Abstract
Rationale
Initial sensitivity to drugs of abuse often predicts subsequent use and abuse but this relationship is not always observed in human studies. Moreover, studies examining the relationship between initial locomotor sensitivity and the rewarding and reinforcing effects of drugs in animal models have also been equivocal. Understanding the relationship between initial drug effects and propensity to continue use, potentially resulting in the development of a substance use disorder, may help to identify key targets for prevention and treatment.
Objectives
We examined intravenous cocaine self-administration in a set of mouse strains that were previously identified to be at the phenotypic extremes for cocaine-induced locomotor activation to determine if initial locomotor sensitivity predicted acquisition, extinction, dose response or progressive ratio (PR) breakpoint.
Methods
We selected 8 inbred mouse strains based on locomotor sensitivity to 20mg/kg cocaine. These strains, designated as low and high responders, were tested in an intravenous self-administration paradigm that included acquisition of 0.5 mg/(kg*inf) under a FR1 schedule, extinction, re-acquisition, dose response to 0.125, 0.25, 0.5, 1 and 2 mg/(kg*inf) and progressive ratio.
Results
We observed overall differences in self-administration behavior between high and low responders. Low responders self-administered less cocaine and had lower breakpoints under the PR schedule. However, we also observed strain differences within each group. Self-administration in the low responder, LG/J, more closely resembled the behavior of the high-responding group and the high responder, P/J, had self-administration behavior that more closely resembled the low-responding group.
Conclusions
We conclude that acute cocaine-induced locomotor activation does predict self-administration behavior, but in a strain-specific manner. These data support the idea that genetic background influences the relationship among addiction-related behaviors.
Keywords: Cocaine, inbred mice, locomotor sensitivity, intravenous self-administration
Introduction
Substance use disorders (SUDs) are a significant health concern worldwide and result in considerable suffering for affected individuals and their families. Moreover, SUDs are now the leading cause of accidental death and are associated with a tremendous societal burden, including increased health care and criminal justice expenditures and lost productivity (Miller & Hendrie, 2008). Billions of dollars are spent each year to combat the consequences of drug use, yet there are very few effective treatment options or prevention strategies and no clear understanding of the underlying etiology of addiction ((CASA), 2009). A better understanding of biological mechanisms that increase risk for addiction and contribute to relapse would aid in the development of more effective treatments.
SUDs are multi-stage disorders that include initial drug exposure, increased drug taking, transition from controlled to compulsive use and cycles of withdrawal and relapse. Great strides have been made in understanding the neural and molecular pathways in all stages of addiction (Koob & Volkow, 2010; Russo et al., 2010; Volkow, Koob, & Baler, 2015), but studying the entire spectrum in humans is difficult due to lack of control over the environment, including previous drug exposures, and the inherent heterogeneity of the disease. As a result, animal models have become a useful tool for studying addiction-related behaviors (Crabbe, 2016; Lynch, Nicholson, Dance, Morgan, & Foley, 2010). Animal models provide the ability to control genetic background and the environment (Crabbe, 2016; Falcone, Lee, Lerman, & Blendy, 2016; Vargas-Irwin, van den Oord, Beardsley, & Robles, 2006); both of which contribute significantly to increased risk for addiction (Robison & Nestler, 2011; Volkow & Morales, 2015).
Animal models have been developed to interrogate the addiction cycle from initial sensitivity (measured by locomotor activation) to intravenous self-administration (IVSA) protocols that measure acquisition, extinction, reinstatement and motivation to obtain the drug (Campbell & Carroll, 2000; Katz & Higgins, 2003; D. C. Roberts, Morgan, & Liu, 2007). Although animal models of addiction have a genetic basis, very few genes have been identified (Kumar et al., 2013; Yazdani et al., 2015). Moreover, the field lacks a thorough understanding of shared and unique genetic and biological pathways that contribute to each behavior.
A link between initial sensitivity to psychostimulants and the rewarding and reinforcing effects of drugs has been reported in humans (de Wit & Phillips, 2012) and rodents (Deminiere, Piazza, Le Moal, & Simon, 1989; Yamamoto et al., 2013). In rodents, correlations between initial sensitivity and drug reward and reinforcement have not been consistently observed (de Wit & Phillips, 2012; Mandt, Johnston, Zahniser, & Allen, 2012); possibly due to different genetic backgrounds used across studies. Furthermore, a link between initial sensitivity and the development of drug dependence has not been conclusively established in either species (de Wit & Phillips, 2012).
We recently published a survey of initial sensitivity to the locomotor stimulating effects of cocaine in a panel of 45 inbred mouse strains and found significant strain effects in locomotor response (Wiltshire et al., 2015). In order to investigate the relationship between locomotor sensitivity and drug reward and reinforcement behavior, we identified several high and low responding strains and tested them in an expanded self-administration protocol that included acquisition, extinction, dose response, re-acquisition and motivation to self-administer under a progressive ratio schedule of reinforcement. The design of our IVSA protocol allowed us to compare initial sensitivity and various drug-taking behaviors on stable, yet diverse genetic backgrounds to study the relationship between these two animal models of addiction-like behavior.
Materials and Methods
Animals
C57BL/6J (B6), C57BR/cdJ, I/LnJ, P/J, LG/J, FVB/NJ, LP/J and BTBR T+ tf/J male mice were purchased from the Jackson Laboratory (Bar Harbor, ME) at 5–7 weeks of age and shipped to The Scripps Research Institute’s (TSRI) Mouse Behavioral Assessment Core in La Jolla, CA for self-administration or the University of North Carolina (UNC) for locomotor dose response studies. Only male mice were tested for comparison to previously published data on initial sensitivity to cocaine (Wiltshire et al., 2015). Mice were maintained in AAALAC-accredited vivaria under sanitary conditions in ventilated cages (Scripps, Ancare, NY; UNC, Tecniplast, PA). At both locations, temperature was controlled at 21 +/− 2°C. At TSRI, mice were on a reverse 12-hr light cycle (8:00PM on, 8:00AM off). At UNC, mice were on a 12-hr light cycle with lights on at 7:00AM. All mice had access to cotton nestlets for enrichment. Food (TSRI; Harlan Teklad LM-485, Harlan, Indianapolis, IN, UNC; Pico rodent chow 20; Purina, St. Louis, MO, USA) and water were provided ad libitum. Catheterization surgeries were performed when mice were 8–9 weeks of age. Mice were group housed throughout locomotor dose response studies. For self-administration studies, mice were group housed prior to surgery and then individually housed for the duration of the experiment. A number of mice were eliminated from the self-administration study at various time points due to loss during or after catheterization surgery, inability to acquire cocaine self-administration or loss of catheter patency. Information on mouse numbers from onset of the self-administration study through progressive ratio testing is provided in Table 1. Any strains with 2 or fewer mice remaining were excluded from statistical analyses.
Table 1.
Number of mice from arrival at the Scripps Research Institute through completion of progressive ratio testing including percent meeting acquisition criteria. Attrition after acquisition was mainly due to loss of patency
| Arrived | Survived Surgery | Survived Recovery | Completed Acquisition | Failed to Meet Criteria | Pct Meeting Criteria | Extinction | Reacquisition | Dose Response | Progressive Ratio | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low Responders | BTBR T+ tf/J | 20 | 19 | 17 | 14 | 5 | 64 | 5 | 4 | 3 | 3 |
| FVB/NJ | 40 | 35 | 34 | 24 | 18 | 25 | 6 | 1 | 1 | 0 | |
| LG/J | 28 | 24 | 17 | 17 | 4 | 76 | 13 | 8 | 7 | 4 | |
| LP/J | 32 | 26 | 24 | 22 | 12 | 45 | 9 | 4 | 4 | 3 | |
| High Responders | C57BL/6J | 58 | 49 | 44 | 36 | 8 | 78 | 24 | 12 | 10 | 5 |
| C57BR/cdJ | 20 | 13 | 13 | 13 | 3 | 77 | 10 | 4 | 4 | 2 | |
| I/LnJ | 19 | 16 | 15 | 15 | 0 | 100 | 14 | 10 | 10 | 10 | |
| P/J | 19 | 18 | 17 | 16 | 6 | 63 | 8 | 4 | 2 | 1 |
Drugs
For locomotor behavior and self-administration experiments, cocaine hydrochloride (Sigma-Aldrich, St. Louis, MO) was dissolved in 0.9% saline. For locomotor dose response experiments, cocaine or saline were administered by intraperitoneal (IP) injection at a volume of 0.01ml/g. For self-administration experiments, mice were injected intravenously (IV) with 15 μL solution at each occurrence with varying concentrations for different doses as well as to account for strain differences in body weights. A ketamine/midazolam combination (15 mg/ml; Ketathesia, Butler and 0.75 mg/ml; Hospira) was used for catheter patency checks each week.
Dose response of locomotor behavior
All of the low responding strains listed above and two of the high responding strains (B6 and I/LnJ) were tested for locomotor activation over a range of doses lower and higher than the 20 mg/kg dose reported in our previous publication (Wiltshire et al., 2015). The C57BR/cdJ and P/J strains were either unavailable or in short supply from the Jackson Laboratory at the time of we conducted these studies and were, therefore, excluded. The procedure for measuring locomotor response to cocaine is described in detail in the Wiltshire et al. paper (Wiltshire et al., 2015). Briefly, mice were tested over 3 days; on Days 1 and 2, mice were injected with saline and placed in an open field apparatus (ENV-515-16, Med Associates, Fairfax, VT, USA) for 30-minutes. On Day 3, mice were injected with either saline or one of 4 doses of cocaine (5, 10, 30 and 40 mg/kg) and placed in the open field for 30-minutes. Locomotor activity measured as distance moved in centimeters was measured by infrared detection. Locomotor response to cocaine was expressed as a difference score by subtracting basal activity on Day 2 from cocaine-induced locomotor activity on Day 3. All behavioral testing occurred between the hours of 8AM – 12PM.
Intravenous Self Administration
Catheters were surgically placed into the jugular vein as previously described (A. J. Roberts, Polis, & Gold, 1997) and animals were allowed to recover for a minimum of 5 days prior to testing. Catheters were flushed with saline/heparin (3 USPU, 0.05ml) daily and mice were checked weekly (2 hours post self-administration testing on Fridays) for catheter patency. Mice not showing signs of sedation within 3 sec of intravenous ketamine/midazolam (0.02–0.04ml) administration were assumed to have malfunctioning catheters and were removed from the study.
Experimental sessions were conducted in mouse operant conditioning chambers (ENV-307W, Med Associates). Each chamber is equipped with two levers, above which are cue lights, linked to software (MED-PC, Med Associates) that controls infusions. The active lever was at the front of the operant chamber and was kept constant throughout the experiment. Presses of the active lever activated the infusion pump and cue light above the lever. The infusion pump provided 15 μL of cocaine hydrochloride over 3 sec in varying concentrations to the intravenous (IV) catheter via a liquid swivel and syringe. The cue light remained on for the 3 sec infusion as well as the ensuing 33 sec timeout period in which presses were recorded, but had no scheduled consequences. Inactive lever pressing also was recorded but had no scheduled consequences. Tygon microbore tubing connected the components of the infusion apparatus and was connected to the catheter port.
Acquisition trials of 1-hour duration were conducted for up to 10 days using a fixed ratio 1 (FR1) schedule of reinforcement for a 0.5 mg/(kg*inf) cocaine with a 33 sec timeout period. All mice were primed with a cocaine infusion on their first session. Mice were moved to the next phase once they were obtaining more than 8 infusions per session, or at least 5 infusions per session for FVB/NJ mice, were making > 75% of their responses on the active lever and when infusions on 2 consecutive days were within 20% of each other. See Table 1 for numbers of mice achieving these criteria. Baseline responding was defined as the average number of infusions received over the final 2 days of acquisition.
After achieving acquisition criteria, mice started an extinction protocol. Extinction was conducted over 10 days in 1-hour sessions with an FR1 schedule. The infusion pump was turned off, mice were not connected to the pump tubing and the cue-light was inactive. Successful extinction occurred when mice pressed the active lever at less than half the level of baseline infusions during the last two days of acquisition. Mice continued the extinction phase for no more than 10 days or until they met criteria if that occurred prior to 10 days.
After extinction, mice were re-exposed to trials using an FR1 schedule and 0.5 mg/kg cocaine for up to three days. Following reacquisition, dose response was measured during daily 1-hour sessions on an FR1 schedule. Cocaine doses (0.125, 0.25, 0.5, 1 and 2 mg/kg/inf) were randomly introduced by subject in a Latin square design. Only one dose was tested each day.
Following dose response testing, mice were reacclimated to a standard dose of 0.5 mg/kg during 2 daily 1-hour sessions using a FR1 schedule. Mice were then tested in a progressive ratio (PR) schedule to examine reward strength. Presses required=(5 •einjection #•0.20) −5, rounded to the nearest integer; the first few values in the series were 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, etc. Mice were removed from the PR schedule session when they failed to press any lever for more than 2 hours. See Table 1 for numbers of mice making it through each of these phases.
Assessment of lever-pressing behavior in B6 and I/LnJ strains
In order to investigate the high rate of lever pressing observed in I/LnJ mice during extinction and progressive ratio testing, we examined this behavior in a group of male I/LnJ (N=12) and B6 (N=12) mice. Mice that were naïve for behavioral testing and cocaine exposure and that had not been implanted with catheters were placed into the self-administration chambers on each of 4 consecutive days for a one-hour session. No cue lights were present and lever-pressing behavior was quantified. Three days after the final one-hour session, mice were placed into the self-administration chambers under the same conditions described above for a single 6-hr session to mimic the length of a progressive ratio session and lever-pressing behavior was quantified.
Statistical Analysis
Data were analyzed using SPSS® Statistics package (IBM®, Windows v. 21 or Macintosh v. 16). Individual strain and group (high or low locomotor activation, see below) effects on self-administration behaviors were analyzed by one-way ANOVA. Repeated measures ANOVA were used for analysis of IVSA dose response data and B6 and I/LnJ lever pressing studies. Significant differences involving group or strain were further evaluated by post-hoc Tukey’s HSD. Significance for all comparisons was set at α=0.05.
Results
Grouping of strains based on initial locomotor sensitivity to cocaine
Strains were grouped as either high or low responders based on their locomotor response to a single, acute dose of 20mg/kg cocaine from the previously published study by (Wiltshire et al., 2015). C57BL/6J (B6), C57BR/cdJ, I/LnJ and P/J were high responders and LG/J, FVB/NJ, LP/J and BTBR T+ tf/J were low responders.
Dose response of locomotor behavior
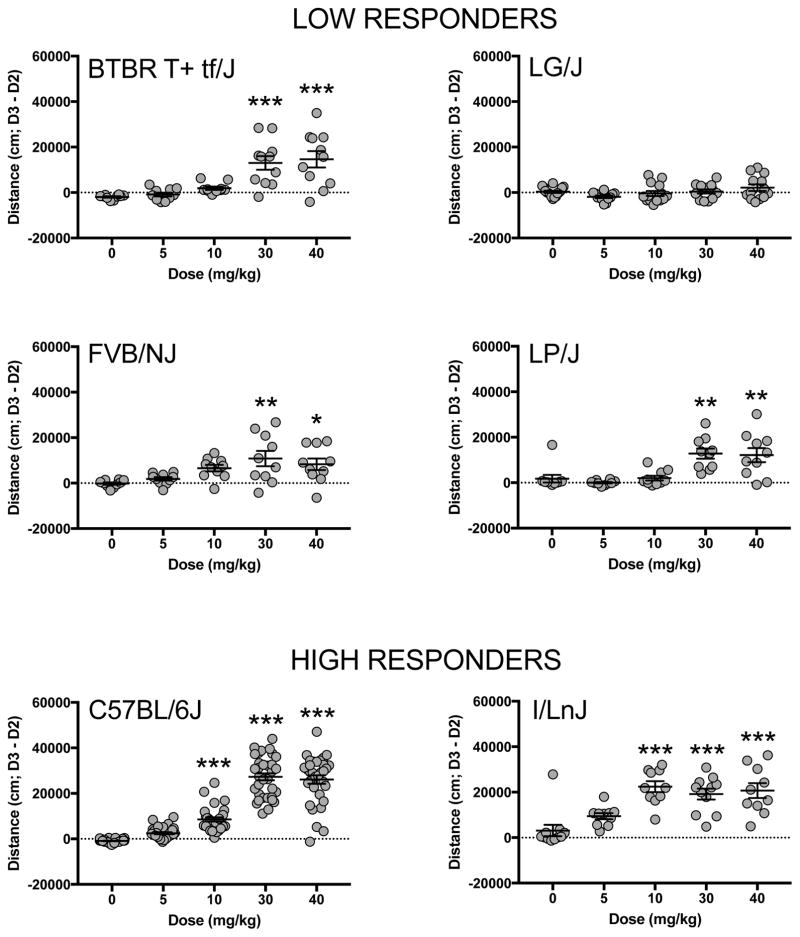
Significant strain (F(6,423) = 52.7;p<0.001), dose (F(4,423) = 74.8;p<0.001) and strain by dose interaction (F(24,423) = 10.0;p<0.001) effects were observed for locomotor response to cocaine as measured by the difference score between locomotor activity on day 3 minus day 2. Post hoc analyses indicate that no strain showed significant locomotor activation to 5mg/kg cocaine in comparison to saline. Both B6 and I/LnJ strains showed significant locomotor activation at 10 mg/kg but low responding strains only showed significant locomotor response to cocaine at 30 and 40 mg/kg (Fig 1). B6 and I/LnJ did not differ from each other at either the 30 or 40 mg/kg dose, however, the low responding strains did have significantly lower cocaine-induced activation compared to B6 at both doses. I/LnJ mice also had a higher locomotor response than low responders at 30 and 40 mg/kg, but the difference was not statistically significant (Supplemental Fig 1).
Figure 1. Dose response of cocaine locomotor activation in high and low responding inbred mouse strains.
Locomotor response to cocaine displayed as the difference between locomotor activity on Day 3 minus Day 2. Each data point represents an individual animal and error bars are standard error of the mean. Asterisks denote significant difference from saline control. *p<0.05, **p<0.01, ***p<0.001.
Acquisition
The percent of mice meeting criteria for acquisition varied across strains (Table 1). FVB/NJ had the fewest mice meeting acquisition criteria (25%) even though the acquisition criteria used for this strain was lower than for the other strains. I/LnJ mice had the highest number of mice meeting criteria (100%). In general, fewer mice met criteria in the low responder group (average 53%) compared to the high responder group (average 79%).
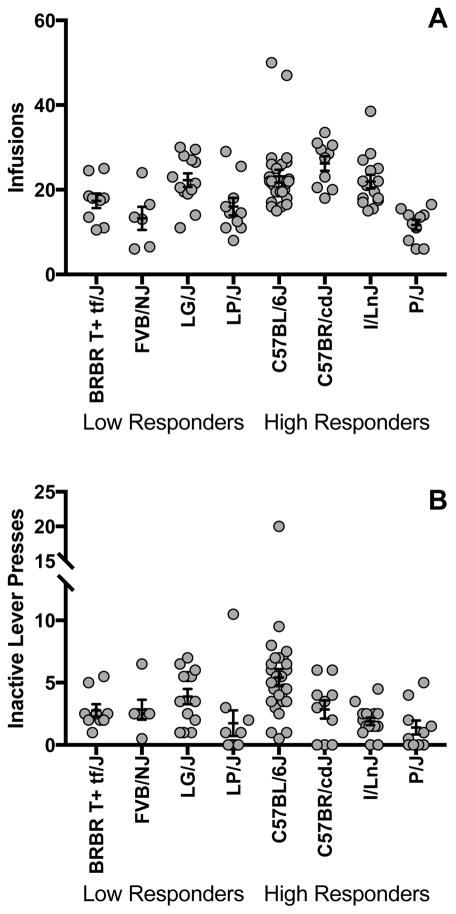
There was no significant strain difference in rate of acquisition (F(7,100) = 1.2; p>0.05) and no significant difference was observed between high and low responding groups on this measure (F(1,100) = 0.1; p>0.05). However, a significant difference was observed for average number of infusions over the last two days of acquisition (Fig 2A) for both group (F(1,100) = 5.2; p<0.05) and strain (F(7,100) = 6.8; p<0.001). As a group, low responders self-administered less cocaine than high responders, but this finding was not consistent across individual strains in each group. Post hoc analyses revealed that the low-responding strain, LG/J, more closely resembled the self-administration behavior of the high-responding group and the high responder, P/J, more closely resembled the low-responding group (Fig 2A). There were no significant differences between the high- and low-responding groups for inactive lever presses (F(1,100) = 1.1; p>0.05) although significant strain effects were observed (Fig 2B, (F(7,100) = 4.7; p<0.001)).
Figure 2. Acquisition of cocaine self-administration.
Average number of infusions during the last two days of acquisition (A) and number of inactive lever presses (B). Each data point represents an individual animal and error bars are standard error.
Extinction
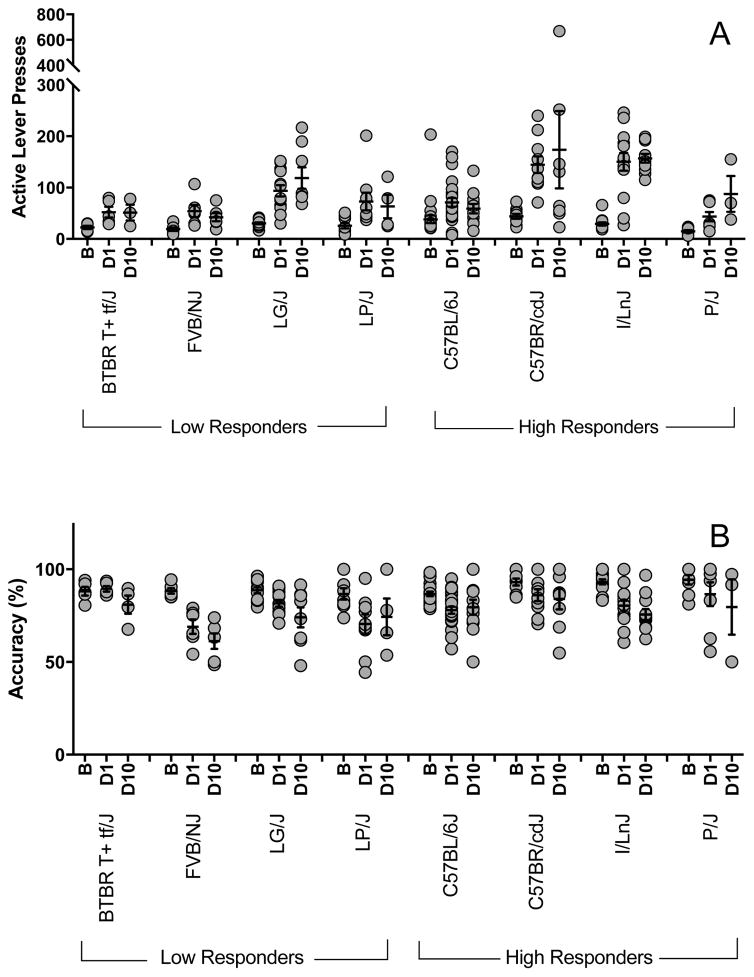
After successfully acquiring FR self-administration, mice were exposed to a 10-day extinction protocol. All strains showed a significant burst of responding on the active lever on the first day of extinction (D1, F(1,177) = 82.4; p<0.001) compared to the average number of active lever presses on the final two days of acquisition (B, Fig 3A).
Figure 3. Extinction of self-administration.
(2A) Baseline (“B”) number of active lever presses during the last two days of acquisition compared to active lever presses on Day 1 (“D1”) and Day 10 (“D10”) of the extinction protocol. (2B) Percent accuracy responding on the active lever at acquisition baseline, Day 1 and Day 10 of extinction. Each data point represents an individual animal and error bars are standard error of the mean.
Of the 68 mice that completed the extinction protocol (maintained patency), only 14 (21%) met the criteria for extinction by day 10. Successful extinction of self-administration behavior varied across strains and ranged from 0% (BTBR T+ tf/J, FVB/NJ and I/LnJ) to 43% (LP/J). There were significant strain differences for both active (F(7,714) = 40.3; p<0.001) and inactive (F(7,713) = 29.5; p<0.001) lever pressing during extinction, but no significant decrease in responding on either lever over the 10-day protocol (D10, Fig 3A, active: (F(9,713) = 1.2; p>0.05) and inactive: (F(9,713) = 0.93; p>0.05)). There was a significant decrease in active lever discrimination on the first day of extinction training compared to average accuracy during the last two days of acquisition regardless of strain (F(1,177) = 40.0;p<0.001; Fig 3B). However, after the initial decrease, discrimination remained stable across the 10 day protocol (F(9,712) = 1.47; p>0.05).
Re-acquisition
Although less than a quarter of the mice extinguished self-administration behavior, all were exposed to up to 3 days of re-acquisition. A two-way ANOVA of strain by test session indicated that both inactive and time out lever pressing decreased in frequency from the last day of extinction (D10) to the final day of re-acquisition (R, inactive: F(1,91) = 23.0;p<0.001 and time out: F(1,91) = 10.3; p<0.01). A significant strain by test session interaction was also observed for both behaviors (inactive: F(6,91) = 4.8;p<0.01 and time out: F(6,91) = 2.9; p<0.05) and post hoc analyses revealed that only one strain, I/LnJ, showed a significant reduction in both behaviors (Supplemental Figure 2A & 2B). Discrimination for the active lever also improved across all strains during reacquisition in comparison to the final day of extinction (F(1,90) = 15.3; p<0.001:data not shown).
Dose response
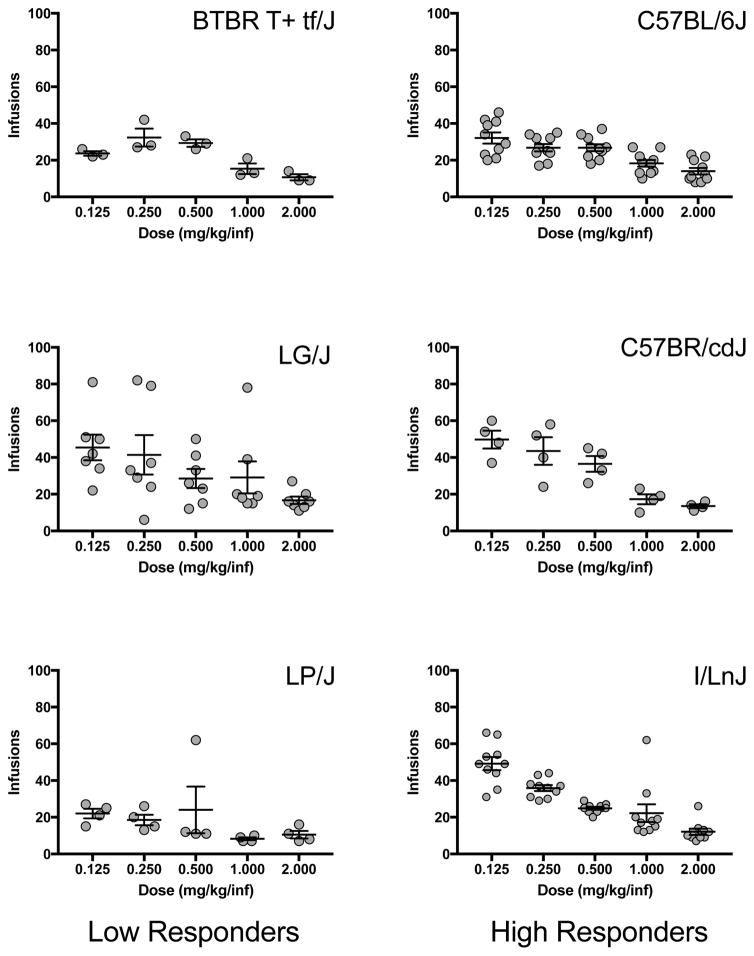
Dose response data were analyzed by repeated measures ANOVA to determine the effects of strain and group (high vs. low responders) on responding for the 5 cocaine doses. Only strains with at least 3 mice completing the dose response portion of the study were included in the analysis (high responders – C57BL/6J, C57BR/cdJ, I/LnJ & low responders – BTBR T+tf/J, LG/J, LP/J (Fig 4). There was an overall effect of strain (F(5,32) = 3.0; p<0.05) as well as a strain by dose interaction (F(20,128) = 2.1; p<0.05). These effects were mostly driven by a large strain difference in responding for the lowest cocaine dose (0.125 mg/kg/infusion) and a moderate strain difference in responding for the second lowest cocaine dose (0.25 mg/kg/infusion). LP/J and BTBR T+tf/J mice took less cocaine at the lowest dose than LG/J, I/LnJ, and C57BR/cdJ mice (p<0.01) and LP/J mice took less cocaine at the 0.25 mg/kg/infusion dose than LG/J, I/LnJ, and C57BR/cdJ mice (p<0.05). There was no significant effect of group (low vs high responders) on the dose effect curve; however, the low responding strains, LP/J and BTBR T+tf/J, took less cocaine than high responding C57BR/cdJ and I/LnJ strains.
Figure 4. Dose response.
Number of infusions of cocaine in 3 low responding strains (BTBR T+ tf/J, LG/J and LP/J) and 3 high responding strains (C57BL/6J, C57BR/cdJ and I/LnJ) at 5 doses. Each data point represents an individual animal and error bars are standard error of the mean.
Looking at the dose response data individually for each strain, only the LP/J mice showed no significant effect of dose, suggesting that cocaine is not reinforcing in this strain. The other strains showed effects of dose on responding, with significantly reduced infusions at the highest dose(s).
Progressive ratio
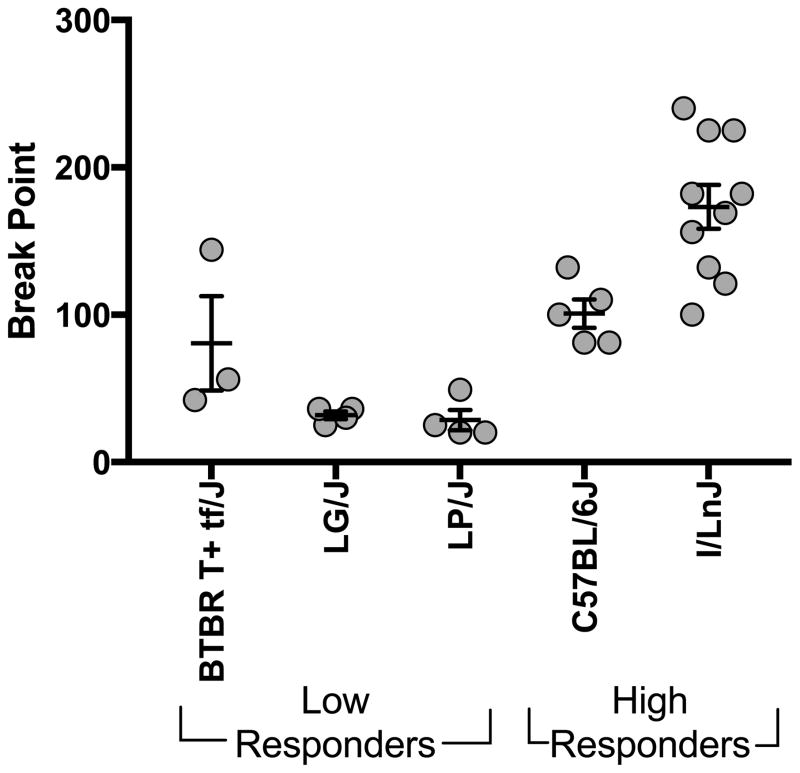
As a group, low responders had significantly lower breakpoints (F(1,25 = 32.5; p<0.001) than high responders. Individually, the strains also differed significantly for breakpoint (F(4,25) = 17.1; p<0.001). Post hoc Tukey’s analysis indicated that the I/LnJ strain had a higher breakpoint than the low responding strains, LG/J and LP/J, but did not differ significantly from C57BL/6J (Fig 5).
Figure 5. Self-administration under progressive ratio schedule.
Break point during the progressive ratio session. Each data point is an individual animal and error bars are standard error of the mean.
Lever pressing behavior in B6 and I/LnJ strains
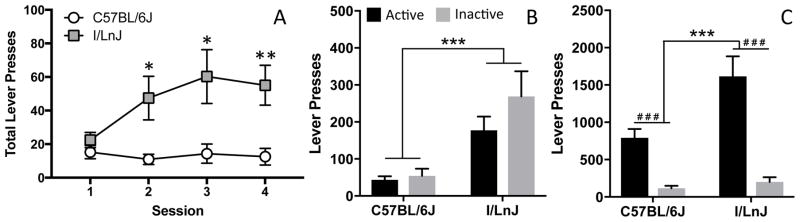
Lever pressing behavior during the 4 one-hour sessions was analyzed using repeated measures ANOVA to determine the effect of strain. There was an overall effect of session (F(3,66) = 4.0; p<0.05) and a session by strain interaction (F(3,66) = 4.9; p<0.01). I/LnJ mice exhibit more lever pressing than B6 mice during each session. While lever pressing behavior in B6 mice was consistent across all sessions, lever pressing increased across sessions in I/LnJ mice, although this increase was not significant (Fig 6A, F(3,47) = 1.9; p = 0.14). Lever pressing during the 6-hr session was analyzed by ANOVA with strain and lever (left vs. right lever) as independent variables. I/LnJ mice also showed significantly higher lever pressing behavior during the 6-hr session in comparison to B6 mice (Fig 6B, F(1,47) = 18.9; p<0.001) but this behavior was not specific for either lever (Fig 6B, F(1,47) = 1.6; p = 0.21). These data are in contrast to the high specificity for the active lever in both strains observed during the 6-hr PR protocol during which mice received an infusion of the drug in response to lever pressing (Fig 6C). These data indicate that although I/LnJ mice show significantly higher lever pressing in the absence of the drug, they are able to distinguish rewarded drug responses after exposure to cocaine.
Figure 6.
Assessment of lever pressing behavior in C57BL/6J and I/LnJ mice during (A) four one-hour sessions and (B) a single six-hour session. Six hour data are compared to (C) lever pressing during the progressive ratio session for the same strains. Error bars are SEM. ***p<0.001 difference between B6 and I/LnJ. # # # p<0.001 difference between active and inactive lever.
Discussion
Evidence from human studies often indicates that the subjective response to an initial drug exposure predicts future drug use (Davidson, Finch, & Schenk, 1993; Haertzen, Kocher, & Miyasato, 1983; Lambert, McLeod, & Schenk, 2006). However, the relationship between initial subjective responses and drug dependence is less clear (de Wit & Phillips, 2012). Similarly, rodent studies have not always identified a significant association between initial locomotor sensitivity and behaviors that model the rewarding and reinforcing effects of drugs (de Wit & Phillips, 2012). Many of the previously published animal studies have been conducted using rat models and show positive, negative or no relationship between locomotor sensitivity and self-administration (reviewed in (Yamamoto et al., 2013). These diverse outcomes might be due to the heterogeneous genetic background and the combination of alleles that are present in these populations and contribute to high and low responding behaviors. We undertook the present study to examine the relationship between initial locomotor response to cocaine and IVSA behavior in stable and defined genetic backgrounds as a first step toward understanding genetic mechanisms underlying addiction-related animal behaviors.
Based on the differences observed across other studies, we hypothesized that although strains at extremes of the phenotypic distribution shared similar phenotypes for initial locomotor sensitivity to cocaine, the relationship between that behavior and IVSA would not be consistent due to the varied genetic backgrounds among the eight inbred strains. We found that 3 of the 4 strains that were high locomotor responders showed characteristics associated with increased cocaine reward, and, conversely, 3 of the 4 low sensitivity strains showed characteristics associated with reduced cocaine reward. Characteristics of increased cocaine reward included higher percentages of mice reaching criteria for self-administration, higher baseline self-administration levels, and higher breakpoints in the progressive ratio test. Attributes observed in 3 of 4 of the low responders were the opposite; fewer achieving criteria for successful self-administration, lower baseline self-administration levels, and lower progressive ratio breakpoints.
One strain per sensitivity group exhibited cocaine self-administration behavior that differed from the others in that cluster. For example, LG/J mice had the lowest locomotor response to cocaine of any strain studied (Wiltshire et al., 2015) and that lack of response persisted across doses higher than 20mg/kg (Fig 1). However, LG/J mice self-administered cocaine at the same rate as most of the high responding strains during acquisition (Fig 2) indicating that locomotor sensitivity in this strain did not predict acquisition or maintenance of self-administration behavior. Interestingly, however, the progressive ratio breakpoint for the LG/J strain was similar to that of other low responding strains, BTBR T+ tf/J and LP/J, suggesting low motivation to obtain cocaine.
Conversely, P/J mice were among the highest locomotor responders to cocaine (Wiltshire et al., 2015) but self-administered fewer infusions than any of the other high responding strains during acquisition. Unfortunately, neither dose response nor breakpoint data were available for P/J mice as this strain was unable to maintain catheter patency throughout the entire IVSA protocol. The remainder of the strains showed a positive relationship between initial locomotor sensitivity and self-administration with high responders self-administering more infusions and achieving higher PR breakpoints. Identifying and exploring strain-specific convergent and divergent patterns for these two commonly used animal models of addiction-related behaviors was the impetus for these studies. These data will be used to design more in-depth experiments in these strains aimed at understanding the relationship between these behaviors on a deeper level.
Intravenous cocaine self-administration behavior for most of the inbred strains examined in this study has not previously been reported in the literature. Multiple publications have reported IVSA behavior for C57BL/6J mice and several other inbred strains including DBA/2J, BALB/cByJ and a few 129 substrains (Carney, Landrum, Cheng, & Seale, 1991; Deroche et al., 1997; Grahame & Cunningham, 1995; Grahame, Phillips, Burkhart-Kasch, & Cunningham, 1995; Griffin & Middaugh, 2003; Kuzmin & Johansson, 2000; A. J. Roberts et al., 1997; Thomsen & Caine, 2006, 2011a, 2011b) and, similar to our results, have observed significant strain differences. Previously published studies also highlight procedural differences that could affect interpretation of the data. The experimental procedures implemented here were chosen for several reasons.
Operant training for food reinforcement often precedes drug self-administration to facilitate acquisition of operant behavior. We performed no operant training prior to cocaine exposure as we were interested in measuring differences in acquisition of drug self-administration behavior. We did not want prior exposure to a food reinforcer to alter subsequent responding for cocaine. All of the strains were able to meet criteria for self-administration behavior; however, as we had anticipated, we did observe strain variation in the number of animals per strain that successfully acquired the behavior and designation as a low or high locomotor responder corresponded with success of acquisition.
Following acquisition, we exposed the animals to a series of sessions with no drug available to extinguish self-administration behavior. It has been recommended that an extinction phase followed by a reacquisition phase be inserted early in the experimental design for mouse intravenous self-administration studies in order to decrease the chance of false positives in acquisition criteria associated with non goal-directed persistence of initial high rates of responding (Thomsen & Caine, 2011a). This recommendation was prompted by the observation that C57BL/6J mice maintained a high rate of lever pressing for a prolonged period of time even in the absence of drug reinforcement (Thomsen & Caine, 2011a). Prolonged responding in the absence of cocaine has been observed across multiple studies in mice (Paneda et al., 2009; Thomsen & Caine, 2006; Thomsen, Han, Gu, & Caine, 2009; Ward, Rosenberg, Dykstra, & Walker, 2009) and our extinction data replicate these observations. However, successful extinction protocols in mice have also been reported for cocaine (Grahame et al., 1995; Gutierrez-Cuesta et al., 2014; Nugent, Anderson, Larson, & Self, 2017; Thanos et al., 2011) nicotine (Contet, Whisler, Jarrell, Kenny, & Markou, 2010) and methamphetamine (Sharpe, Varela, Bettinger, & Beckstead, 2014; Yan, Nitta, Mizoguchi, Yamada, & Nabeshima, 2006)). It bears noting that although all of the studies referenced above used C57BL/6 mice, the particular self-administration and extinction protocols used varied. Response manipulanda (nose poke vs lever pressing), extinction criteria and experimental protocol leading up to extinction may all affect both success and the speed at which mice extinguish.
It is possible that inserting extinction sessions after acquisition and before dose response could alter dose response in a strain specific manner, making interpretation of our data more complicated. We do not think that extinction weakened self-administration behavior since all strains return to “pre-extinction” levels of responding after extinction trials (Supplemental Fig 2C). Although we observed a trend for increased responding post-extinction, the strain order from acquisition was maintained at re-acquisition with the exception of the P/J strain that showed a greater increase in responding post extinction than did other strains (Supplemental Fig 2C).
The presence of cues associated with drug administration can also result in maintenance of lever pressing across many days of extinction (Olsen & Winder, 2009). However, our extinction protocol included neither cue lights nor saline infusions (pump noise) and, thus, we do not believe that the high level of responding was cue-induced. Mice show several spontaneous perseverative behaviors as well repetitive and stereotyped actions during interactions with artificial objects (Ahmari, 2016), which may explain persistent lever pressing behavior. It is also possible that strain differences in lever pressing may contribute to differences in self-administration behavior. In fact, we have observed significant differences in lever pressing between B6 and I/LnJ mice exposed to levers with no prior or current consequences (Fig 6A). Although both strains show similar behavior upon the first exposure, I/LnJ mice increased lever pressing upon repeated exposures. However, this lever pressing behavior is directed equally to both “active” and “inactive” levers (Fig 6B) whereas after exposure to cocaine, we observed a high level of specificity for the active lever (Fig 6C). These data suggest that differences in responding could be due to a combination of the drug and other, as yet unexplored, strain-specific behaviors that will need to be more completely characterized in future studies.
It has been suggested that in the absence of extinction, additional criteria be evaluated in order to determine whether cocaine served as a positive reinforcer (Thomsen & Caine, 2011a). Importantly, we did observe a burst of responding on the first day of the extinction protocol and a decrease in discrimination for the active lever. These observations along with improved discrimination for the active lever during reacquisition provide evidence that the mice did distinguish drug availability from no drug availability.
Visual cues alone can also act as reinforcers (Olsen & Winder, 2009) and strain differences in responding to the cue light may contribute to some of the strain effects we observed. Data from a small study in our laboratory indicate that B6 mice increase lever pressing in response to a light cue but that this behavior is not maintained beyond 3 sessions indicating that the cue light itself may not be reinforcing (data not shown). In the present study we did not include an experimental group that was exposed to the cue light in the absence of cocaine and we cannot rule out the possibility that the light may be acting alone or additively with cocaine to increase responding on the active lever. A study by Contet et al. (Contet et al., 2010) suggests that even though rodents will respond to visual stimuli in a manner that mimics responding for a drug reinforcer, patterns of responding differ and can be used to interrogate cue vs drug responding. Although not currently the norm, future studies with inbred strains should include a “cue only” control group along with detailed analysis of selectivity for the active lever and time-out responding to allow for assessment of strain specific differences in salience of the visual cue.
Even in the face of these caveats, our data provide a starting point from which to study shared and unique genetic influences on initial sensitivity and the rewarding and reinforcing effects of psychomotor stimulants. We observed a relationship between locomotor response to cocaine and its reinforcing effects in the self-administration assay. However, we focused on extreme locomotor responders and that may have biased our study toward the observation of a positive phenotypic relationship (Preacher, Rucker, MacCallum, & Nicewander, 2005). Additional studies examining more strains across the phenotypic distribution are required to gain a more complete understanding of the relationship between these two animal models of drug response. We can expand our understanding of the relationship between locomotor sensitivity and self-administration, and probe shared and distinct mechanisms by expanding these studies to include additional strains including those reported by Wiltshire et al. (Wiltshire et al., 2015). Moreover, these studies provide information on strain differences that can be used to design genetic crosses for identifying underlying genes.
In addition to standard inbred strains, new experimental populations have been developed to maximize genetic diversity and provide a tool for systems genetics approaches, such as the Collaborative Cross or Diversity Outbred (Churchill et al., 2004; Dickson et al., 2015). These populations would also provide an additional platform on which to continue genetic and mechanistic studies. Only one of the 8 inbred founder strains for the Collaborative Cross and Diversity Outbred, C57BL/6J, was examined in our self-administration study. Assessing self-administration behavior in the remainder of the founder strains would be the first step in moving forward with these unique populations. For any future studies, our data indicate that careful consideration of experimental sample sizes will be necessary based on strain differences in variance observed for these behaviors.
Our study was limited to 8 inbred strains, but represents the first report of intravenous cocaine self-administration behavior for many of the strains. Even among these 8 strains, we observed complex relationships between the locomotor stimulating effects of cocaine and its reinforcing properties. Obviously, additional studies are necessary to both replicate and expand upon these findings. The genetic and genomic tools available in the mouse are ever expanding and provide unique and innovative opportunities for examining gene function and mechanistic relationships between gene and phenotype. In addition, these tools are also expanding at a rapid rate in other model organisms, particularly the rat (Parker et al., 2014). We believe that a growing body of data from rats and mice as well as other experimental models can only be beneficial and will continue to inform the field.
With our data as a starting point, we can begin to untangle the relationships between initial locomotor sensitivity and the rewarding and reinforcing properties of cocaine using the genetic and genomic tools available in animal models that are more accessible for understanding brain development and function. As our understanding of these processes becomes clearer, we can apply this knowledge to the human condition to develop better preventative and therapeutic strategies.
Supplementary Material
Cocaine-induced locomotor activation dose response for saline control, 30 and 40 mg/kg. Asterisks indicate significant difference from C57BL/6J at the same dose. Error bars are SEM.
Number of inactive lever presses (A) and time out responding (B) during the last day of extinction (“D10”) and the final day of re-acquisition (“R”). Data points are individual animals and error bars are SEM. (C) Number of infusions self-administered on the last day of acquisition and during reacquisition. Each data point is a strain mean.
Acknowledgments
Funding Statement: The work described in this manuscript was funded by a grant from the National Institute on Drug Abuse, R01DA023690
Footnotes
Conflict of Interest: The authors declare no conflicts of interest
References
- (CASA), National Ctr on Addiction and Substance Abuse at Columbia University. Shoveling Up II: the Impact of Substance Abuse on Federal, State and Local Budgets. New York, NY: Columbia University; 2009. [Google Scholar]
- Ahmari SE. Using mice to model Obsessive Compulsive Disorder: From genes to circuits. Neuroscience. 2016;321:121–137. doi: 10.1016/j.neuroscience.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell UC, Carroll ME. Acquisition of drug self-administration: environmental and pharmacological interventions. Exp Clin Psychopharmacol. 2000;8(3):312–325. doi: 10.1037//1064-1297.8.3.312. [DOI] [PubMed] [Google Scholar]
- Carney JM, Landrum RW, Cheng MS, Seale TW. Establishment of Chronic Intravenous Drug Self-Administration in C57bl/6j Mouse. Neuroreport. 1991;2(8):477–480. doi: 10.1097/00001756-199108000-00017. [DOI] [PubMed] [Google Scholar]
- Churchill GA, Airey DC, Allayee H, Angel JM, Attie AD, Beatty J … Complex Trait, Consortium. The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nat Genet. 2004;36(11):1133–1137. doi: 10.1038/ng1104-1133. [DOI] [PubMed] [Google Scholar]
- Contet C, Whisler KN, Jarrell H, Kenny PJ, Markou A. Patterns of responding differentiate intravenous nicotine self-administration from responding for a visual stimulus in C57BL/6J mice. Psychopharmacology (Berl) 2010;212(3):283–299. doi: 10.1007/s00213-010-1950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC. Progress With Nonhuman Animal Models of Addiction. J Stud Alcohol Drugs. 2016;77(5):696–699. doi: 10.15288/jsad.2016.77.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson ES, Finch JF, Schenk S. Variability in subjective responses to cocaine: initial experiences of college students. Addict Behav. 1993;18(4):445–453. doi: 10.1016/0306-4603(93)90062-e. [DOI] [PubMed] [Google Scholar]
- de Wit H, Phillips TJ. Do initial responses to drugs predict future use or abuse? Neurosci Biobehav Rev. 2012;36(6):1565–1576. doi: 10.1016/j.neubiorev.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deminiere JM, Piazza PV, Le Moal M, Simon H. Experimental approach to individual vulnerability to psychostimulant addiction. Neurosci Biobehav Rev. 1989;13(2–3):141–147. doi: 10.1016/s0149-7634(89)80023-5. [DOI] [PubMed] [Google Scholar]
- Deroche V, Caine SB, Heyser CJ, Polis I, Koob GF, Gold LH. Differences in the liability to self-administer intravenous cocaine between C57BL/6xSJL and BALB/cByJ mice. Pharmacology Biochemistry and Behavior. 1997;57(3):429–440. doi: 10.1016/S0091-3057(96)00439-X. [DOI] [PubMed] [Google Scholar]
- Dickson PE, Ndukum J, Wilcox T, Clark J, Roy B, Zhang L, … Chesler EJ. Association of novelty-related behaviors and intravenous cocaine self-administration in Diversity Outbred mice. Psychopharmacology (Berl) 2015;232(6):1011–1024. doi: 10.1007/s00213-014-3737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone M, Lee B, Lerman C, Blendy JA. Translational Research on Nicotine Dependence. Curr Top Behav Neurosci. 2016;28:121–150. doi: 10.1007/7854_2015_5005. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Cunningham CL. Genetic differences in intravenous cocaine self-administration between C57BL/6J and DBA/2J mice. Psychopharmacology (Berl) 1995;122(3):281–291. doi: 10.1007/BF02246549. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Phillips TJ, Burkhart-Kasch S, Cunningham CL. Intravenous cocaine self-administration in the C57BL/6J mouse. Pharmacol Biochem Behav. 1995;51(4):827–834. doi: 10.1016/0091-3057(95)00047-z. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Middaugh LD. Acquisition of lever pressing for cocaine in C57BL/6J mice: effects of prior Pavlovian conditioning. Pharmacol Biochem Behav. 2003;76(3–4):543–549. doi: 10.1016/j.pbb.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Cuesta J, Burokas A, Mancino S, Kummer S, Martin-Garcia E, Maldonado R. Effects of genetic deletion of endogenous opioid system components on the reinstatement of cocaine-seeking behavior in mice. Neuropsychopharmacology. 2014;39(13):2974–2988. doi: 10.1038/npp.2014.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haertzen CA, Kocher TR, Miyasato K. Reinforcements from the first drug experience can predict later drug habits and/or addiction: results with coffee, cigarettes, alcohol, barbiturates, minor and major tranquilizers, stimulants, marijuana, hallucinogens, heroin, opiates and cocaine. Drug Alcohol Depend. 1983;11(2):147–165. doi: 10.1016/0376-8716(83)90076-5. [DOI] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology (Berl) 2003;168(1–2):21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Kim K, Joseph C, Kourrich S, Yoo SH, Huang HC, … Takahashi JS. C57BL/6N mutation in cytoplasmic FMRP interacting protein 2 regulates cocaine response. Science. 2013;342(6165):1508–1512. doi: 10.1126/science.1245503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, Johansson B. Reinforcing and neurochemical effects of cocaine: differences among C57, DBA, and 129 mice. Pharmacol Biochem Behav. 2000;65(3):399–406. doi: 10.1016/s0091-3057(99)00211-7. [DOI] [PubMed] [Google Scholar]
- Lambert NM, McLeod M, Schenk S. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction. 2006;101(5):713–725. doi: 10.1111/j.1360-0443.2006.01408.x. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Nicholson KL, Dance ME, Morgan RW, Foley PL. Animal models of substance abuse and addiction: implications for science, animal welfare, and society. Comp Med. 2010;60(3):177–188. [PMC free article] [PubMed] [Google Scholar]
- Mandt BH, Johnston NL, Zahniser NR, Allen RM. Acquisition of cocaine self-administration in male Sprague-Dawley rats: effects of cocaine dose but not initial locomotor response to cocaine. Psychopharmacology (Berl) 2012;219(4):1089–1097. doi: 10.1007/s00213-011-2438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T, Hendrie D. Substance Abuse Prevention Dollars and Cents: A Cost-Benefit Analysis. Rockville MD: 2008. SMA) 07-4298. [Google Scholar]
- Nugent AL, Anderson EM, Larson EB, Self DW. Incubation of cue-induced reinstatement of cocaine, but not sucrose, seeking in C57BL/6J mice. Pharmacol Biochem Behav. 2017;159:12–17. doi: 10.1016/j.pbb.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, Winder DG. Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacology. 2009;34(7):1685–1694. doi: 10.1038/npp.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paneda C, Huitron-Resendiz S, Frago LM, Chowen JA, Picetti R, de Lecea L, Roberts AJ. Neuropeptide S reinstates cocaine-seeking behavior and increases locomotor activity through corticotropin-releasing factor receptor 1 in mice. Journal of Neuroscience. 2009;29(13):4155–4161. doi: 10.1523/JNEUROSCI.5256-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CC, Chen H, Flagel SB, Geurts AM, Richards JB, Robinson TE, … Palmer AA. Rats are the smart choice: Rationale for a renewed focus on rats in behavioral genetics. Neuropharmacology. 2014;76(Pt B):250–258. doi: 10.1016/j.neuropharm.2013.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, MacCallum RC, Nicewander WA. Use of the extreme groups approach: a critical reexamination and new recommendations. Psychol Methods. 2005;10(2):178–192. doi: 10.1037/1082-989X.10.2.178. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Polis IY, Gold LH. Intravenous self-administration of heroin, cocaine, and the combination in Balb/c mice. European Journal of Pharmacology. 1997;326(2–3):119–125. doi: 10.1016/S0014-2999(97)85405-2. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Morgan D, Liu Y. How to make a rat addicted to cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(8):1614–1624. doi: 10.1016/j.pnpbp.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12(11):623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33(6):267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AL, Varela E, Bettinger L, Beckstead MJ. Methamphetamine self-administration in mice decreases GIRK channel-mediated currents in midbrain dopamine neurons. Int J Neuropsychopharmacol. 2014;18(5) doi: 10.1093/ijnp/pyu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Subrize M, Lui W, Puca Z, Ananth M, Michaelides M, … Volkow ND. D-cycloserine facilitates extinction of cocaine self-administration in C57 mice. Synapse. 2011;65(10):1099–1105. doi: 10.1002/syn.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Cocaine self-administration under fixed and progressive ratio schedules of reinforcement: comparison of C57BL/6J, 129X1/SvJ, and 129S6/SvEvTac inbred mice. Psychopharmacology (Berl) 2006;184(2):145–154. doi: 10.1007/s00213-005-0207-0. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. False positive in the intravenous drug self-administration test in C57BL/6J mice. Behav Pharmacol. 2011a;22(3):239–247. doi: 10.1097/FBP.0b013e328345f8f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Psychomotor stimulant effects of cocaine in rats and 15 mouse strains. Exp Clin Psychopharmacol. 2011b;19(5):321–341. doi: 10.1037/a0024798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Han DD, Gu HH, Caine SB. Lack of cocaine self-administration in mice expressing a cocaine-insensitive dopamine transporter. J Pharmacol Exp Ther. 2009;331(1):204–211. doi: 10.1124/jpet.109.156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Irwin C, van den Oord EJ, Beardsley PM, Robles JR. A method for analyzing strain differences in acquisition of IV cocaine self-administration in mice. Behav Genet. 2006;36(4):525–535. doi: 10.1007/s10519-006-9068-5. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob G, Baler R. Biomarkers in substance use disorders. ACS Chem Neurosci. 2015;6(4):522–525. doi: 10.1021/acschemneuro.5b00067. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Morales M. The Brain on Drugs: From Reward to Addiction. Cell. 2015;162(4):712–725. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Rosenberg M, Dykstra LA, Walker EA. The CB1 antagonist rimonabant (SR141716) blocks cue-induced reinstatement of cocaine seeking and other context and extinction phenomena predictive of relapse. Drug Alcohol Depend. 2009;105(3):248–255. doi: 10.1016/j.drugalcdep.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltshire T, Ervin RB, Duan H, Bogue MA, Zamboni WC, Cook S, … Tarantino LM. Initial locomotor sensitivity to cocaine varies widely among inbred mouse strains. Genes Brain Behav. 2015;14(3):271–280. doi: 10.1111/gbb.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto DJ, Nelson AM, Mandt BH, Larson GA, Rorabaugh JM, Ng CM, … Zahniser NR. Rats classified as low or high cocaine locomotor responders: a unique model involving striatal dopamine transporters that predicts cocaine addiction-like behaviors. Neurosci Biobehav Rev. 2013;37(8):1738–1753. doi: 10.1016/j.neubiorev.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Nitta A, Mizoguchi H, Yamada K, Nabeshima T. Relapse of methamphetamine-seeking behavior in C57BL/6J mice demonstrated by a reinstatement procedure involving intravenous self-administration. Behav Brain Res. 2006;168(1):137–143. doi: 10.1016/j.bbr.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Yazdani N, Parker CC, Shen Y, Reed ER, Guido MA, Kole LA, … Bryant CD. Hnrnph1 Is A Quantitative Trait Gene for Methamphetamine Sensitivity. PLoS Genet. 2015;11(12):e1005713. doi: 10.1371/journal.pgen.1005713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cocaine-induced locomotor activation dose response for saline control, 30 and 40 mg/kg. Asterisks indicate significant difference from C57BL/6J at the same dose. Error bars are SEM.
Number of inactive lever presses (A) and time out responding (B) during the last day of extinction (“D10”) and the final day of re-acquisition (“R”). Data points are individual animals and error bars are SEM. (C) Number of infusions self-administered on the last day of acquisition and during reacquisition. Each data point is a strain mean.