Abstract
Purpose
To elucidate the effects of growth differentiation factor-6 (GDF6) on: (i) gene expression of inflammatory/pain-related molecules and structural integrity in the rabbit intervertebral disc (IVD) degeneration model, and (ii) sensory dysfunction and changes in pain-marker expression in dorsal nerve ganglia (DRGs) in the rat xenograft radiculopathy model.
Methods
Forty-six adolescent rabbits received anular-puncture in two non-consecutive lumbar IVDs. Four weeks later, phosphate-buffered saline (PBS) or GDF6 (1, 10 or 100 μg) was injected into the nucleus pulposus (NP) of punctured discs and followed for four weeks for gene expression analysis and 12 weeks for structural analyses. For pain assessment, eight rabbits were sacrificed at four weeks post-injection and NP tissues of injected discs were transplanted onto L5 DRGs of 16 nude rats to examine mechanical allodynia. The rat DRGs were analyzed immunohistochemically.
Results
In GDF6-treated rabbit NPs, gene expressions of interleukin-6, tumor necrosis factor-α, vascular endothelial growth factor, prostaglandin-endoperoxide synthase 2, and nerve growth factor were significantly lower than those in the PBS group. GDF6 injections resulted in partial restoration of disc height and improvement of MRI disc degeneration grades with statistical significance in rabbit structural analyses. Allodynia induced by xenograft transplantation of rabbit degenerated NPs onto rat DRGs was significantly reduced by GDF6 injection. Staining intensities for ionized calcium binding adaptor molecule-1 and calcitonin gene-related peptide in rat DRGs of the GDF6 group were significantly lower than those of the PBS group.
Conclusion
GDF6 injection may change the pathological status of degenerative discs and attenuate degenerated IVD-induced pain.
Keywords: intervertebral disc degeneration, growth differentiation factor-6, rabbit anular puncture model, rat xenograft radiculopathy model, pain behavior, inflammatory cytokines
INTRODUCTION
Degenerative disc disease is a common disorder causing low back pain and dysfunction [1, 2]. During intervertebral disc (IVD) degeneration, the inflammatory response modulated degradation of the extracellular matrix (ECM) with increased levels of matrix-degrading enzymes [3]. In human IVD cells, pro-inflammatory cytokines, such as interleukin-1β (IL-1β) and tumor necrosis factor α (TNFα), stimulated nerve growth factor (NGF) [4] and vascular endothelial growth factor (VEGF) [5, 6], both of which can promote nerve and vascular ingrowth into IVDs. Degenerating and painful human IVDs released increased levels of NGF [7-9]. Furthermore, NGF increased the expression of the nociceptive neuropeptide calcitonin gene-related peptide (CGRP) in the dorsal root ganglion (DRG); these DRG neurons innervate the IVD, acting as a sensitizer of discogenic pain [10, 11]. Consequently, this evidence suggests a structural and biological role of IVD degeneration associated with inflammation and pain. The optimal treatment for IVD degeneration should not only restore biomechanical properties and biological features of the IVD matrix, but should also ensure relief from IVD-associated pain.
Recent studies revealed that growth factors, such as bone morphogenetic protein 2 (BMP2) [2], BMP7 [12], and growth differentiation factor 5 (GDF5) [13] had positive effects on ECM metabolism in vitro and induced structural repair of IVDs in vivo [14]. GDF6 (also known as BMP13) played an essential role in skeletal development by enhancing cartilage growth and down-regulating bone formation [15-18]. Endogenous GDF6 was found to be expressed in human degenerative IVDs [19]. GDF6 increased the expression of type I collagen (Col-I) and Col-II with increasing proteoglycan (PG) accumulation by human nucleus pulposus (NP) and endplate cells in vitro [19]. Moreover, a single injection of GDF6 at the time of anular stab in an in vivo sheep model resulted in preventing the loss of disc height, increasing PG and collagen synthesis, and maintaining cell number in the IVD [20].
However, due to the difficulty of assessing pain and behavior change in large animals, outcome measures have been limited to biochemical and structural features of IVDs, which are different from the primary outcomes (pain and activity) anticipated for use in clinical studies for discogenic pain patients. A rat model, in which degenerated IVDs placed on nerve roots could produce painful radiculopathy and alter pain-related behavior, has been established by Kawakami et al. [21] The authors have taken advantage of this rat model to test the potency of rabbit discs, recovered from the anular-puncture disc degeneration model, to induce pain in the two-step disc xenograft radiculopathy model.
In this study, we hypothesized that structural and biological modifications of IVDs by GDF6 injection will be associated with relief from pain induced by degenerated IVDs. First, discs after GDF6 injection in the rabbit anular-puncture model were characterized. The recovered NP tissues were transplanted onto nude rat DRGs as a xenograft transplant to assess potency for pain generation; the status of pain markers in DRGs was then assessed. Finally, the long-term effects of GDF6 injection were assessed for structural modifications of degenerative discs.
MATERIALS AND METHODS
The study protocol was approved by the Institutional Animal Care and Use Committee (IACUC).
Rabbit Anular-puncture Disc Degeneration Model
Surgeries and Injection of GDF6
The rabbit anular-puncture disc degeneration model was utilized in the current study [22]. Under general anesthesia, lumbar IVDs of female New Zealand white rabbits (n=46, 3.3–4.5 kg, five months-old) were exposed, and anular-puncture (18-gauge, 5 mm depth) was performed at two non-continuous discs (L2/3 and L4/5), with the disc (L3/4) between the punctured discs left intact as a control. Four weeks after the initial puncture, either vehicle (phosphate-buffered saline [PBS]; 10 μL per disc) or GDF6 (1, 10 or 100 μg in 10 μL PBS per disc; Carrier free; PEPROTECH, Rocky Hill, NJ, USA) was injected into the center of the NP using a fine tip needle (approximately 26.5-gauge, OD 0.43, XX*MS16, Ito Corporation, Japan) attached to MS*GFN25 syringe (Ito Corporation, Shizuoka, Japan). Eight weeks after puncture (four weeks after injection of PBS or GDF6 [100 μg/disc]), six rabbits were euthanized for quantitative real-time polymerase chain reaction (qPCR) analysis and eight rabbits were euthanized for the rat xenograft radiculopathy model described below. Sixteen weeks after puncture (12 weeks after injection of PBS or GDF6 [1, 10, or 100 μg/disc]), the remaining 32 rabbits were euthanized for micro-computed tomography (μCT), magnetic resonance imaging (MRI) and histological analyses (Fig. 1).
Fig. 1.
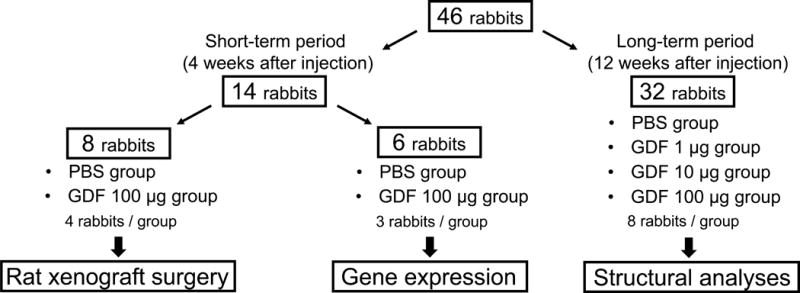
Schema for experimental rabbits.
Gene Expression Analyses of Degenerated/Treated Rabbit Disc Tissues Four Weeks after Injection
Four weeks after injection (either PBS or 100 μg/disc of GDF6), three rabbits from each group were euthanized and the experimental IVDs were removed. Total RNA in NP and anulus fibrosus (AF) tissues was extracted by bead disruption and Qiazol (Qiagen, Valencia, CA, USA) with further purification by chloroform separation followed by the MinElute Cleanup kit (Qiagen). Whole transcriptome cDNA libraries were synthesized using the QuantiTect Whole Transcriptome kit (Qiagen). After preamplification using SsoAdvanced™ PreAmp Supermix (Bio-Rad, Hercules, CA, USA), qPCR using a SYBR Green kit (Qiagen) was carried out on a Rotor-Gene Q (Qiagen) for quantification of the mRNA. Gene expressions for aggrecan (ACAN), Col-II, IL-1β, IL-6, TNFα, VEGF, prostaglandin-endoperoxide synthase 2 (PTGS2), and NGF in both NP and AF tissues were analyzed with standards using pre-designed primers (Table 1). Gene expressions were calculated as the number of copies of each gene relative to that for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and further normalized to relative gene expressions in the non-punctured disc (L3/4). The data are represented on a log scale.
Table 1.
Primer Information
| Company | Target gene | Assay/Catalog ID |
|---|---|---|
| Biorad | GAPDH | qOcuCED0019227 |
| Biorad | Col-II | qOcuCED0015663 |
| Biorad | IL-1β | qOcuCED0009632 |
| Biorad | TNFα | qOcuCED0015500 |
| Biorad | PTGS2 | qOcuCED0018228 |
| Qiagen | IL-6 | PPN00115A |
|
| ||
| Company | Target gene | Primer sequence (From 5′to 3′) |
|
| ||
| IDT | ACAN | Forward: GTC TAC CAC CAG CTA CGA AAT AG |
| Reverse: CCA GAT AGG TCT CCA CTG ACT | ||
| IDT | VEGF | Forward: TGG CAG AAG AAG GAG ACA ATA AA |
| Reverse: GAA GAT GTC CAC CAA GGT CTC | ||
| IDT | NGF | Forward: AGT GGG TTC CAC GCT TAT TC |
| Reverse: CGT CTC AGT GTT GCA GTG T | ||
GAPDH, Glyceraldehyde-3-Phosphate Dehydrogenase; Col-II, type II collagen; IL-1β, interleukin-1β; TNFα, tumor necrosis factor α; PTGS2, prostaglandin-endoperoxide synthase 2; ACAN, aggrecan; VEGF, vascular endothelial growth factor; NGF, nerve growth factor; IDT, Integrated DNA Technology
Radiographic Analysis of Disc Height
Lateral radiographs of the lumbar spine were obtained at two-week intervals up to 16 weeks after initial puncture. IVD height was expressed as disc height index (DHI), which was calculated as previously described [23]. The average percent change in DHI of injected discs (both L2/3 and L4/5) was calculated for each postoperative disc as a ratio to its preoperative DHI [%DHI = (postoperative DHI/preoperative DHI) ×100] and further normalized to the DHI of the non-punctured disc (L3/4): [Normalized %DHI = (punctured %DHI/non-punctured %DHI) ×100]. All radiographs were assessed by an observer blinded to this experiment.
Micro-computed Tomography (μCT) and Three-dimensional Disc Height Distribution (DHD)
After sacrifice at 16 weeks, isolated spine segments were imaged by a μCT scanner (Skyscan 1076, Bruker micro CT, Kontich, Belgium). Using Mimics (Materialise, Plymouth, MI, USA), three-dimensional (3D) surfaces were reconstructed and the minimum distance between apposing bony endplates was then calculated (Fig. 7Error! Reference source not found.a) and averaged to determine disc height distribution (DHD) using a custom-written Microsoft Visual C++ software [24]. Within each spine segment, the normalized DHD was calculated by dividing the average DHD of punctured discs (L2/3 and L4/5) by the DHD of the non-punctured control disc (L3/4). To determine regional repair variations, DHDs in five sub-regions were computed: posterior, central, anterior, right-lateral, and left-lateral regions (Fig. 7b). These analyses were performed by three observers blinded to this experiment.
Fig. 7.
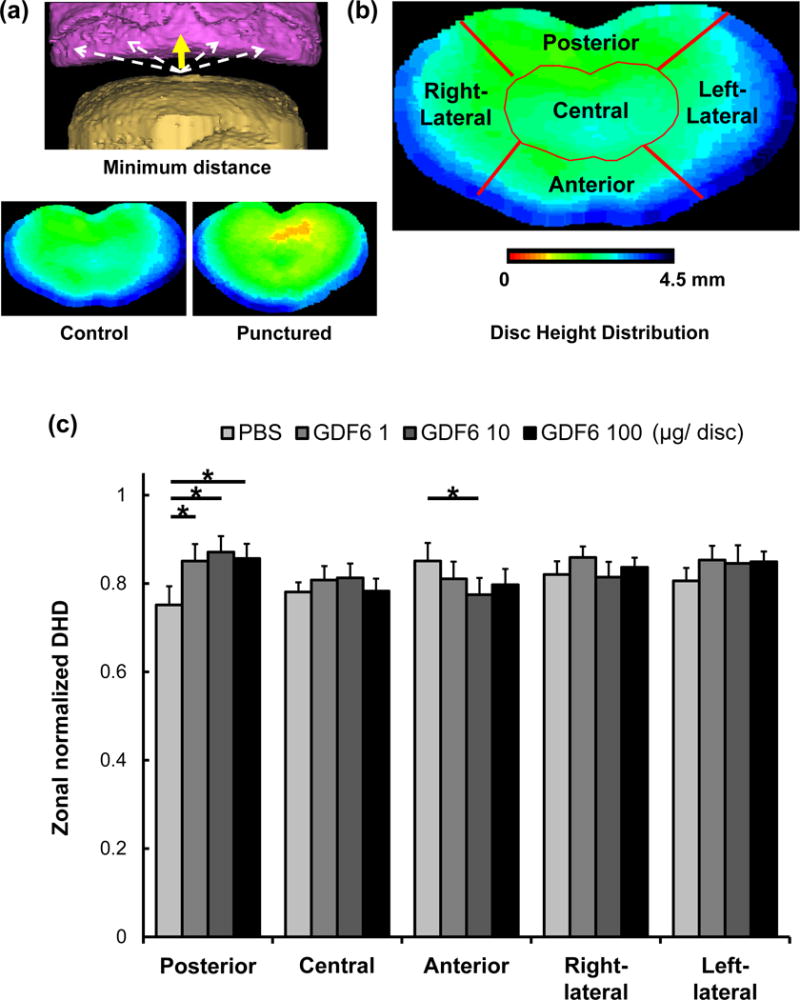
The effect of growth differentiation factor-6 (GDF6) injection on three-dimensional (3D) disc height distribution (DHD) of the lumbar disc in the rabbit anular-puncture model. a. The minimum distance between apposing bony endplates using reconstructed 3D surfaces (yellow arrow) with 3D images from non-punctured control and punctured discs. b. DHDs in five sub-regions. c. Zonal normalized DHDs 16 weeks after puncture (12 weeks after injections of phosphate-buffered saline [PBS] or GDF6 [1, 10, or 100 μg/disc]). Data are expressed as the mean ± standard error (n = 8). Two-way measurement analysis of variance with the least significant difference as a post hoc test was used. *p < 0.05.
Magnetic Resonance Imaging Analyses
After sacrifice at 16 weeks, MRI examinations on isolated spine segments were performed using a 7-Tesla BioSpec 70/30 (BRUKER, Billerica, MA, USA). The MRI degeneration grade of IVDs was classified according to Pfirrmann grade [25] using T2 weighted sagittal images; the evaluations were performed by two observers blinded to the experimental groups. The average degeneration grade of injected discs (L2/3 and L4/5) was calculated.
Histological Analyses
Midsagittal sections (5 μm) of each experimental IVD from rabbits sacrificed at 16 weeks were stained with either hematoxylin and eosin or safranin-O. An observer blinded to this experiment analyzed the histologic sections and graded them using our established protocol (Table 2) [13]. In addition, cellular changes including cell cloning or the presence of chondrocyte-like cells in either the inner or outer AF or in the NP were analyzed.
Table 2.
Definition of Histological Grading Scale
|
I. Anulus fibrosus:
|
| Grade: |
|
|
II. Border between the anulus fibrosus and nucleus pulposus: |
| Grade: |
|
|
III. Cellularity of the nucleus pulposus: |
| Grade: |
|
|
IV. Matrix of the nucleus pulposus: |
| Grade: |
|
Histological grading scale based on four categories of degenerative changes with scores ranging from a normal disc with 4 points (1 point in each category) to a severely degenerated disc with 12 points (3 points in each category).
Nude Rat Disc Xenograft Radiculopathy Model
Xenograft Surgery
Rabbit NP tissues recovered four weeks after injection were used to test pain induction in the rat radiculopathy model as previously described with modification as a xenograft transplantation model [21]. Female NIH-Foxn1rnu nude rats (n=16, 150–200 g, two months-old) were used for this study. The right L5 DRG was exposed by partial laminectomy and facetectomy under general anesthesia. Degenerated NPs from punctured/injected IVDs (either L2/3 or 4/5) of anular-punctured rabbits (either PBS or 100 μg/disc of GDF6 injected group; four rabbits per group) were placed on exposed DRGs as xenografts (Fig. 2). Mechanical allodynia was assessed up to three weeks after surgery, and all rats were then sacrificed for DRG collection with perfusion fixation.
Fig. 2.
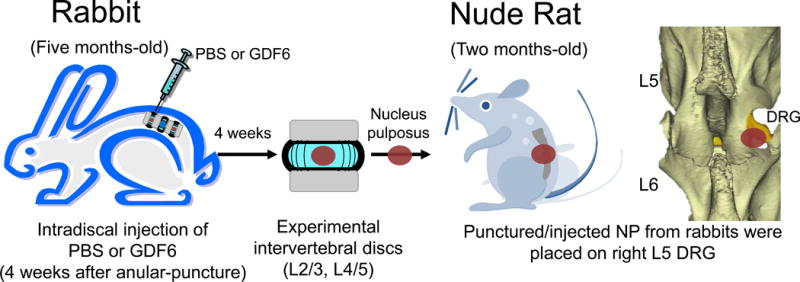
The two-step disc xenograft radiculopathy model using rabbits and nude rats. Four weeks after injection, degenerated nucleus pulposus (NP) tissue from punctured/injected (either phosphate buffered saline [PBS] or 100 μg/disc of growth differentiation factor-6 [GDF6]) intervertebral discs (either L2/3 or 4/5) of anular-punctured rabbits was placed on an exposed nude rat L5 dorsal root ganglion (DRG) as a xenograft.
Analyses of Mechanical Allodynia
Mechanical allodynia was evaluated by the threshold of 50% paw withdrawal response to mechanical stimulation with Von Frey hair filaments (Muromachi Kikai, Tokyo, Japan) for both ipsilateral (right) and contralateral hind paws. The rats were placed in a plastic cage with a wire mesh bottom and acclimated for 15 minutes. Von Frey filaments (0.4, 0.6, 1, 2, 4, 6, 8, and 15 g) were pressed onto the plantar surface using the up-down method [26, 27].
Nude Rat Dorsal Root Ganglion Immunohistochemistry
The expressions of ionized calcium binding adaptor molecule-1 (Iba-1), which is a microglia/macrophage specific calcium binding protein, and CGRP in the nude rat DRGs were analyzed. Cryosections (10 μm) were incubated with primary antibody for Iba-1 (Waco Chemical, Richmond, VA, USA) and CGRP (Thermo Fisher Scientific, Carlsbad, CA, USA) overnight at 4°C, and then in the appropriate secondary antibody, Alexa Fluor® 594 or 488 (Molecular Probes, Eugene, OR, USA) for 1 hour at room temperature followed by nuclear staining using 4′,6-diamidin-2-phenylindol (DAPI). Images were taken using fluorescence microscope Leica AF6000 (Leica Microsystems Inc, Buffalo Grove, IL, USA). At least four sections from each rat were used and two random fields were analyzed with Image-J. The numbers of Iba-1 positive microglia were quantified and expressed as microglia/mm2. The numbers of CGRP-positive or -negative neurons in DRGs were counted and represented as percentages of CGRP-positive neurons.
Statistical Analysis
Data are shown as the mean ± standard error. Two-way repeated measurement analysis of variance (ANOVA) with the least significant difference (LSD) as a post hoc test was used to assess DHI and mechanical allodynia. One or two-way ANOVA with LSD test was used for gene expression and DHD analyses. The Kruskal-Wallis test was used to assess MRI and histological grading analyses. The unpaired t-test was used for analysis of immunohistochemistry. Statistical analysis was performed using IBM SPSS (IBM, Chicago, IL, USA). Statistical significance was established as p < 0.05.
RESULTS
The Effect of GDF6 (100 μg) on Gene Expressions in NP and AF Tissues Four Weeks after Injection (Short-term Period) in the Rabbit Anular Puncture Model
ECM Genes
In both NP and AF tissues, there were no significant differences in gene expressions of ACAN and Col-II between the PBS and GDF6 groups (100 μg) (Fig. 3a, 3b).
Fig. 3.
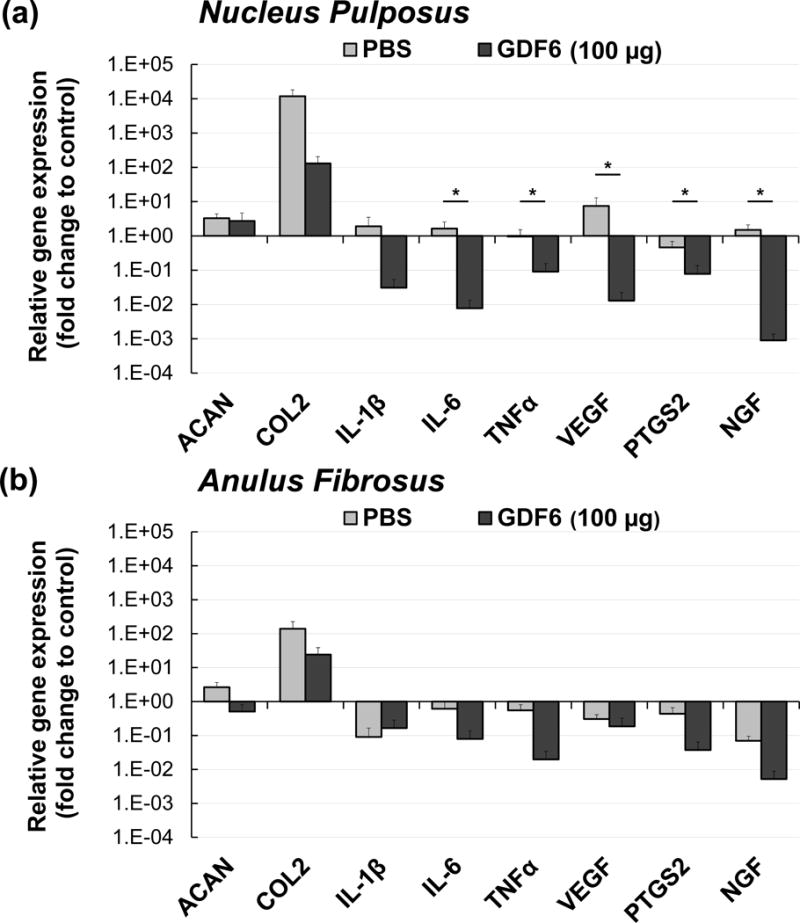
Quantitative real-time polymerase chain reaction analyses for gene expressions of aggrecan (ACAN), type II collagen (Col-II), interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNFα), vascular endothelial growth factor (VEGF), prostaglandin endoperoxide synthase 2 (PTGS2), and nerve growth factor (NGF) eight weeks after anular-puncture (four weeks after injection of either phosphate-buffered saline [PBS] or 100 μg/disc of growth differentiation factor-6 [GDF6]) in both nucleus pulposus (a) and anulus fibrosus (b). Gene expressions were calculated as the number of copies of each gene relative to that for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and further normalized to those of the non-punctured control disc. The normalized gene expressions are represented on a log scale. Data are expressed as the mean ± standard error (n = 6). One-way analysis of variance with least significant difference as a post hoc test was used. *p < 0.05.
Pro-inflammatory Genes
In the NP, IL-6 and TNFα gene expressions with GDF6 treatment were significantly lower than those with PBS treatment (p < 0.05). Although IL-1β gene expression was similarly down-regulated by GDF6 treatment in the NP, there were no significant differences between the groups (Fig. 3a). In the AF, there were no significant differences in gene expressions of IL-1β, IL-6, and TNFα between the PBS and GDF6 groups (Fig. 3b).
Pain-related Molecules
In the NP, gene expressions of VEGF, PTGS2, and NGF were significantly down-regulated by GDF6 treatment compared to the PBS group (p < 0.05, Fig. 3a). In the AF, there were no significant differences in VEGF, PTGS2, and NGF gene expressions between the PBS and GDF6 groups (Fig. 3b).
The Nude Rat Xenograft Radiculopathy Model
Mechanical Allodynia
Our preliminary experiment demonstrated that rats transplanted with rabbit non-punctured NPs or fat tissue did not exhibit mechanical allodynia (data not shown). In both PBS and GDF6 (100 μg) groups, the 50% paw withdrawal threshold in the ipsilateral hind paw was significantly reduced at day 7 compared to baseline values (p < 0.05). In the PBS group, the threshold continued to reduce until day 10, but had largely recovered on day 14. On the other hand, in the GDF6 group, this reduction recovered from day 10 and had essentially returned to baseline by day 14. Significant differences in the threshold withdrawal force between the groups were observed on both days 10 and 14 (p < 0.05) (Fig. 4).
Fig. 4.
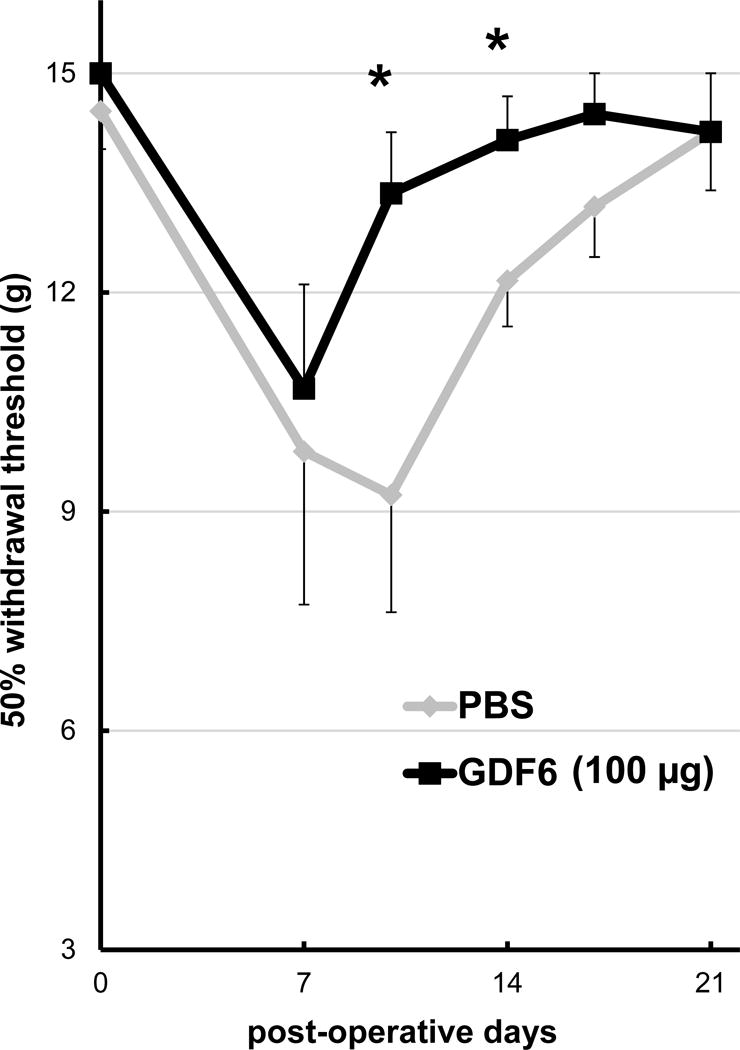
Time course of mechanical allodynia in the nude rat xenograft radiculopathy model. The effect of degenerated nucleus pulposus tissues from either phosphate-buffered saline (PBS) control or 100 μg/disc of growth differentiation factor-6 (GDF6) injected rabbit intervertebral discs on the paw withdrawal threshold of nude rats was measured with von Frey filaments to assess mechanical allodynia for up to 21 days after xenograft surgery. Data are expressed as the mean ± standard error (n = 8). Two-way repeated measurement analysis of variance with the least significant difference as a post hoc test was used. *p < 0.05.
Expression of Iba-1 and CGRP by Nude Rat DRGs
Representative images of nude rat DRGs transplanted with NPs from both PBS and GDF6 (100 μg) injected groups are shown in Fig. 5a. The average number of Iba-1-positive microglia/mm2 in the GDF6 group was significantly lower than that in the PBS group (p < 0.05, Fig. 5b). The average percentage of CGRP-positive neurons in the GDF6 group was also significantly lower compared to the PBS group (p < 0.01) (Fig. 5b).
Fig. 5.
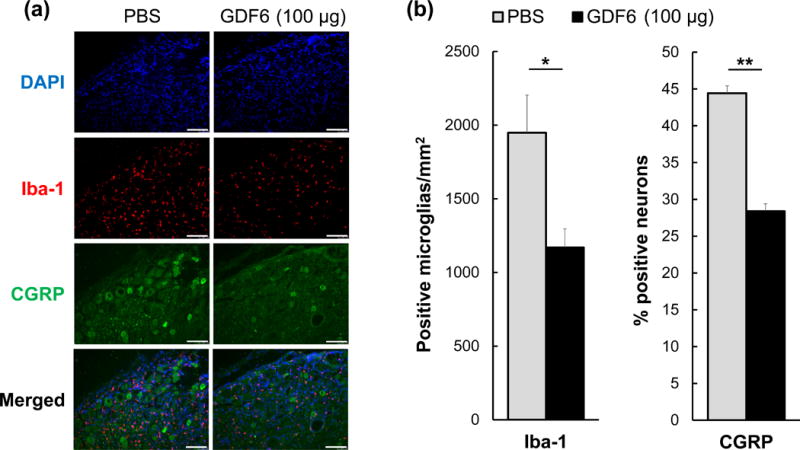
Dorsal root ganglion (DRG) immunohistochemistry in the nude rat xenograft radiculopathy model. The effect of degenerated nucleus pulposus tissues from either phosphate-buffered saline (PBS) control or 100 μg/disc of growth differentiation factor-6 (GDF6) injected rabbit intervertebral discs on the immunohistochemistry of rat DRGs in the rat xenograft model. a. Representative images of immunofluorescence for 4′,6-diamidin-2-phenylindol (DAPI) (blue), ionized calcium binding adaptor molecule-1 (Iba-1) (red), calcitonin gene-related peptide (CGRP) (green), and merged signals in experimental and control DRGs. Bar scale is 100 μm. b. On day 21 after xenograft, the number of Iba-1-positive microglia/mm2 and percentage of CGRP-positive neurons in DRGs of PBS and GDF6-injected discs transplanted onto nude rat DRGs are shown. Data are expressed as the mean ± standard error (n = 5). Unpaired t-test was used. *p < 0.05, **p < 0.01.
Structural Analyses 12 Weeks after Injection (the Rabbit Anular Puncture Model: Long-term Period)
Radiographic Assessment of Disc Degeneration
In all groups, normalized %DHI four weeks after anular puncture decreased by approximately 25% with no significant differences among the groups, indicating that disc degeneration was sufficiently achieved after initial puncture. Treatment with GDF6 (10 or 100 μg/disc) significantly affected normalized %DHI (GDF6 1 μg; p =0.17, 10 μg; p < 0.05, 100 μg; p < 0.01 vs. PBS group, two-way repeated ANOVA). Sixteen weeks after puncture, GDF6 10 and 100 μg groups showed significantly higher normalized %DHI than the PBS group (GDF6 1 μg; p =0.07, 10 μg; p < 0.01, 100 μg; p < 0.01 vs. PBS group) (Fig. 6a, b).
Fig. 6.
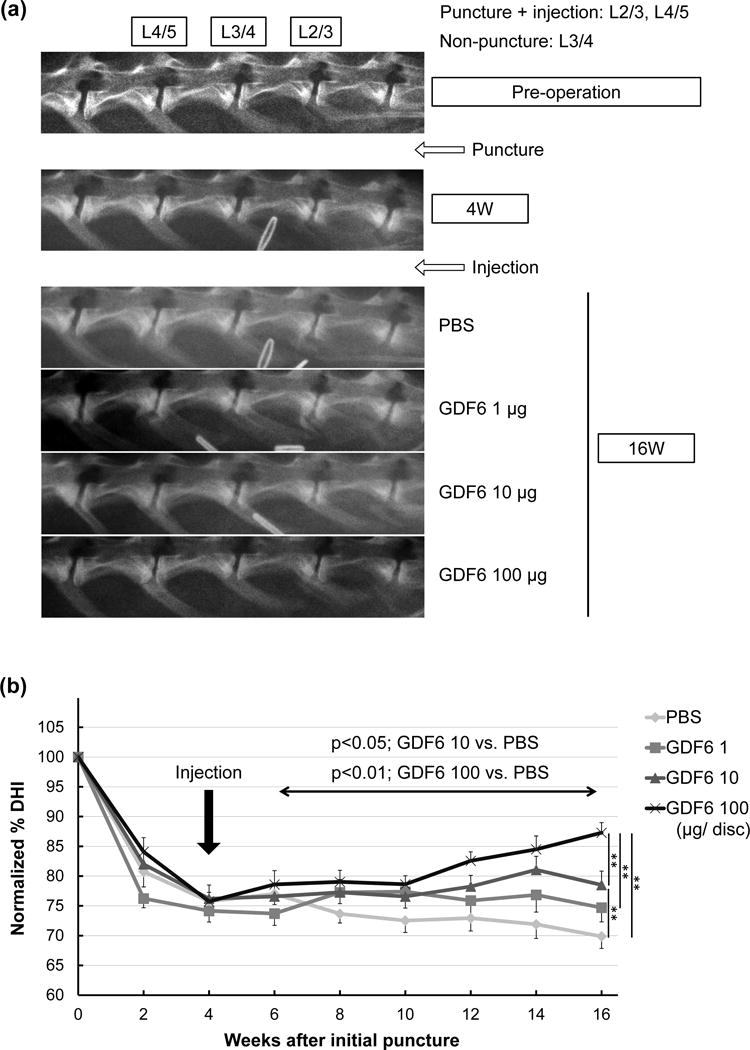
Radiographic analyses of the effect of growth differentiation factor-6 (GDF6) injection on lumbar disc height in the rabbit anular-puncture model. a. Representative lateral radiograms of a rabbit lumbar spine before (pre-operative), four weeks after anular-puncture with an 18-gauge needle, and 16 weeks after puncture (12 weeks after injections of phosphate-buffered saline [PBS] or GDF6 [1, 10, or 100 μg/disc]). b. Change in intervertebral disc height index (DHI) after anular-puncture and injections of either PBS or GDF6. Data are expressed as the mean ± standard error (n = 8). Two-way repeated measurement analysis of variance (ANOVA) with the least significant difference as a post hoc test was used for comparison among the groups during post-injection periods. At week 16, significant differences were observed among treatment groups using one-way ANOVA with the least significant difference. **p < 0.01.
Micro-computed Tomography (μCT) Assessment of 3D Disc Height Distribution (DHD)
There were no significant differences in average normalized DHD 12 weeks after injections of PBS or GDF6 (1, 10 or 100 μg/disc). However, the normalized zonal DHD of the posterior region in GDF6 groups was significantly higher than that in the PBS group (GDF6 1 μg; p < 0.05, 10 μg; p < 0.01, 100 μg; p < 0.01 vs. PBS group) (Fig. 7c).
MRI Assessment of Disc Degeneration Grade
Twelve weeks after PBS or GDF6 injection, L3/4 control discs in all groups did not exhibit disc degeneration (Fig. 8a). Significantly lower Pfirrmann scores (less degeneration) were observed in GDF6 10 and 100 μg groups compared to the PBS group (GDF6 1 μg; p =0.15, 10 μg; p < 0.05, 100 μg; p < 0.05 vs. PBS group) (Fig. 8b).
Fig. 8.
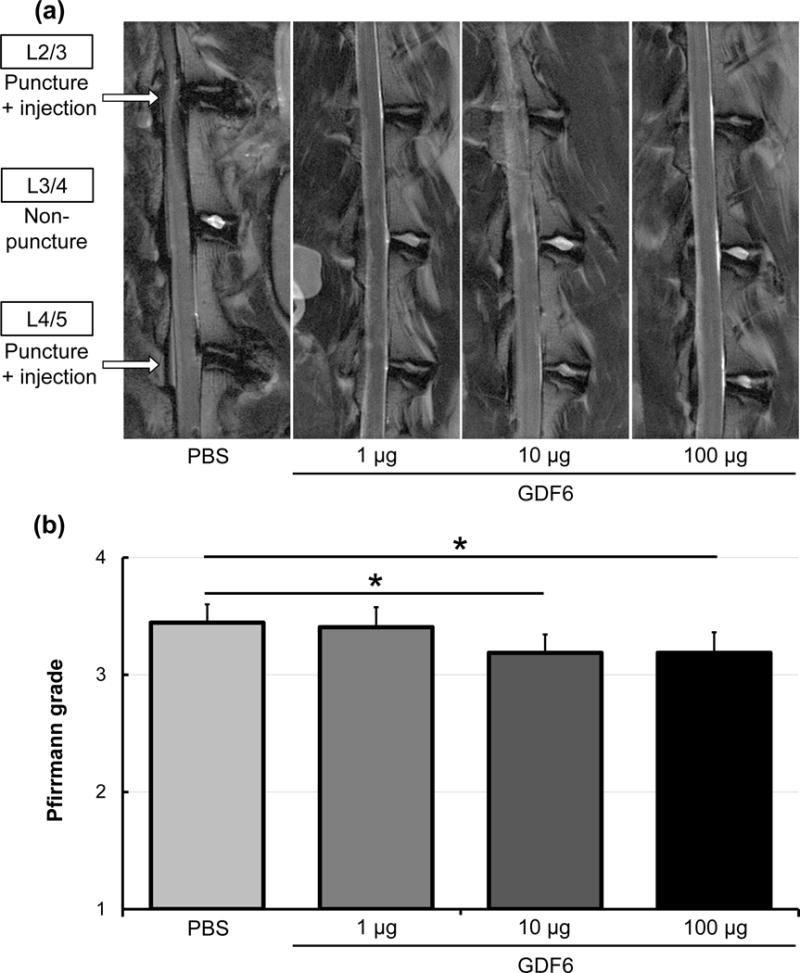
The effect of growth differentiation factor-6 (GDF6) injection on magnetic resonance imaging (MRI) degeneration grade of the lumbar disc in the rabbit anular-puncture model. a. MRI T2 weight sections in the sagittal plane 16 weeks after anular-puncture (12 weeks after injections of phosphate-buffered saline [PBS] or GDF6 [1, 10, or 100 μg/disc]). b. Pfirrmann MRI grade scores of discs after PBS or GDF6 injections. Data are expressed as the mean ± standard error (n = 8). The Kruskal-Wallis test was used. *p < 0.05.
Histological Grading
The injection of GDF6 did not show a significant effect on the overall histological score of disc degeneration 12 weeks after PBS or GDF6 injection (data not shown). However, the analysis of cell morphology revealed that chondrocyte-like cell cloning occurred in some GDF6 1- and 100 μg-treated discs. On the other hand, these cell clones were not found in non-puncture control and PBS-treated discs (PBS; 0%, GDF6 1 μg; 12.5%, 10 μg; 0%, 100 μg; 37.5%, Fig. 9).
Fig. 9.
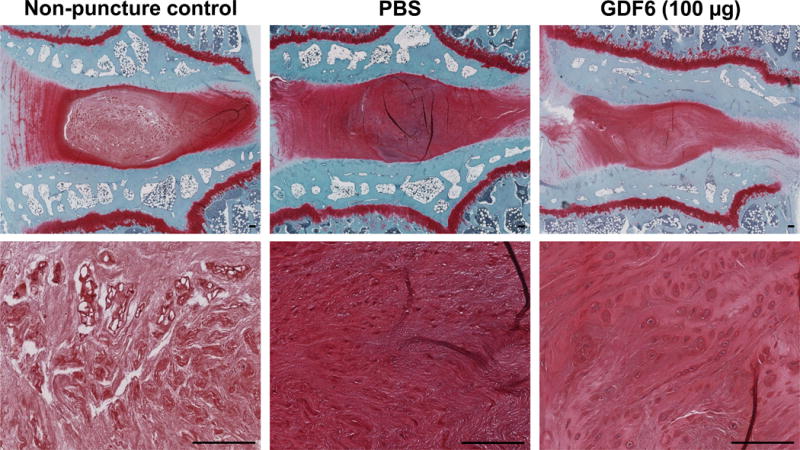
Cell morphology changes 16 weeks after anular-puncture (12 weeks after injections of phosphate-buffered saline [PBS] or growth differentiation factor-6 [GDF6]) in lumbar discs in the rabbit anular-puncture model. On safranin-O stained sections of control (non-puncture), PBS-, and GDF6 100 μg-treated discs, cell clones were not found in non-puncture and PBS-treated discs, while chondrocyte-like cell clone formation was found in GDF6 100 μg-treated discs (37.5%). Bar scale is 100 μm.
DISCUSSION
Our newly developed two-step xenograft rat radiculopathy model revealed that rabbit NP tissues with excessive expression of pro-inflammatory cytokines and pain-related molecules generated by the rabbit anular-puncture model can induce mechanical allodynia. Importantly, the intradiscal injection of GDF6 (100 μg/disc) into these rabbit IVDs attenuated degenerated disc tissue-induced pain.
Gene expression analyses of four-week post-GDF6 injection NP tissues from the rabbit anular-puncture model demonstrated significant inhibitory effects of GDF6 on gene expressions of IL-6, TNFα, VEGF, PTGS2, and NGF, compared to PBS injection, while, in AF tissues, there were no significant differences in these gene expressions between the GDF6 and PBS groups. Notably, these results aligned with the effect of GDF6 on mechanical allodynia in the subsequent rat radiculopathy model. Because recent literature indicates that degenerated discs are often associated with upregulation of inflammatory cytokines and catabolic enzymes [3, 28, 29], the involvement of pro-inflammatory factors, such as IL-1β, IL-6, and TNFα, in the biological repair of the IVD needs to be addressed. Moreover, the release of these inflammatory cytokines induced significant increases of NGF and VEGF in the IVD, which can promote neuronal and vascular ingrowth resulting in painful IVDs [4, 5, 7]. In the current study, the decreased inflammatory condition, supported by gene expression analyses, corresponded with less pain generation in the rat radiculopathy model. The pain status in these rats was further supported by the immunohistochemistry results (low Iba-1 and CGRP staining) in DRGs of the GDF6-injected IVD transplanted group. Thus, these results suggest that pain generation induced by molecules released by degenerated discs may be ameliorated by treatment with GDF6 through the inhibition of inflammatory/pain-related molecules. Our previous work indicated that GDF6 had a predominant effect on chondrocyte-like cells in the human NP as opposed to fibroblastic cells in the human AF [19, 20]. Therefore, these distinct responses to GDF6 in NP cells compared with those in AF cells may be due to different sensitivities dependent on cell phenotype.
From the structural analyses at 12 weeks after injection in this in vivo rabbit disc degeneration model, this study demonstrated that a single injection of GDF6 (10 and 100 μg/disc) in the NP induced restoration of disc height in a dose-dependent manner after disc degeneration caused by anular-puncture. The zonal analysis of disc height revealed that this restoration was significant in the posterior zone. Moreover, MRI degeneration grade assessment suggested that GDF6 (10 and 100 μg/disc) protected the IVD from degenerative changes. No apparent acceleration of bone formation was found in μCT analysis. These results suggest that a single injection of GDF6 four weeks after anular-puncture changes the pathological status of degenerated discs. In our previous work, a single injection of GDF6 at the time of anular-puncture could modify structural changes of the IVD following anular-puncture, including protection from disc narrowing and stimulation of PG production in both the NP and AF and collagen production in the inner AF in a sheep model of disc degeneration.[20] Considering prior evidence, GDF6 may also be effective at stimulating repair of the IVD through ECM production in this rabbit degenerative disc model. To prove biological efficacy of GDF6, further metabolic studies of PG and collagen synthesis at multiple time points may shed light on the mechanism of disc repair. The current work demonstrated that GDF6 injection at 100 μg/disc induced inhibition of inflammatory/pain-related molecules in rabbit NP cells at four weeks after injection; this supports our contention that GDF6 shifts the metabolic status to anabolism, thus inducing structural repair and reducing the progression of disc degeneration. Interestingly, the delay in disc height recovery (four-six weeks post-injection) is similar to that of BMP7 [12, 13] or GDF5 [22]. This may be because of a delayed biological response of IVD cells to the injection. Furthermore, the stimulation of GDF6 may induce a cascade of events eventually resulting in the recovery of disc height.
While the issue of superiority of one BMP molecule or the other can only be determined in a clinical setting with long term follow up of human data; Clark et al. demonstrated, in a series of elegant in vitro studies, that stem cells of varying origin demonstrated a much superior response to GDF6 stimulation when compared to GDF5 [30]. The established use in the clinic of BMP2 and BMP7 for bone formation may be a cause of concern for their use in regenerating the disc; hence a disc cell-forming molecule with potential for pain relief, like GDF6, has potential for clinical use.
To apply our findings to a clinical investigation, additional studies may be required. The current experimental analyses were conducted at limited time points (four weeks after an injection for gene expression analysis and xenograft surgery and 12 weeks for structural analyses). Further investigations at different time points would be informative to compare chronic pathology in the human. In addition, this study did not address the bio-distribution of GDF6 that was injected into the center of the NP. Because the presence of endogenous GDF6 made an experimental design to address this question difficult without the use of radiolabeled GDF6, a decision was made to elucidate the half-life of injected GDF6 in a separate experiment. These results may provide the frequency of injections needed for effective clinical treatment.
In conclusion, an injection of GDF6 may change the pathological status of degenerated discs and attenuate degenerated IVD-induced pain. It serves as a new therapeutic approach for degenerative disc disease. This study provides preclinical evidence that GDF6 injection may be effective in humans.
Acknowledgments
The authors would like to thank Erikka Linn, Edward Abarado, and Weibo Jiang, M.D. for their generous assistance.
Footnotes
Disclosures/Conflicts of interest:
The authors did not receive outside funding or support for this work, and have no actual or potential conflicts of interest to disclose.
References
- 1.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 2.Gilbertson L, Ahn SH, Teng PN, Studer RK, Niyibizi C, Kang JD. The effects of recombinant human bone morphogenetic protein-2, recombinant human bone morphogenetic protein-12, and adenoviral bone morphogenetic protein-12 on matrix synthesis in human annulus fibrosis and nucleus pulposus cells. Spine J. 2008;8:449–456. doi: 10.1016/j.spinee.2006.11.006. doi: S1529-9430(06)01068-0 [pii] 10.1016/j.spinee.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204:47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
- 4.Abe Y, Akeda K, An HS, Aoki Y, Pichika R, Muehleman C, Kimura T, Masuda K. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine. 2007;32:635–642. doi: 10.1097/01.brs.0000257556.90850.53. [DOI] [PubMed] [Google Scholar]
- 5.Binch AL, Cole AA, Breakwell LM, Michael AL, Chiverton N, Cross AK, Le Maitre CL. Expression and regulation of neurotrophic and angiogenic factors during human intervertebral disc degeneration. Arthritis Res Ther. 2014;16:416. doi: 10.1186/s13075-014-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JM, Song JY, Baek M, Jung HY, Kang H, Han IB, Kwon YD, Shin DE. Interleukin-1beta induces angiogenesis and innervation in human intervertebral disc degeneration. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2011;29:265–269. doi: 10.1002/jor.21210. [DOI] [PubMed] [Google Scholar]
- 7.Freemont AJ, Watkins A, Le Maitre C, Baird P, Jeziorska M, Knight MT, Ross ER, O’Brien JP, Hoyland JA. Nerve growth factor expression and innervation of the painful intervertebral disc. The Journal of pathology. 2002;197:286–292. doi: 10.1002/path.1108. [DOI] [PubMed] [Google Scholar]
- 8.Krock E, Rosenzweig DH, Chabot-Dore AJ, Jarzem P, Weber MH, Ouellet JA, Stone LS, Haglund L. Painful, degenerating intervertebral discs up-regulate neurite sprouting and CGRP through nociceptive factors. Journal of cellular and molecular medicine. 2014;18:1213–1225. doi: 10.1111/jcmm.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis research & therapy. 2008;10:R99. doi: 10.1186/ar2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoki Y, An HS, Takahashi K, Miyamoto K, Lenz ME, Moriya H, Masuda K. Axonal growth potential of lumbar dorsal root ganglion neurons in an organ culture system: response of nerve growth factor-sensitive neurons to neuronal injury and an inflammatory cytokine. Spine. 2007;32:857–863. doi: 10.1097/01.brs.0000259810.48681.90. [DOI] [PubMed] [Google Scholar]
- 11.Aoki Y, Takahashi Y, Ohtori S, Moriya H, Takahashi K. Distribution and immunocytochemical characterization of dorsal root ganglion neurons innervating the lumbar intervertebral disc in rats: a review. Life Sci. 2004;74:2627–2642. doi: 10.1016/j.lfs.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Masuda K, Imai Y, Okuma M, Muehleman C, Nakagawa K, Akeda K, Thonar E, Andersson G, An HS. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine (Phila Pa 1976) 2006;31:742–754. doi: 10.1097/01.brs.0000206358.66412.7b. doi: 10.1097/01.brs.0000206358.66412.7b 00007632-200604010-00006 [pii] [DOI] [PubMed] [Google Scholar]
- 13.Chujo T, An HS, Akeda K, Miyamoto K, Muehleman C, Attawia M, Andersson G, Masuda K. Effects of growth differentiation factor-5 on the intervertebral disc–in vitro bovine study and in vivo rabbit disc degeneration model study. Spine (Phila Pa 1976) 2006;31:2909–2917. doi: 10.1097/01.brs.0000248428.22823.86. [DOI] [PubMed] [Google Scholar]
- 14.Masuda K, Oegema TR, Jr, An HS. Growth factors and treatment of intervertebral disc degeneration. Spine. 2004;29:2757–2769. doi: 10.1097/01.brs.0000146048.14946.af. [DOI] [PubMed] [Google Scholar]
- 15.Chang SC, Hoang B, Thomas JT, Vukicevic S, Luyten FP, Ryba NJ, Kozak CA, Reddi AH, Moos M., Jr Cartilage-derived morphogenetic proteins. New members of the transforming growth factor-beta superfamily predominantly expressed in long bones during human embryonic development. J Biol Chem. 1994;269:28227–28234. [PubMed] [Google Scholar]
- 16.Shen B, Bhargav D, Wei A, Williams LA, Tao H, Ma DD, Diwan AD. BMP-13 emerges as a potential inhibitor of bone formation. International journal of biological sciences. 2009;5:192–200. doi: 10.7150/ijbs.5.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei A, Shen B, Williams LA, Bhargav D, Gulati T, Fang Z, Pathmanandavel S, Diwan AD. Expression of growth differentiation factor 6 in the human developing fetal spine retreats from vertebral ossifying regions and is restricted to cartilaginous tissues. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2016;34:279–289. doi: 10.1002/jor.22983. [DOI] [PubMed] [Google Scholar]
- 18.Williams LA, Bhargav D, Diwan AD. Unveiling the bmp13 enigma: redundant morphogen or crucial regulator? International journal of biological sciences. 2008;4:318–329. doi: 10.7150/ijbs.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulati T, Chung SA, Wei AQ, Diwan AD. Localization of bone morphogenetic protein 13 in human intervertebral disc and its molecular and functional effects in vitro in 3D culture. J Orthop Res. 2015;33:1769–1775. doi: 10.1002/jor.22965. [DOI] [PubMed] [Google Scholar]
- 20.Wei A, Williams LA, Bhargav D, Shen B, Kishen T, Duffy N, Diwan AD. BMP13 prevents the effects of anular injury in an ovine model. International journal of biological sciences. 2009;5:388–396. doi: 10.7150/ijbs.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawakami M, Tamaki T, Hayashi N, Hashizume H, Matsumoto T, Minamide A, Kihira T. Mechanical compression of the lumbar nerve root alters pain-related behaviors induced by the nucleus pulposus in the rat. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2000;18:257–264. doi: 10.1002/jor.1100180214. [DOI] [PubMed] [Google Scholar]
- 22.Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, Andersson GB, An HS. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine (Phila Pa 1976) 2005;30:5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 23.Mwale F, Masuda K, Pichika R, Epure LM, Yoshikawa T, Hemmad A, Roughley PJ, Antoniou J. The efficacy of Link N as a mediator of repair in a rabbit model of intervertebral disc degeneration. Arthritis research & therapy. 2011;13:R120. doi: 10.1186/ar3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Espinoza Orias AA, Mammoser NM, Triano JJ, An HS, Andersson GB, Inoue N. Effects of Axial Torsion on Disc Height Distribution: An In Vivo Study. J Manipulative Physiol Ther. 2016;39:294–303. doi: 10.1016/j.jmpt.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 26.Dixon WJ. Efficient analysis of experimental observations. Annual review of pharmacology and toxicology. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 27.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. doi: 0165-0270(94)90144-9 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiler C, Nerlich AG, Bachmeier BE, Boos N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine. 2005;30:44–53. doi: 10.1097/01.brs.0000149186.63457.20. discussion 54. [DOI] [PubMed] [Google Scholar]
- 30.Clarke LE, McConnell JC, Sherratt MJ, Derby B, Richardson SM, Hoyland JA. Growth differentiation factor 6 and transforming growth factor-beta differentially mediate mesenchymal stem cell differentiation, composition, and micromechanical properties of nucleus pulposus constructs. Arthritis Res Ther. 2014;16:R67. doi: 10.1186/ar4505. [DOI] [PMC free article] [PubMed] [Google Scholar]


