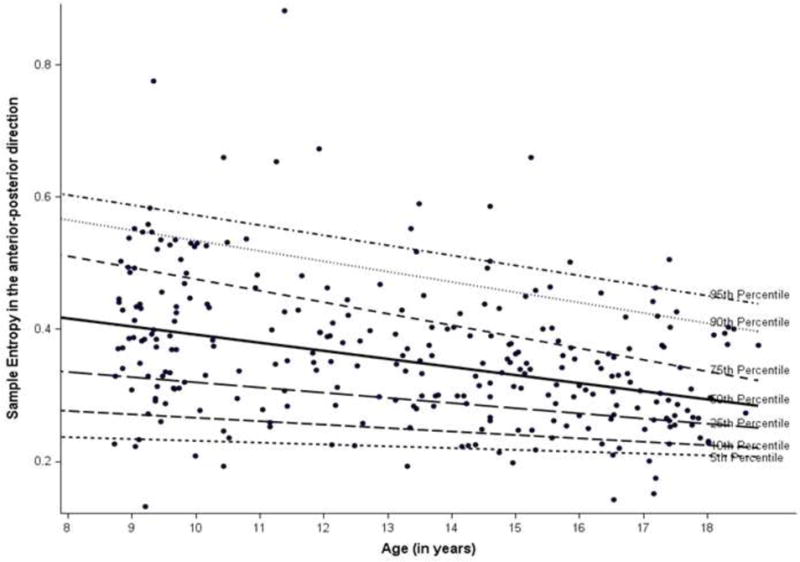
Quantile regression plot for sample entropy in anterior-posterior direction
Figure 3.
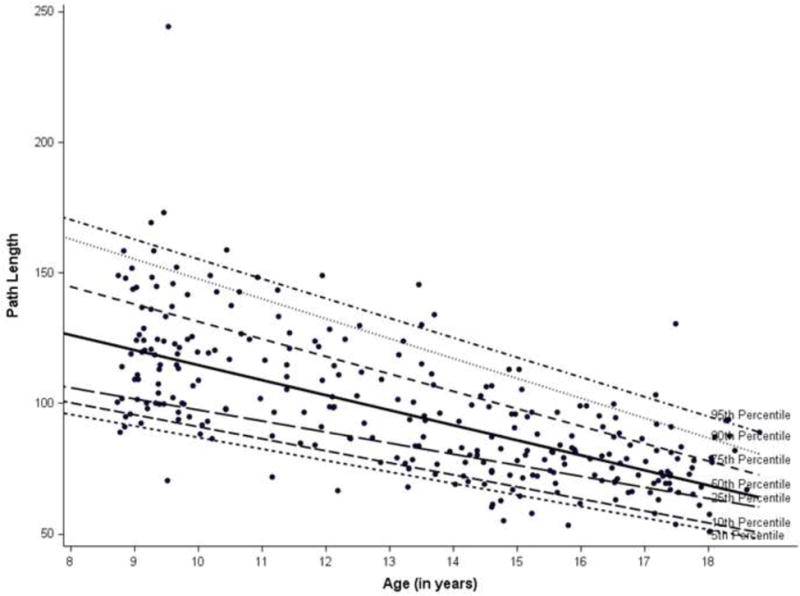
Quantile regression plot for path length
Table 2.
Linear regression and Quantile regression results
| Variable | Linear Regression Mean (SE) | Quantile | ||
|---|---|---|---|---|
| 10% Est (SE) |
50% Est (SE) |
90% Est (SE) |
||
| SampEn-AP* | ||||
| Constant | 0.57 (0.03) | 0.31 (0.03) | 0.51 (0.03) | 0.69 (0.04) |
| Age | −0.006 (0.002) | −0.005 (0.002) | −0.01 (0.002) | −0.01 (0.003) |
| SampEn-ML* | ||||
| Constant | 0.67 (0.04) | 0.39 (0.04) | 0.61 (0.05) | 0.88 (0.09) |
| Age | −0.01 (0.003) | −0.01 (0.002) | −0.02 (0.003) | −0.02 (0.006) |
| Path Length* | ||||
| Constant | 188.12 (5.80) | 136.90 (5.01) | 172.18 (6.24) | 223.76 (10.31) |
| Age | −4.56 (0.49) | −4.60 (0.37) | −5.75 (0.45) | −7.62 (0.75) |
These estimates were the same for the eyes open and eyes closed conditions and for body mass and with body mass index adjusted after rounding to the nearest decimal.
SE: Standard error; Est: Quantile regression estimates
In essence, Figures 1–3 provide supplementary information to the linear regression approach. While linear regression provides the population average effects on entropy and PL measures, the quantile regressions (as reflected in Figures 1–3) reflect the relationship beyond the average effects of age based on normal distributions (which are used in linear regression). With a distribution free assumption, the quantile regressions provide an illustration of the relationship between age and the entire distribution of entropy and PL values. The figures, thus, help capture the negative magnitude and relative variability of entropy at each percentile in relation to age.
4. Discussion
The purpose of this study was to investigate the impact of adolescent development on COP displacement in quiet stance for a large cohort of healthy children. Even after accounting for BMI, age was correlated with SampEn and PL values. Older adolescents exhibited less sway (smaller path length) and less complex, more deterministic sway patterns (lower SampEn-AP and SampEn-ML values) compared to the younger children under both eyes opened and eyes closed conditions. These findings support prior studies suggesting that developmental changes in postural control abilities may continue throughout adolescence and beyond. Moreover, while the physical changes of increased height and body mass can account for some of the age-related differences observed during adolescence, ongoing subtle refinements of the sensory integration and motor processing elements postural maintenance strategies could be a source of developmental differences observed throughout adolescence.
An advantage of fully developed and healthy postural control coordination is the ability to adjust rapidly to new challenges like a sudden change in task, an unexpected nudge from behind, or safe navigation of unstable terrain. SampEn values for COP data are theorized to be reflective of how well the body is able to integrate coordinate sensory, motor, task, and environmental inputs and outputs and are thought to be indicative of a person’s capacity to generate adaptive responses to expected or unexpected perturbations such as these. Optimal SampEn values are hypothesized to fall somewhere within a continuum characterized by two undesirable extremes. On one end of the continuum, very high SampEn would represent excessive randomness and poor regulation of postural maintenance while very low SampEn would be indicative of postural maintenance that is overly rigid and less adaptable than might be desired. As such, healthy, well-developed postural control is thought to be characterized by a theoretical “sweet spot” that is comprised of both some regularity and some randomness but not too much of either.(Stergiou et al., 2006)
The direction of the SampEn changes are opposite of what some may expect. Infants with developmental delay have been shown to display diminished regularity in their sway patterns compared to typically developing, healthy peers. (Deffeyes et al., 2010) Studies also indicate that quiet sway entropy can be lower following concussion for youth compared to typically developing, healthy peers.(Quatman-Yates et al., 2015) Therefore, it is tempting to assume that as a typically developing, healthy child goes through adolescence, higher SampEn values should be observed. However, this study’s results indicate that this may not be the case. Prior pediatric study designs may have masked the developmental trends that naturally occur for typically developing, healthy children. Additional studies are needed to further clarify what happens as adolescents continue to mature through the aging continuum and how the theoretical “sweet spot” applies to COP displacement and functional abilities.
Likewise, the results of this study underscore a need for additional studies to consider the way developmental changes in postural dynamics may affect the clinical utility and interpretability of entropy and PL postural assessments performed with youth. For example, there has been a cautious, yet growing optimism supporting entropy assessments as a means to help evaluate concussion-related recovery. A finding noted in prior studies utilizing entropy measures for comparable patient populations is an observable decline in entropy scores following a concussion.(Cavanaugh, Guskiewicz, Giuliani, et al., 2005; Cavanaugh, Guskiewicz, & Stergiou, 2005; McCrory et al., 2013; Quatman-Yates et al., 2015) Likewise, a decline in PL after concussion using the same protocol used in this study has also been reported.(Quatman-Yates et al., 2015). Given the results of the current study, further work is needed to help clarify how typical developmental changes may impact the clinical utility and interpretability of identifying and monitoring states of impairment (e.g., post-concussion postural control) based on COP displacement dynamics.
It is important to note that several elements of the current study may limit the generalizability and interpretability of these findings. First, the study had a cross-sectional rather than a longitudinal design. Future longitudinal study designs would provide a strong complement to the findings of this exploratory study. Also, the postural control task used in this study was static, and relatively simple in nature. Further exploration of the developmental nature of dynamic and suprapostural control tasks may prove worthwhile. Finally, for this particularly study, we opted to focus on the eyes open condition, the positional analysis of the COP data, and SampEn and PL measures. Future work with this dataset could yield interesting insights relative to developmental trends for the eyes-closed condition, analysis of the COP velocity data, and other types of linear and nonlinear or time variant/invariant analyses (e.g., spectral analyses, fractal analyses, recurrence quantification analyses).
5. Conclusion
The results of this study indicate that postural control abilities are still being refined throughout the adolescent physical development period. Older youth have more regularity in their quiet COP displacement as measured by the temporal order and structure of the variability in sway trajectories, posing potential challenges to the clinical interpretability of postural control assessments for youth.
Figure 2.
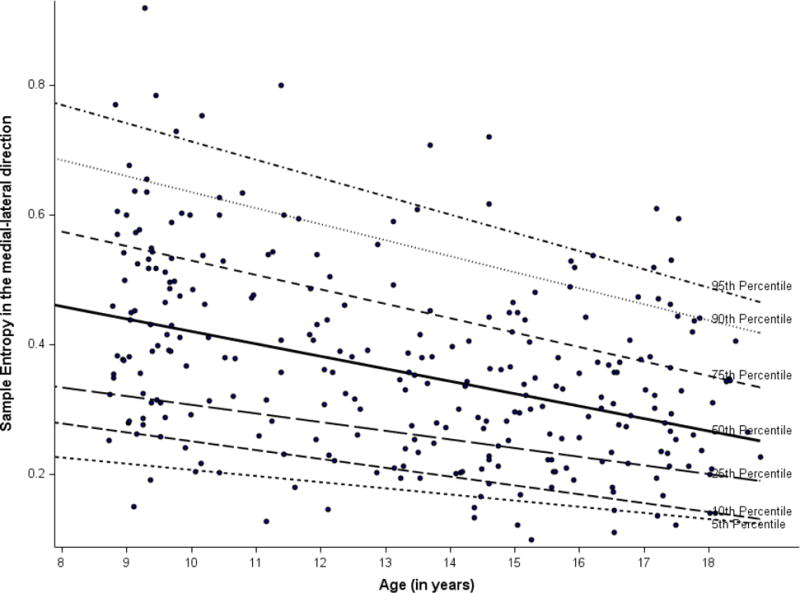
Quantile regression plot for sample entropy in the medial-lateral direction
Research Highlights.
Chronological age was predictive of temporal order and structure of sway
Older adolescents exhibited less sway and more regular sway patterns
Developmental changes sway may continue throughout adolescence
Acknowledgments
The authors would like to acknowledge Meredith Vegh PT, DPT, Barbara Janiszewski PT, DPT, SCS, Staci Thomas, MS, Joubert Lucas, MS and Carolyn Cassaro for their assistance with data collection and data management for the project.
Funding Sources:
Sources of funding supporting the project included: the Ohio Physical Therapy Association’s Research Grant, Cincinnati Children’s Hospital Research in Patient Services PS2 Grant, and the National Institutes of Health KL2 TR001426-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barozzi S, Socci M, Soi D, Di Berardino F, Fabio G, Forti S, Cesarani A. Reliability of postural control measures in children and young adolescents. Eur Arch Otorhinolaryngol. 2014;271(7):2069–2077. doi: 10.1007/s00405-014-2930-9. [DOI] [PubMed] [Google Scholar]
- Cavanaugh JT, Guskiewicz KM, Giuliani C, Marshall S, Mercer V, Stergiou N. Detecting altered postural control after cerebral concussion in athletes with normal postural stability. Br J Sports Med. 2005;39(11):805–811. doi: 10.1136/bjsm.2004.015909. doi: 39/11/805 [pii] 10.1136/bjsm.2004.015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh JT, Guskiewicz KM, Giuliani C, Marshall S, Mercer VS, Stergiou N. Recovery of postural control after cerebral concussion: new insights using approximate entropy. J Athl Train. 2006;41(3):305–313. [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh JT, Guskiewicz KM, Stergiou N. A nonlinear dynamic approach for evaluating postural control: new directions for the management of sport-related cerebral concussion. Sports Med. 2005;35(11):935–950. doi: 10.2165/00007256-200535110-00002. doi: 35112 [pii] [DOI] [PubMed] [Google Scholar]
- Chesnokov YV. Complexity and spectral analysis of the heart rate variability dynamics for distant prediction of paroxysmal atrial fibrillation with artificial intelligence methods. Artif Intell Med. 2008;43(2):151–165. doi: 10.1016/j.artmed.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Cumberworth VL, Patel NN, Rogers W, Kenyon GS. The maturation of balance in children. J Laryngol Otol. 2007;121(5):449–454. doi: 10.1017/S0022215106004051. [DOI] [PubMed] [Google Scholar]
- Deffeyes JE, Harbourne RT, Stuberg WA, Stergiou N. Approximate entropy used to assess sitting postural sway of infants with developmental delay. Infant Behav Dev. 2010 doi: 10.1016/j.infbeh.2010.10.001. doi: S0163-6383(10)00119-0 [pii] 10.1016/j.infbeh.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber-Viart C, Ionescu E, Morlet T, Froehlich P, Dubreuil C. Balance in healthy individuals assessed with Equitest: maturation and normative data for children and young adults. Int J Pediatr Otorhinolaryngol. 2007;71(7):1041–1046. doi: 10.1016/j.ijporl.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Goldberger AL. Fractal variability versus pathologic periodicity: complexity loss and stereotypy in disease. Perspect Biol Med. 1997;40(4):543–561. doi: 10.1353/pbm.1997.0063. [DOI] [PubMed] [Google Scholar]
- Goldberger AL, Amaral LA, Hausdorff JM, Ivanov P, Peng CK, Stanley HE. Fractal dynamics in physiology: alterations with disease and aging. Proc Natl Acad Sci U S A. 2002;99(Suppl 1):2466–2472. doi: 10.1073/pnas.012579499. doi: 10.1073/pnas.012579499 99/suppl_1/2466 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouleme N, Ezane MD, Wiener-Vacher S, Bucci MP. Spatial and temporal postural analysis: a developmental study in healthy children. Int J Dev Neurosci. 2014;38:169–177. doi: 10.1016/j.ijdevneu.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Hirabayashi S, Iwasaki Y. Developmental perspective of sensory organization on postural control. Brain Dev. 1995;17(2):111–113. doi: 10.1016/0387-7604(95)00009-z. doi: 038776049500009Z [pii] [DOI] [PubMed] [Google Scholar]
- Hytonen M, Pyykko I, Aalto H, Starck J. Postural control and age. Acta Otolaryngol. 1993;113(2):119–122. doi: 10.3109/00016489309135778. [DOI] [PubMed] [Google Scholar]
- Ko JH, Newell KM. Aging and the complexity of center of pressure in static and dynamic postural tasks. Neurosci Lett. 2016;610:104–109. doi: 10.1016/j.neulet.2015.10.069. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Goldberger AL. Loss of ‘complexity’ and aging. Potential applications of fractals and chaos theory to senescence. JAMA. 1992;267(13):1806–1809. [PubMed] [Google Scholar]
- McCrory P, Meeuwisse WH, Aubry M, Cantu B, Dvorak J, Echemendia RJ, Turner M. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250–258. doi: 10.1136/bjsports-2013-092313. [DOI] [PubMed] [Google Scholar]
- Quatman-Yates CC, Bonnette S, Hugentobler JA, Mede B, Kiefer AW, Kurowski BG, Riley MA. Postconcussion Postural Sway Variability Changes in Youth: The Benefit of Structural Variability Analyses. Pediatr Phys Ther. 2015;27(4):316–327. doi: 10.1097/PEP.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quatman-Yates CC, Lee A, Hugentobler JA, Kurowski BG, Myer GD, Riley MA. Test-retest consistency of a postural sway assessment protocol for adolescent athletes measured with a force plate. Int J Sports Phys Ther. 2013;8(6):741–748. [PMC free article] [PubMed] [Google Scholar]
- Quatman-Yates CC, Quatman CE, Meszaros AJ, Paterno MV, Hewett TE. A systematic review of sensorimotor function during adolescence: a developmental stage of increased motor awkwardness? Br J Sports Med. 2011 doi: 10.1136/bjsm.2010.079616. doi: bjsm.2010.079616 [pii]10.1136/bjsm.2010.079616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdani S, Seigle B, Lagarde J, Bouchara F, Bernard PL. On the use of sample entropy to analyze human postural sway data. Med Eng Phys. 2009;31(8):1023–1031. doi: 10.1016/j.medengphy.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Riley MA, Balasubramaniam R, Turvey MT. Recurrence quantification analysis of postural fluctuations. Gait Posture. 1999;9(1):65–78. doi: 10.1016/s0966-6362(98)00044-7. doi: S0966-6362(98)00044-7 [pii] [DOI] [PubMed] [Google Scholar]
- Riley MA, Turvey MT. Variability and determinism in motor behavior. J Mot Behav. 2002;34(2):99–125. doi: 10.1080/00222890209601934. [DOI] [PubMed] [Google Scholar]
- Schlenstedt C, Muthuraman M, Witt K, Weisser B, Fasano A, Deuschl G. Postural control and freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. 2016;24:107–112. doi: 10.1016/j.parkreldis.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Schmit JM, Riley MA, Dalvi A, Sahay A, Shear PK, Shockley KD, Pun RY. Deterministic center of pressure patterns characterize postural instability in Parkinson’s disease. Exp Brain Res. 2006;168(3):357–367. doi: 10.1007/s00221-005-0094-y. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Woollacott MH. The growth of stability: postural control from a development perspective. J Mot Behav. 1985;17(2):131–147. doi: 10.1080/00222895.1985.10735341. [DOI] [PubMed] [Google Scholar]
- Steindl R, Kunz K, Schrott-Fischer A, Scholtz AW. Effect of age and sex on maturation of sensory systems and balance control. Dev Med Child Neurol. 2006;48(6):477–482. doi: 10.1017/S0012162206001022. [DOI] [PubMed] [Google Scholar]
- Stergiou N, Harbourne R, Cavanaugh J. Optimal movement variability: a new theoretical perspective for neurologic physical therapy. J Neurol Phys Ther. 2006;30(3):120–129. doi: 10.1097/01.npt.0000281949.48193.d9. [DOI] [PubMed] [Google Scholar]
- Wolff DR, Rose J, Jones VK, Bloch DA, Oehlert JW, Gamble JG. Postural balance measurements for children and adolescents. J Orthop Res. 1998;16(2):271–275. doi: 10.1002/jor.1100160215. [DOI] [PubMed] [Google Scholar]


