Abstract
Backgrounds
Elucidating the causal effects of common intermediate risk factors on the onset of age-related diseases is indispensable for developing prevention and intervention procedures.
Methods
We conducted two-stage time-to-event Mendelian randomization meta-analyses combining five large-scale longitudinal cohorts to investigate the dynamic causal effects of cardiovascular disease risk factors including body mass index (BMI), systolic blood pressure (SBP), and lipids on the age-at-onset of age-related diseases. We constructed weighted polygenic scores based on genetic markers from previously reported genome-wide association studies as instrumental variables to estimate the causal effects. To examine potential pleiotropic effect, we also performed a leave-one-out sensitivity analysis and an MR-Egger sensitivity analysis that we expanded to the survival context.
Results
Our results show that elevated BMI increases the absolute risk of type 2 diabetes (T2D) (p=7.68e-04), heart failure (p=9.03e-03), and cardiovascular diseases (CVD) (p=1.69e-03) and the effects start at different ages. A significant association between BMI and the risk of stroke is observed; however, the sensitivity analyses show that the association with stroke is attributed to the potential pleiotropic effects of rs2867125 and rs1558902 on BMI and stroke. Raised SBP levels are significantly associated with the development of atrial fibrillation (p=6.42e-03). Low-density lipoprotein cholesterol (LDL-C) levels are inversely associated with the age-at-onset of T2D (p=1.05e-02). In addition, LDL-C and triglycerides are inversely associated with the hazards of cancer and T2D, respectively. Nevertheless, the sensitivity analyses suggest that these associations are probably due to pleiotropic effects of several single-nucleotide polymorphisms including rs4970834 and rs1260326.
Conclusions
Our results highlight the involvement of BMI in the development of multiple age-related diseases. We found that some observed causal associations can attribute to pleiotropic effects of some genetic variations. These findings have important implications in unraveling causal effects of common risk factors on age-related diseases and guiding effective intervention strategies to reduce the incidence of these diseases.
Keywords: Mendelian randomization, body mass index, causal effect, age-related disease, time-to-event analysis
Introduction
Common aging-associated diseases including cancer, various cardiovascular diseases (CVDs), type 2 diabetes (T2D) and neurodegenerative diseases (NDs) such as Alzheimer’s disease (AD) are the leading causes of death and major contributors to morbidity, disability, and mortality at old age (Sahyoun et al., 2001). Evidence has shown that most of these diseases are correlated with certain common intermediate risk factors such as body mass index (BMI), blood pressure (BP) and lipids. A fundamental hypothesis in gerontology is that the biological aging process that leads to physical dysfunction and deviation from normal physiological indices and levels of some crucial biomarkers would be implicated in the onset and progression of multiple diseases (Arbeev et al., 2011; Kaeberlein et al., 2015). Nevertheless, it is still elusive whether the deviation from the optimal values of the biomarkers directly results in onset of these diseases or they are concurrent ramifications of certain more fundamental molecular mechanisms underlying the biological aging process, i.e., pleiotropy. Thus, elucidating the potential causal relationship between the intermediate risk factors and the onset of the age-related diseases will not only contribute to the development of effective intervention procedure and management policy that may lead to remarkable improvement of human healthspan and lifespan, but also provide more insights into the underlying biological implications in aging process and pinpointing potential physiological determinants of these age-related diseases.
Despite the evidence of association, it is unclear whether these intermediate biomarkers play a causal role in the etiology of many of these age-related diseases and, more interestingly, how the effects vary with increasing age if they exist. This is because inferring causation from general association analyses is often not straightforward as it is complicated by potentially unmeasured confounders and reverse causality (Pearl, 2000). One of the feasible approaches for causal inference is to construct an instrumental variable (IV) under a parametric model. With the advent of sequencing and microarray techniques in molecular biology, causal effects can be evaluated with the Mendelian randomization (MR) analyses that leverage a large number of identified genetic variants from genome-wide association analyses (GWAS) as IVs (Davey Smith and Hemani, 2014; Didelez and Sheehan, 2007). Previous MR studies based on cross-sectional or longitudinal datasets have reported that intermediate risk factors such as BMI, BP and lipids may have causal effects on the incidence of multiple age-related diseases (Hägg et al., 2015; Holmes et al., 2015, 2014; Østergaard et al., 2015; Proitsi et al., 2014).
However, to our knowledge, few MR studies have focused on the causal effects on the age-at-onset of these diseases. Most recently, several methods using IVs based on the additive hazards model (Aalen, 1978) have been proposed (Li et al., 2015; Tchetgen Tchetgen et al., 2015), which are characterized by straightforward estimation of the dynamic effects over age and more intuitive interpretation of the estimated parameters. MR using the additive hazards model enjoys robustness due to collapsibility compared to proportional hazards model (Martinussen and Vansteelandt, 2013). Compared to the MR analyses in cross-sectional studies, MR analyses with time-to-event outcomes leverage more information from the longitudinal data than those using binary outcomes, so that it can provide an estimate of time-varying causal effects on risk of diseases. This gives more information about the age at which the effects start and how long they persist, which is of importance because in many cases intermediate phenotypes are measured at different ages across the sample at the baseline. In the survival context using the additive hazards model, the assumption of a constant causal effect at different ages, which is not always realistic, can be relaxed (Tchetgen Tchetgen et al., 2015). For example, large BMI at early stage of life may have different effects on T2D compared to middle age. Therefore, it is of enormous interest to investigate how the causal effects are changed and modulated by other environmental factors during the life course.
In this work, we perform MR analyses to investigate the causal effects of five cardiovascular disease risk factors including BMI, systolic blood pressure (SBP), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and triglycerides on eight age-related diseases including DM, coronary heart disease (CHD), heart failure (HF), myocardial infarction (MI), stroke, cancer, atrial fibrillation (AF) and AD. These diseases are major causes of death in developed societies. We include more than 30,000 individuals from five NIH-funded large-scale longitudinal cohorts (Atherosclerosis Risk in Communities study (ARIC), the Framingham Heart Study (FHS), the Multi-Ethnic Study of Atherosclerosis (MESA), the Cardiovascular Health Study (CHS), and the Women’s Health Initiative (WHI)) and perform a meta-analysis to combine the causal effects estimated from each cohort. We estimate the causal effects on the age-at-onset of these diseases based on an additive hazards model. As some factors such as pleiotropy and population stratification can violate the assumption in the MR analysis (Burgess et al., 2015, 2016; Taylor et al., 2014), we construct polygenic scores based on well recognized genetic loci with known mechanisms as IVs and conduct various sensitivity analyses to minimize the possibility of reporting false positives.
Material and Methods
Study cohorts
We collected datasets from five large-scale longitudinal cohorts including ARIC, FHS, MESA, CHS, and WHI (total 30,505 individuals included) (Table 1). In each study, we only included non-Hispanic Caucasian subjects. For FHS, we only included the datasets of the FHS Original cohort (FHS cohort 1) and the FHS Offspring cohort (FHS cohort 2) because the third generation was too young to observe sufficient informative outcomes. Details of the study design, collection of samples, measurement of intermediate phenotypes and diagnosis of diseases have been described in previous publications (ARIC: (Sharrett, 1992; The ARIC investigators, 1989), FHS: (Cupples et al., 2009; Govindaraju et al., 2008; Splansky et al., 2007), MESA: (Bild et al., 2002), CHS: (Gottdiener et al., 2000), WHI: (The Women’s Health Initiative Study Group, 1998)). Table 1 summarizes the basic characteristics of the samples and provides a list of the phenotypes we investigated.
Table 1.
The basic characteristics of the cohorts included in the MR analyses. Sample size: the number includes non-Caucasian whites. Diseases: the age-related diseases examined in each cohort. AF is only available in ARIC and WHI. AD is only available in FHS and CHS. Cancer is not included in MESA due to its age-at-onset information is missing in the original data. Covariate: the covariates adjusted in the main MR analysis. ‘Cohort’ in FHS is the indicator for generation, i.e., FHS or FHSO. Other covariates: the covariates adjusted in the follow-up sensitivity analyses. BC: birth cohort. SM: smoking history (current smoker, ex-smoker, non-smoker). DR: alcohol consumption history (the concrete definition of it varies among the cohorts). EDU, education level (in FHS and CHS, the binary variable indicating low education level is adjusted due to large missing data in education level).
| Study | Sample size | Number of families | Male (%) | Average age at baseline (±sd) | Diseases | Covariate | Other confounder |
|---|---|---|---|---|---|---|---|
| ARIC | 9,810 | 9,105 | 47.12538 | 54.32956 (5.668133) | T2D, CHD, HF, MI, AF, cancer, stroke, CVD | Site | BC, Sex, SM, DR, EDU |
| FHS | 4,700 | 1,482 | 45.2766 | 35.90638 (9.247683) | T2D, CHD, HF, MI, cancer, stroke, AD, CVD | Cohort | BC, Sex, SM, DR, EDU |
| CHS | 3,310 | 3,310 | 39.75831 | 72.40634 (5.409841) | T2D, CHD, HF, MI, cancer, stroke, AD, CVD | - | BC, Sex, SM, DR, EDU |
| MESA | 2,685 | 2,649 | 47.85847 | 62.74413 (10.15761) | T2D, CHD, HF, MI, stroke, CVD | Site | BC, Sex, SM, EDU |
| WHI | 10,000 | 9,990 | 0 | 67.0469 (6.447857) | T2D, CHD, HF, MI, AF, cancer, stroke, CVD | Region | BC, SM, DR, EDU |
Phenotypes and covariates
In each cohort, we used the measurements of BMI, SBP, HDL-C, LDL-C and triglycerides at the entry of enrollment, so that the precedence of the factor to the diagnosis of diseases was guaranteed to minimize the possibility of reverse causation. For the disease outcomes, we included T2D, CHD, HF, MI, stroke and CVD in all five cohorts. The CVD variables were constructed separately in each cohort from CHD, HF, MI and stroke to harmonize the definition across the cohorts (The details of the construction of the CVD variable are provided in Table S9). The age at onset of T2D in WHI was reckoned from self-reported treatment history or the age at diagnosis that was categorized into age groups. We included cancer in ARIC, FHS, CHS and WHI, which was not included in MESA due to missing information on age at onset in the original data. In FHS and WHI, we used the cancer variable that excluded skin cancer because the information was either not available or inaccurate. Additionally, we included AF in ARIC and WHI, and AD in FHS and CHS in which we selected AD or mild dementia. In the sensitivity analyses adjusted for potential confounders (described later), we included sex, birth cohort, which were non-heritable variables, and further education level, smoking and drinking status as covariates. In CHS and FHS, we determined a smoker and a drinker at the baseline according to cigarettes per day (>0) and alcohol consumption (>0), respectively. In CHS and FHS, we used an indicator of low education as there were large missing data in the variable of education level. The inclusion of diseases, covariates and confounders in each cohort is summarized in Table 1.
Accession numbers
This manuscript was prepared using a limited access datasets obtained though dbGaP (accession numbers phs000007.v22.p8, phs000280.v2.p1, phs000209.v12.p3, phs000287.v3.p1).
Genotyping, quality control and imputation
Genotyping of 12,771 ARIC participants (N=9,633 whites, N=9,618 included) and 8,224 MESA participants (N=2,686 whites, N=2,455 included) was conducted using an Affymetrix 6.0 array (1,000K SNPs). Genotyping of 9,167 participants (N=4,594 included) in FHS was conducted using an Affymetrix 500K array. Genotyping of 3,043 participants in CHS was done using an Illumina Human Omni1-Quad array. In WHI, genotyping were performed in two separate groups that used an Illumina Human Omni1-Qud array (1M SNPs) and an Illumina Human Omni Express array (~730K SNPs). All genotyped SNPs were further filtered for the analysis according to the exclusion criteria of the Hardy-Weinberg equilibrium test p<1e-05 and missing rate <5%. We imputed the missing genotypes of the SNPs that were used in the construction of the polygenic scores for the MR analysis (details provided in the following subsection). The imputation was performed using IMPUTE2 (Howie et al., 2009) with a 1000 Genomes Project Phase I reference panel and those SNPs with an imputation information score<0.8 were excluded. The imputation information score and minor allele frequency (MAF) for each SNP in each cohort is provided in the Supplementary materials Tables S1–S5.
Construction of polygenic scores
We created weighted genetic scores based on a group of selected independent genetic variants that have been recognized to show biological link to each phenotype. Following the same strategy as previous studies (Østergaard et al., 2015; Richmond et al., 2014) for including associated variants, we adopted 32 variants and 25 variants with their effect sizes estimated from large-scale GWAS meta-analyses (Speliotes et al., 2010; The International Consortium for Blood Pressure Genome-Wide Association Studies, 2011) for BMI and SBP, respectively. For lipids, we included the SNPs that were reported in a large-scale meta-analysis of blood lipid traits (Benjamin F. Voight et al., 2012) and were carefully selected in a recent MR study (Holmes et al., 2015). These included 48 variants for HDL-C, 42 variants for LDL-C and 67 variants for triglycerides. The lists of the variants used for construction of the polygenic scores in each cohort are provided in Supplementary materials Tables S1–S5. Denote βj the effect size estimated from the meta-analyses for a SNP j, and gij its genotype (either genotyped or imputed) for an individual i assuming additive genetic model. The polygenic score Gi for a specific phenotype with n associated variants for the individual i was calculated according to
Time-to-event MR analysis
For the time-to-event MR analysis, we adopt a two-stage regression approach utilizing an additive hazards model proposed by (Tchetgen Tchetgen et al., 2015). Specifically, we first fitted the following linear regression model for an intermediate risk factor M
in which ε is the random error term following a zero–mean normal distribution and X stands for other observed covariates such as cohorts and regions that are assumed to be independent of the polygenic score G. Thereby we obtained a predicted mean of the phenotype . In the second stage, given the age at an event T* = min(T, C), where T is the failure time and C is the assumed censoring time that is independent of the failure time, and the variable δ ∈ {0,1} indicating T* = T or C, we fitted the following additive hazards model
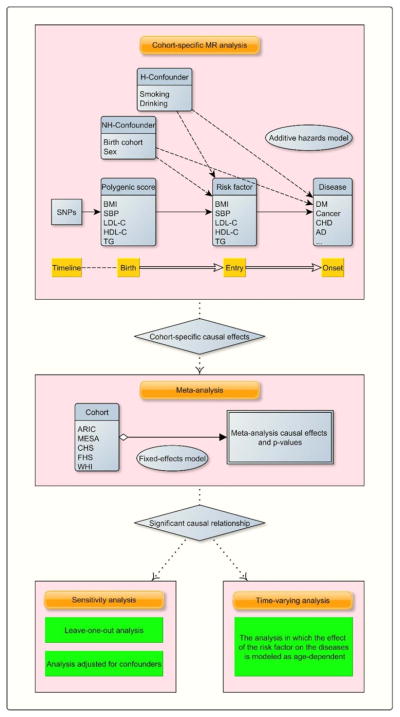
in which h(t|G, X) is the conditional hazard function evaluated at t given only G and X, b0(t) is the baseline hazard, b1(t) is the time-varying causal effect of the risk factor M̂ (Thus, is the cumulative causal effect up to the time s) and b2(t) is the effect of the covariates. Following the similar spirit of (Lamarca et al., 1998), we adopted age as the time scale because age per se was of primary interest and could be a potential confounder. We used the age at entry into the study as left censoring. The effect size b1(t) of our interest is interpreted as the change of the absolute hazard with respect to one unit change of M̂. In our main MR analysis, we fitted a restricted model in which we assume an age-invariant causal effect over age (i.e., b1(t) = b1), and then relaxed this assumption for significant findings (p<0.05) in the follow-up analyses in pursuit of its dynamic effects. The estimate of the effect size was computed using the R package timereg (Martinussen and Scheike, 2007). The package also provides a Kolmogorov–Smirnov test for the hypothesis of a constant effect (b1(t) = b1). As pointed out previously (Tchetgen Tchetgen et al., 2015), the standard error of should take into account both the uncertainty of the estimation in the first stage and family structure in some studies such as FHS. We estimated the standard error using a non-parametric bootstrap method with 500 bootstrap samples that were drawn based on the family structure. In the analysis assuming a constant effect size, we conducted a meta-analysis based on a fixed-effects model combining the estimates from the five cohorts. More specifically, we calculated the combined causal effect size as and its standard error as , in which k is the index of the studies and is the weight for the study k. The overall strategy of the MR analysis is depicted in Figure 1.
Figure 1.
A flowchart of the time-to-event MR meta-analyses. H-confounder: heritable confounders such as age and sex. NH-confounder: non-heritable confounders. Entry: the age at the entry into the follow-up cohort study. Significant causal relationship: the associations with a p-value<0.05.
Sensitivity analysis
We performed several sensitivity analyses to assess the robustness of the identified causal effects and ruled out potential false positives. To examine whether and the extent to which the findings were affected by other potential confounders that were not included in the main analysis, we performed a sensitivity analysis further including age, sex, education level, smoking and alcohol consumption history up to the enrollment as covariates. This is particularly relevant for the risk factors that vary over age such as SBP or differs in sex as inclusion of these covariates improves the estimate of the effect size of the polygenic score. As the stringency of criteria for the IV assumption might not be maintained for all variants in the allele score (Burgess and Thompson, 2013), we used an MR-Egger method (Bowden et al., 2015) as a sensitivity analysis to examine the influence of potential pleiotropic effects of the SNPs in the polygenic scores. As MR-Egger is originally proposed for a linear model (Bowden et al., 2015), we extended it for the additive hazards model (The detailed derivation is given in the supplementary materials). We further conducted a leave-one-out sensitivity analysis for the identified associations to check that the causal effect was not driven by a specific variant, in which the MR analysis was repeated with each SNP removed from the polygenic score. This analysis could discover those SNPs that harbor independent pleiotropic effects on both the intermediate risk factor and the disease. We also performed a meta-analysis for T2D without WHI because the estimated age at onset of T2D was less reliable in WHI. Another sensitivity analysis was conducted for FHS alone, in which we chose the age at genotyping as the starting point of the follow-up. This removed the potential selection bias for some diseases during the long interval in FHS between the entry into the study and the genotyping, although consequently we did not observe evident difference in the results.
Results
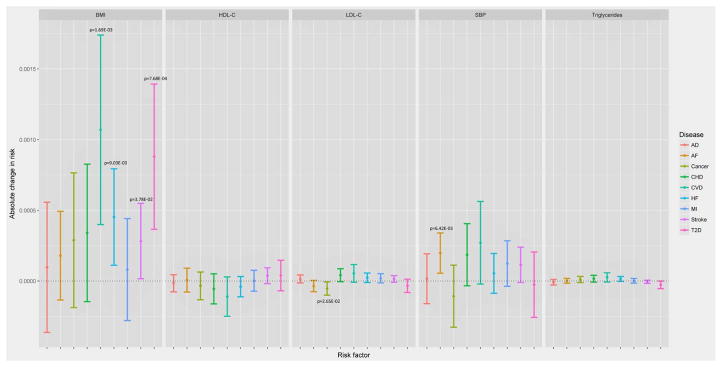
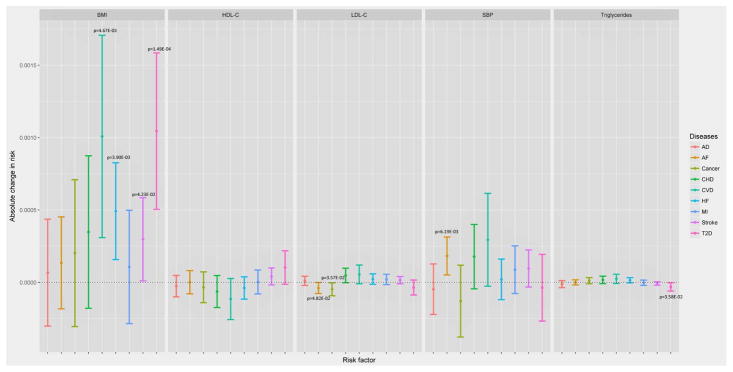
Each constructed polygenic score was significantly (p<0.05) associated with its risk factor (Table S7) (except for SBP in CHS when adjusted for the covariates and this was due to smaller sample size resulting from exclusion of missing data on the covariates), validating the assumption of the prediction of IVs for the risk factors. Conditional upon the risk factors, the vast majority of the associations between the polygenic scores and the age-at-onset were non-significant (Table S10), indicating no direct evidence of the violation of the conditional independence assumption between most polygenic scores and outcomes (except for HDL-C and T2D, TG and T2D, LDL-C and AD in several studies which will be discussed later separately). Table S10 also shows that the vast majority of the risk factors were significantly associated with the hazards of the diseases. The results from the main MR meta-analysis (without the adjustment for other covariates) of causal effects of the five risk factors on the hazards of the diseases are plotted in Figure 2 (The detailed results are given in Table S12). From the main analysis, we observed 6 significant associations including BMI with T2D, HF, stroke and CVD, SBP with AF, and LDL-C with cancer. Two additional significant associations (triglycerides and T2D, LDL-C and AF) were identified in the sensitivity analysis adjusted for the other covariates (Figure 3). From the analysis of T2D excluding WHI, we found one more significant association between LDL-C and T2D. For these significant associations, the results from the leave-one-out sensitivity analyses and the MR-Egger analysis, which scrutinized the reliability of the findings, suggest that some of the associations probably were originated from potential pleiotropic effects of certain genetics variants.
Figure 2.
Results from the main meta-analysis and the sensitivity meta-analyses for the association between five genetically predicted risk factors and onset of seven age-related diseases. The model used in this analysis assumes a constant causal effect over age. The statistically significant associations (p<0.05) are annotated.
Figure 3.
Results from the sensitivity meta-analyses adjusted for sex, birth cohort, education and smoking and alcohol consumption history for the association between five genetically predicted risk factors and onset of seven age-related diseases. The model used in this analysis assumes a constant causal effect over age. The statistically significant associations (p<0.05) are annotated.
Elevated BMI levels associated with development of DM, HF and CVD
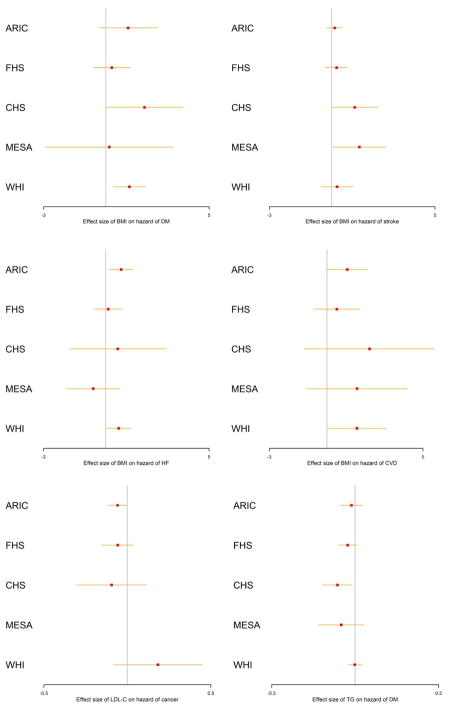
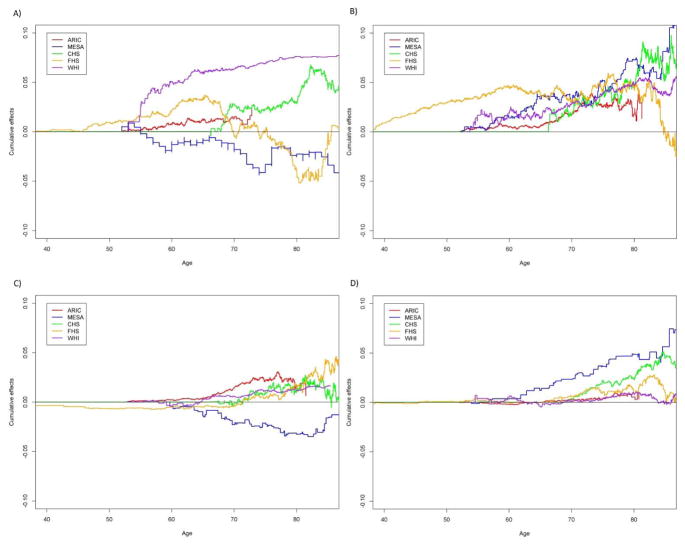
From the main MR analyses, we observed that per 1 kg/m2 increase of BMI significantly (p<0.05) causally increased the hazard of T2D by 8.80e-04 (increasing 0.88 cases per 1000 person-year) (beta=8.80e-04, p=7.68e-04). This causal effect of BMI became more significant after adjusted for the covariates (beta=1.04e-03, p=1.49e-04) (Table S12). The result was consistent with the estimate from MR-Egger, which also suggested no evidence of directional pleiotropy (Table S11). This association remained significant in the analyses without WHI when adjusted for the covariates (p=2.28E-2) (Table 2). The leave-one-out sensitivity analyses suggested that no single SNP in the polygenic score was predominantly responsible for the causal association (Table S8). Most of the diagnosis of T2D occurred within 10 years after the enrollment in ARIC, MESA, CHS and WHI and between 20–30 years in FHS (Figure S1). The increasing effects of BMI on the hazards of T2D were consistent across the five studies (Figure 4). A steep growth of the dynamic effects estimated from the age-dependent analysis became evident from age 50 and shared similar patterns in ARIC, CHS, FHS and WHI until age 70 (Figure 5), although the test for age-invariant effects suggested that the age-dependent effects of BMI during the follow-up interval did not significantly deviate from a constant in all cohorts except WHI (Table S6).
Table 2.
Results from the sensitivity analysis of association between five genetically predicted risk factors and the onset of T2D, in which the WHI cohort is excluded. The statistically significant associations (p<0.05) are highlighted in boldface.
| Risk factor | Main analysis | Analysis adjusted for all covariates | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Change in absolute risk | SD | P | Change in absolute risk | SD | P | |
|
|
||||||
| BMI | 6.70E-04 | 3.50E-04 | 5.57E-02 | 9.07E-04 | 3.99E-04 | 2.28E-02 |
|
|
||||||
| SBP | −9.52E-05 | 1.68E-04 | 5.71E-01 | −1.66E-04 | 1.96E-04 | 3.98E-01 |
|
|
||||||
| HDL-C | 3.99E-05 | 6.69E-05 | 5.51E-02 | 9.98E-05 | 7.55E-05 | 1.86E-01 |
|
|
||||||
| LDL-C | −7.98E-05 | 3.12E-05 | 1.05E-02 | −8.14E-05 | 3.32E-05 | 1.42E-02 |
|
|
||||||
| Triglycerides | −4.78E-05 | 1.88E-05 | 1.09E-02 | −5.11E-05 | 2.05E-05 | 1.25E-02 |
Figure 4.
Forest plots of the estimated effect sizes with the 95%CIs in the five cohorts for the six significant associations identified from the main meta-analysis. X-axis: the effect size (10−3) in the additive hazards model.
Figure 5.
Age-dependent cumulative causal effects of BMI on T2D, HF, CVD and stroke from age 40 to 85 in the five cohorts. Cumulative effect is the cumulative change of the absolute hazard with one unit change of the risk factor. A) BMI on T2D, B) BMI on CVD, C) BMI on HF, D) BMI on stroke.
We found elevated BMI levels also significantly (p<0.05) increased the hazard of HF (beta=4.53e-04, p=9.03e-03) in the main MR analyses, and this causal association became more significant after the adjustment for the covariates (beta=4.92e-04, p=3.90e-03) (Table S12). The detrimental effects of BMI were observed in all cohorts except MESA (Figure 4). The result was consistent with the estimate from the MR-Egger analysis (Table S11). The leave-one-out sensitivity analyses suggested that no single SNP in the polygenic score dominated the causal association (Table S8). Similar to T2D, the dynamic effects shared similar patterns in ARIC, CHS, FHS and WHI (Figure 5). The test for the age-invariant effects suggested that the effects of BMI during the follow-up interval did not significantly deviate from a constant across all cohorts (Table S6).
We observed that higher BMI levels were associated with the risk of CVD in the main MR analyses (beta=1.07e-03, p=1.69e-03) and the association became more significantly when adjusted for the covariates (beta=1.01e-03, p=4.67e-03) (Table S12). The effects on the hazards of CVD were consistent across all of the cohorts included (Figure 4). The result was consistent with the estimate from MR-Egger (Table S11). The age-dependent analyses suggested that the accumulative effects of BMI on the hazards of CVD started to rise immediately after the entry into the follow-up across these cohorts (Figure 5). We also observed that the effect was age-dependent only in FHS (p=9.0e-03) (Table S6) and dropped dramatically after age ~80.
In addition, we found that elevated BMI levels seemed to be significantly (p<0.05) associated with the increasing hazards of stroke (beta=2.83e-04, p=3.78e-02), and this association remained significant after adjusted for the covariates (beta=2.98e-04, p=4.23e-02) (Table S12). The effects of BMI on the hazards of stroke were consistent across the five cohorts in the main analyses (Figure 4). Nevertheless, this association became non-significant after removing certain SNPs (e.g., rs2867125 or rs7359397 (located on 16p11.2)) from the polygenic score (Table S8). The result from MR-Egger also showed significant directional pleiotropic effects (Table S11). The test for age-invariant effects suggested that the effects of BMI across the follow-up interval did not significantly deviate from a constant in ARIC, MESA, CHS and WHI (Table S6). The dynamic effects started to rise from age ~65 and shared very similar pattern across the five cohorts (Figure 3).
Elevated SBP levels associated with development of AF
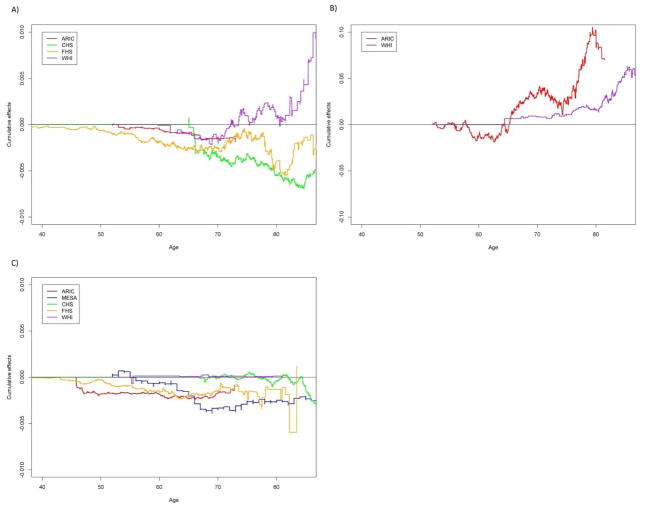
We observed that elevated SBP levels increased the hazard of AF (beta=1.98e-04, p=6.42e-03), and after adjusted for the covariates, this association remained significant (beta=1.83e-04, p=6.19e-03) (Table S12). The result from MR-Egger also showed significant directional pleiotropic effects (Table S11). There was no single underlying SNP in the polygenic score that drove this association (Table S8). The age-dependent analyses showed that the effects rose dramatically after age ~65 in both cohorts (ARIC and WHI). We did not observe an association between AF and any of the other intermediate phenotypes.
LDL-C levels are inversely associated with hazards of cancer and T2D
We observed that LDL-C levels were significantly (p<0.05) inversely associated with the hazards of cancer in the main analyses (beta=−5.28e-05, p=2.65e-02). This negative association was replicated after adjusted for the covariates (beta=−4.78e-05, p=3.57e-02) (Table S12). The negative effect on the hazards was observed in ARIC, CHS and FHS, but not in WHI (Figure 4). The age-dependent analyses showed that the dynamic effect estimated from WHI turned into positive and rose steadily after age ~75, which was in an opposite temporal pattern compared to those from the other cohorts (Figure 6). The onset of cancer occurred on average 5 years since the enrollment in ARIC and CHS, almost a decade in WHI, and ~30 years in FHS (Figure S1 (c)). Although the result from MR-Egger showed no evidence of directional pleiotropic effects (Table S11), the leave-one-out analysis showed that the association was no longer significant after excluding rs4970834 (located on 1p13.3) from the polygenic score (p= 0.0613) (Table S8).
Figure 6.
Age-dependent cumulative causal effects of (A) LDL-C on cancer, (B) SBP on AF and (C) triglycerides on T2D from age 40 to 85. Cumulative effect is the cumulative change of the absolute hazard with one unit change of the risk factor. The AF variable is not available. Due to a numerical issue, we used a scaled SBP (0.1*SBP) when fitting the dynamic additive hazards model using timereg.
We also observed that LDL-C was significantly (p<0.05) inversely associated with the hazards of T2D in the analysis without WHI regardless of the covariates adjusted (beta=−7.98e-05, p=1.05E-02 in the main analyses, beta=−8.14E-05, p=1.42E-02 after adjusted for all covariates) (Table 2), and the negative effect was consistent in all of the four cohorts (Table S7). The leave-one-out analysis showed that these two associations still remained significant, indicating no single underlying SNP drove the associations (Table S8).
Relationship between triglycerides and risk of T2D
We did not observed significant inverse association between triglyceride levels and the hazards of T2D in the main MR analysis; however, the association became significant when adjusted for all covariates (Figure 3). And it became even more significant in the sensitivity analysis of T2D without WHI regardless of the adjustment of the covariates (Table 2). The inverse association was observed consistently across all cohorts (Figure 4). The age-dependent analyses suggested that the cumulative negative effects increased immediately after the entry into the enrollment, particularly in ARIC, FHS and MESA, and then leveled off after age of ~70 (Figure 6). However, this association became non-significant after some SNPs (e.g. rs1260326) were removed from the polygenic score (Table S8). In addition, the result from MR-Egger also showed that the association was not significant (Table S11).
No risk factors associated with AD
For AD, we did not observe an association with any of the risk factors except LDL-C, which was significant only in CHS (Table S7); however, the association was no longer significant after excluding rs2075650 or rs10402271 in CHS. The results from MR-Egger also suggest no evidence of causal effects of BMI, SBP, LDLC and HDLC. For triglyceride, both a causal effect and directional pleiotropy were observed.
Discussion
In this study, we perform time-to-event MR analyses to unravel the causality between five intermediate risk factors and the onset of age-related diseases. Combining the results from the MR analyses using the elaborately constructed polygenic scores and the follow-up sensitivity analyses, we found evidence for four associations between genetic predicted biomarkers and risk of diseases. By leveraging the additive hazards model, we have a refined picture of the dynamic pattern of causal effects if any. Combining with the MR-Egger and leave-one-out sensitivity analyses, we further found that some significant associations identified from the score-based two stage MR analyses are probably due to pleiotropic effects of certain SNPs.
Specifically, higher levels of BMI are associated with increased hazards of T2D. It is found that BMI as well as obesity is a strong predictor of T2D (Garber, 2012; Meisinger et al., 2006) and, in particularly, is the risk factor with the highest impact in Indian population (He et al., 2015). Recent MR studies with large sample sizes provide more evidence for the causal relationship between BMI and T2D (Corbin et al., 2016; Holmes et al., 2014; Lyall et al., 2017). An epigenome-wide association study of T2D in Indians also identifies pathways related to obesity (Chambers et al., 2014). Our result is in line with those from the previous studies and further show that obesity had nearly constant detrimental effects on the onset of T2D before age of ~70, and after that, the effects from the five cohorts diverged with different temporal patterns.
Besides BMI, numerous studies report that high triglycerides are frequently associated with increased risk of T2D (He et al., 2015; Hjellvik et al., 2012; Tirosh et al., 2008). However, MR studies either do not confirm (De Silva et al., 2011) or even report negative associations between genetic predisposition to elevated triglyceride levels and higher risk of T2D (Klimentidis et al., 2015; White J et al., 2016). Although our results adjusted for the covariates show that there is a protective effect of triglyceride on the risk of T2D, the leave-one-out analysis and the MR-Egger analysis suggest that the association is due to pleiotropy of certain SNPs including rs1260326. Previous reports (Klimentidis et al., 2015; Klimentidis and Arora, 2016) point out the likely pleiotropic nature of triglycerides-related SNPs such as rs1260326 in GCKR gene as one of several drivers of the pleiotropic effects. The non-synonymous common variant rs1260326, with inverse effects on triglycerides and insulin resistance, is located in glucokinase regulatory protein (GCKR) that plays a critical role for maintaining glucose homeostasis and has pleiotropic effects on multiple metabolic phenotypes (Brouwers et al., 2015; Raimondo et al., 2015; Van Schaftingen et al., 1994). Rs1260326 is in strong LD with other variants in GCKR associated with a range of metabolic phenotypes (Dupuis et al., 2010; Orho-Melander et al., 2008; Sparsø et al., 2008).
Although plasma LDL-C levels are usually normal in T2D (Sniderman et al., 2001), an increased proportion of atherogenic small dense LDL particles and modified LDL (oxidized LDL and glycated LDL) have been reported (Njajou et al., 2009; Vergès, 2009). Insulin resistance and pancreatic β-cell dysfunction are the core problems of T2D. LDL-C has the adverse effects on insulin secretion and modified LDL (e.g., ox-LDL) has the negative effects on pancreatic β-cell survival. Ox-LDL levels increase in diabetic patients over time independently of maintaining optimized levels of LDL-C (Nakhjavani et al., 2010). Recent MR studies employing the MR-Egger regression (Bowden et al., 2015; White J et al., 2016) find that increased LDL-C levels are associated with lower risk of T2D, which is consistent with our results.
A latest large-scale analysis based on the summary statistics from twenty-three prospective studies report strong association between BMI and HF incidence (Aune et al., 2016), which is in accordance with the results from our meta-analyses. However, it is unclear why BMI has a protective effect on the risk of HF in MESA. Although overweight and obesity are linked to HF (Clark et al., 2014), the nature of this association is not clear. For example, the study of 5,881 FHS participants (Kenchaiah et al., 2002) suggests that obesity is an important risk factor for HF. However, the authors observed the smaller effect of BMI on the risk of HF in subjects with hypertension and the lack of the effect of BMI on the risk of HF in subjects with MI, although the latter could be because of the small sample. BMI was not associated with HF risk in (Voulgari et al., 2011). Also, HF patients with increased BMI have reduced mortality rates (Clark et al., 2014; Oreopoulos et al., 2008). The reasons for the obesity paradox in HF and other cardiovascular diseases remain unclear. Furthermore, the obesity paradox can be sensitive to the cardiorespiratory fitness (Clark et al., 2014; Lavie et al., 2013). The results in MESA may, therefore, be plausibly explained by different degree of physical capacity of the study participants.
In our main analysis, we did not find significant causal effect of BMI on the risk of CHD although the effect was significant in the sensitivity analysis using MR-Egger. Multiple recent MR studies report large causal effect of BMI on CHD (Dale et al., 2017; Hägg et al., 2015; Lyall et al., 2017). The difference is probably due to the smaller number of the CHD cases in our study, and consequently there is no enough statistical power to detect the effect.
A previous MR study using a logistic model does not find significant association between BMI and the incidence of stroke (Holmes et al., 2014), while a more recent MR study using Cox proportional hazards model suggests that a causal effect of adiposity on development of ischemic stroke (Hägg et al., 2015), which is consistent with our results from the main MR analysis. Nevertheless, our results from MR-Egger indicated the evidence of directional pleiotropy, and the leave-one-out analysis further suggested that rs2867125 and rs7359397 can be the key SNPs underlying the association with stroke. Rs7359397, located in the 16p11.2 region, is in perfect LD with multiple SNPs in the SH2B1 gene (Jamshidi et al., 2007), including the non-synonymous rs7498665 SNP associated with total fat, waist circumference, body weight and BMI (Speliotes et al., 2010). SH2B1 adapter protein is involved in multiple signaling pathways including insulin and leptin signaling (Li et al., 2007, p. 1; Maures et al., 2007, p. 1). The level of the adipose-derived hormone leptin is associated with the risk of stroke (Kim et al., 2012; Liu et al., 2010; Söderberg et al., 2003).
We found that the inverse association between LDL-C levels and the onset of cancer was consistent across three of the cohorts except WHI, in which the effect turns into positive after age of ~70. As WHI consisted of only female subjects, the positive association was probably due to breast cancer which had the biggest prevalence among all cancers in women. A previous study utilizing in vitro and in vivo models of cholesterol enrichment provides novel mechanistic evidence that high LDL-C levels promote breast cancer progression (Rodrigues dos Santos et al., 2014). Without WHI, our results are in line with a previous study (Lavigne et al., 2012) that assesses the trend of LDL-C for an extended period of time prior to cancer diagnosis without history of lipid-lowering therapy using data from the FHSO. This previous study demonstrates an inverse association between LDL-C and cancer extending over 18 years prior to diagnosis. However, the causal role of LDL-C in the biological etiology of cancer is still elusive. Another MR study suggests that LDL-C levels per se do not cause cancer (Benn et al., 2011), but the non-significant result is probably due to the lack of statistical power as only several SNPs associated with low LDL-C levels are used in this study. One hypothesis for the inverse association is potential reverse causation (Trompet et al., 2009) because cancer may start to develop before the measurement of the exposure, i.e., LDL-C in this case. The observation of an inverse association in our analysis of the FHS cohort seems not to support the reverse causality hypothesis because most measurements of LDL-C in FHS predate cancer diagnosis by three decades as shown in the density plot. Another potential explanation is the effect of treatments such as statin for controlling LDL-C levels. Nevertheless, it is still under debate whether treatment with statin has a beneficial or detrimental effect on the incidence of cancer (Jukema et al., 2012).
BP is one of the main risk factors for the development of AF and is involved in all of the underlying potential risk models for AF (Manolis et al., 2012). We found that the significant associations were observed in both cohort and the cumulative effects soared even at older age. This result seems to conflict with a latest large-scale association study using linked electronic health records, which shows that the significant effect of SBP attenuates with increasing age (Emdin et al., 2016).
Our results showed that the association between LDL-C and AD was no longer significant in CHS when excluding rs2075650 (in TOMM40) and rs10402271 (associated with BCAM). Both SNPs are close to APOE, which has been shown to have pleiotropic effects on lipids and AD (Østergaard et al., 2015; Proitsi et al., 2014). Unlike the previous findings about SBP (Østergaard et al., 2015), we did not observe the significant association between SBP and the hazards of AD despite the observation of the same direction of effect size.
The significant findings from the analysis using the polygenic scores are consistent with the results from the MR-Egger method. However, MR-Egger detected additional significant causal associations, including the causal effects of LDL-C on CHD, HF, CVD, stroke and MI. Although a recent study shows that MR-Egger may be biased and interpretation should be cautious (Burgess and Thompson, 2017), we notice that the major difference in these associations between MR-Egger and the method using polygenic scores results from different estimates in WHI, which only includes female individuals. As LDL-C has been widely reported to have causal influence on CHD (Ference et al., 2012; Holmes et al., 2015; Linsel-Nitschke et al., 2008) and MI (Benjamin F Voight et al., 2012), this indicates that direct use of the polygenic scores constructed from GWAS may not be powerful in an MR analysis for a specific sub-population.
Although much work has been done to minimize the bias and increase the statistical power, the MR study still has some limitations. First, the definitions of the diseases and criteria of diagnosis may vary across these five cohorts. This may explain some of the observed heterogeneity among these cohorts. Second, the constructed polygenic score can only explain a small proportion of the variance of the intermediate risk factors, which may limits the statistical power to detect the causal effects. Third, the medication history during the follow-up can also affect the estimated effects of certain biomarkers on the hazards of diseases. Fourth, the numbers of cases for some diseases are small, so there might be no sufficient statistical power to detect the causal effect for these diseases.
In conclusion, we report significant causal associations between certain cardiovascular intermediate risk factors and the onset of age-related diseases. The estimated causal effects highlight BMI, which is significantly associated with increased risks of T2D, HF and CVD, implying its potential etiological role implicated in these age-related diseases and the importance as the target for prevention procedures. SBP has a causal effect on AF, which manifests even after age ~80. In addition, we provide evidence to show that multiple previously reported associations are probably not driven by its biological causality, but rather result from the potential pleiotropy of certain genetic variants, either biological or spurious (pleiotropy due to design artefact or LD with two different causal variants (Solovieff et al., 2013)). The association of BMI with stroke may be partly attributed to the pleiotropic effects of multiple SNPs including rs1558902, rs2867125 and rs7359397. The identified inverse association between triglyceride levels and the onset of T2D may attribute to the pleiotropy of SNPs like rs1260326. These findings provide more insights into the biological mechanisms underlying the observed associations between these common risk factors and the risk of age-related diseases.
Supplementary Material
Figure S1: The density plot of years from age at the baseline to the onset of the age-related diseases in each cohort.
Text S1: The detailed description of the derivation of the MR-Egger method in the survival context.
Table S1. The detailed information of each SNP included in the MR analyses for the construction of polygenic score for BMI. SNP: rs ID. Gene: the gene associated with the SNP. Chr: the chromosome on which the SNP is located. Pos: the position of the SNP on that chromosome. Weight: the weight used for calculation of the polygenic score for BMI. The weight is obtained from the effect size estimated from the previous reported meta-analysis (Speliotes et al., 2010). Increasing/other allele: The allele corresponding to the positive effect size/the other allele. MAF: the MAF estimated from the samples in a specific cohort. Info score: The imputation quality score from IMPUTE2.
Table S10. The results for checking the assumption of conditional independence required for the MR analyses. ES/SD/P (POLY): the effect sizes/standard errors/p-values of the polygenic score on the age-at-onset of the disease based on the additive hazards model (described in the Materials and Methods section) with the adjustment of the risk factor. ES/SD/P (RF): the effect sizes/standard errors/p-values of the risk factor on the age-at-onset of the disease based on the additive hazards model (described in the Materials and Methods section) without other adjustment.
Table S11. The results from the MR-egger sensitivity analysis. The analysis adjusted for all covariates. The meta-analysis combining the results from the five cohorts was performed by using a fixed-effects model. Risk factor: the risk factor of which the causal effect is estimated. Disease: the disease on which the causal effect is estimated. Coef_intercept: the estimate of the intercept (directional pleiotropy) in MR-Egger. Sd_intercept: the standard error of the intercept estimate in MR-Egger. P_intercept: the p-value for the intercept. Coef_slope: the estimate of the slope (causal effect) in MR-Egger. Sd_slope: the standard error of the slope estimate in MR-Egger. P_slope: the p-value for the slope.
Table S12. The detailed results from the main MR analysis and the sensitivity analysis adjusted for the covariates.
Table S2. The detailed information of each SNP included in the MR analyses for the construction of polygenic score for SBP. SNP: rs ID. Gene: the gene associated with the SNP. Chr: the chromosome on which the SNP is located. Pos: the position of the SNP on that chromosome. Weight: the weight used for calculation of the polygenic score for SBP. The weight is obtained from the effect size estimated from the previous reported meta-analysis (The International Consortium for Blood Pressure Genome-Wide Association Studies, 2011). Increasing/other allele: The allele corresponding to the positive effect size/the other allele. MAF: the MAF estimated from the samples in a specific cohort. Info score: The imputation quality score from IMPUTE2.
Table S3. The detailed information of each SNP included in the MR analyses for the construction of polygenic score for LDL-C. SNP: rs ID. Gene: the gene associated with the SNP. Chr: the chromosome on which the SNP is located. Pos: the position of the SNP on that chromosome. Weight: the weight used for calculation of the polygenic score for LDL-C. The weight is obtained from the effect size estimated from the previous reported meta-analysis (Benjamin F. Voight et al., 2012). Increasing/other allele: The allele corresponding to the positive effect size/the other allele. MAF: the MAF estimated from the samples in a specific cohort. Info score: The imputation quality score from IMPUTE2.
Table S4. The detailed information of each SNP included in the MR analyses for the construction of polygenic score for HDL-C. SNP: rs ID. Gene: the gene associated with the SNP. Chr: the chromosome on which the SNP is located. Pos: the position of the SNP on that chromosome. Weight: the weight used for calculation of the polygenic score for HDL-C. The weight is obtained from the effect size estimated from the previous reported meta-analysis (Benjamin F. Voight et al., 2012). Increasing/other allele: The allele corresponding to the positive effect size/the other allele. MAF: the MAF estimated from the samples in a specific cohort. Info score: The imputation quality score from IMPUTE2.
Table S5. The detailed information of each SNP included in the MR analyses for the construction of polygenic score for triglycerides. SNP: rs ID. Gene: the gene associated with the SNP. Chr: the chromosome on which the SNP is located. Pos: the position of the SNP on that chromosome. Weight: the weight used for calculation of the polygenic score for triglycerides. The weight is obtained from the effect size estimated from the previous reported meta-analysis (Benjamin F. Voight et al., 2012). Increasing/other allele: The allele corresponding to the positive effect size/the other allele. MAF: the MAF estimated from the samples in a specific cohort. Info score: The imputation quality score from IMPUTE2.
Table S6. The results of testing age-invariant causal effects from a time-varying additive hazards model based on a Kolmogorov–Smirnov test. P(B=0): The p-value from the test of no effects. P(B=C): The p-value from the test of an age-invariant effect.
Table S7. The detailed results in each cohort from the discovery meta-analysis and the sensitivity meta-analyses adjusted for sex, birth cohort, education level, smoking and alcohol consumption history for the association between genetically predicted risk factors and onset of age-related diseases. Coef: the effect size from the additive hazards model. SD_boot: the SD calculated from the bootstrap method. P_boot: the p-value calculated based on the effect size and SD. P_polygenic: the p-value for the association between the polygenic score and the risk factor in that specific analysis.
Table S8. The results from the leave-one-out sensitivity analysis, in which each SNP is excluded from the polygenic score. p_meta: the p-values from the meta-analysis. p_ARIC: the p-values from the ARIC (the same for FHS, MESA, CHS and WHI).
Table S9. Details of the construction of the CVD variable in each cohort.
Highlights.
BMI is involved in the development of multiple age-related diseases including type 2 diabetes, heart failure and cardiovascular diseases.
SBP has causal effect on atrial fibrillation, which manifests even after age ~80.
Multiple SNPs that may possess potential pleiotropic effects responsible for some observed associations.
These findings provide more evidence for the potential role of pleiotropic effects of genetic variants underlying the observed associations between these risk factors and the onset of age-related diseases.
Acknowledgments
The research reported in this paper was supported by Grants No P01 AG043352 and R01AG047310 from the National Institute on Aging (NIA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
The Atherosclerosis Risk in Communities Study (ARIC) is carried out as a collaborative study supported by the NHLBI contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contributions. Funding for GENEVA was provided by the National Human Genome Research Institute grant U01HG004402 (E. Boerwinkle).
The Framingham Heart Study (FHS) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with Boston University (Contract No. N01-HC-25195). This manuscript was not prepared in collaboration with investigators of the FHS and does not necessarily reflect the opinions or views of the FHS, Boston University, or NHLBI. Funding for SHARe Affymetrix genotyping was provided by NHLBI Contract N02-HL-64278. SHARe Illumina genotyping was provided under an agreement between Illumina and Boston University.
Multi-Ethnic Study of Atherosclerosis (MESA) and the MESA SHARe project are conducted and supported by the NHLBI in collaboration with MESA investigators. Support for MESA is provided by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169 and CTSA UL1-RR-024156. Funding for SHARe genotyping was provided by NHLBI Contract N02-HL-64278. This manuscript was not prepared in collaboration with MESA investigators and does not necessarily reflect the opinions or views of MESA, or the NHLBI.
The Cardiovascular Health Study (CHS) was supported by contract numbers N01-HC- 85079, N01-HC-85080, N01-HC-85081, N01-HC-85082, N01-HC-85083, N01-HC- 85084, N01-HC-85085, N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC- 55222, N01-HC-75150, N01-HC-45133, N01-HC-85239 and HHSN268201200036C; grant numbers U01 HL080295 from the NHLBI and R01 AG-023629 from the NIA, with additional contribution from the National Institute of Neurological Disorders and Stroke. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. This manuscript was not prepared in collaboration with CHS investigators and does not necessarily reflect the opinions or views of CHS, or the NHLBI. Support for the genotyping through the CARe Study was provided by NHLBI Contract N01-HC-65226.
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
References
- Aalen O. Nonparametric Inference for a Family of Counting Processes. Ann Stat. 1978;6:701–726. doi: 10.1214/aos/1176344247. [DOI] [Google Scholar]
- Arbeev KG, Ukraintseva SV, Akushevich I, Kulminski AM, Arbeeva LS, Akushevich L, Culminskaya IV, Yashin AI. Age trajectories of physiological indices in relation to healthy life course. Mech Ageing Dev. 2011;132:93–102. doi: 10.1016/j.mad.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune D, Sen A, Norat T, Janszky I, Romundstad P, Tonstad S, Vatten LJ. Body Mass Index, Abdominal Fatness and Heart Failure Incidence and Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Circulation CIRCULATIONAHA.115.016801. 2016 doi: 10.1161/CIRCULATIONAHA.115.016801. [DOI] [PubMed] [Google Scholar]
- Benn M, Tybjærg-Hansen A, Stender S, Frikke-Schmidt R, Nordestgaard BG. Low-density lipoprotein cholesterol and the risk of cancer: a mendelian randomization study. J Natl Cancer Inst. 2011;103:508–519. doi: 10.1093/jnci/djr008. [DOI] [PubMed] [Google Scholar]
- Bild DE, Bluemke DA, Burke GL, Detrano R, Roux AVD, Folsom AR, Greenland P, JacobsJr DR, Kronmal R, Liu K, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers MCGJ, Jacobs C, Bast A, Stehouwer CDA, Schaper NC. Modulation of Glucokinase Regulatory Protein: A Double-Edged Sword? Trends Mol Med. 2015;21:583–594. doi: 10.1016/j.molmed.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Burgess S, Butterworth AS, Thompson JR. Beyond Mendelian randomization: how to interpret evidence of shared genetic predictors. J Clin Epidemiol. 2016;69:208–216. doi: 10.1016/j.jclinepi.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32:377–389. doi: 10.1007/s10654-017-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Thompson SG. Use of allele scores as instrumental variables for Mendelian randomization. Int J Epidemiol. 2013;42:1134–1144. doi: 10.1093/ije/dyt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Timpson NJ, Ebrahim S, Smith GD. Mendelian randomization: where are we now and where are we going? Int J Epidemiol. 2015;44:379–388. doi: 10.1093/ije/dyv108. [DOI] [PubMed] [Google Scholar]
- Chambers JC, Lehne B, Loh M. Epigenome-wide association identifies DNA methylation markers in peripheral blood that predict future Type-2 diabetes 2014 [Google Scholar]
- Clark AL, Fonarow GC, Horwich TB. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis. 2014;56:409–414. doi: 10.1016/j.pcad.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Corbin LJ, Richmond RC, Wade KH, Burgess S, Bowden J, Smith GD, Timpson NJ. Body mass index as a modifiable risk factor for type 2 diabetes: Refining and understanding causal estimates using Mendelian randomisation. Diabetes. 2016:db160418. doi: 10.2337/db16-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupples LA, Heard-Costa N, Lee M, Atwood LD. Genetics Analysis Workshop 16 Problem 2: the Framingham Heart Study data. BMC Proc. 2009;3:S3. doi: 10.1186/1753-6561-3-s7-s3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale CE, Fatemifar G, Palmer TM, White J, Prieto-Merino D, Zabaneh D, Engmann JEL, Shah T, Wong A, Warren HR, McLachlan S, Trompet S, Moldovan M, Morris RW, Sofat R, Kumari M, Hyppönen E, Jefferis BJ, Gaunt TR, Ben-Shlomo Y, Zhou A, Gentry-Maharaj A, Ryan A, Caulfield MJ, Jukema JW, Worrall BB, Munroe PB, Menon U, Power C, Kuh D, Lawlor DA, Humphries SE, Mook-Kanamori DO, Sattar N, Kivimaki M, Price JF, Davey Smith G, Dudbridge F, Hingorani AD, Holmes MV, Casas JP UCLEB Consortium; METASTROKE Consortium Mutsert R de Noordam R. Causal Associations of Adiposity and Body Fat Distribution With Coronary Heart Disease, Stroke Subtypes, and Type 2 Diabetes Mellitus: A Mendelian Randomization Analysis. Circulation. 2017;135:2373–2388. doi: 10.1161/CIRCULATIONAHA.116.026560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23:R89–R98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva NMG, Freathy RM, Palmer TM, Donnelly LA, Luan J, Gaunt T, Langenberg C, Weedon MN, Shields B, Knight BA, Ward KJ, Sandhu MS, Harbord RM, McCarthy MI, Smith GD, Ebrahim S, Hattersley AT, Wareham N, Lawlor DA, Morris AD, Palmer CNA, Frayling TM. Mendelian randomization studies do not support a role for raised circulating triglyceride levels influencing type 2 diabetes, glucose levels, or insulin resistance. Diabetes. 2011;60:1008–1018. doi: 10.2337/db10-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didelez V, Sheehan N. Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res. 2007;16:309–330. doi: 10.1177/0962280206077743. [DOI] [PubMed] [Google Scholar]
- Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, Lindgren CM, Mägi R, Morris AP, Randall J, Johnson T, Elliott P, Rybin D, Thorleifsson G, Steinthorsdottir V, Henneman P, Grallert H, Dehghan A, Hottenga JJ, Franklin CS, Navarro P, Song K, Goel A, Perry JRB, Egan JM, Lajunen T, Grarup N, Spars⊘ T, Doney A, Voight BF, Stringham HM, Li M, Kanoni S, Shrader P, Cavalcanti-Proença C, Kumari M, Qi L, Timpson NJ, Gieger C, Zabena C, Rocheleau G, Ingelsson E, An P, O’Connell J, Luan J, Elliott A, McCarroll SA, Payne F, Roccasecca RM, Pattou F, Sethupathy P, Ardlie K, Ariyurek Y, Balkau B, Barter P, Beilby JP, Ben-Shlomo Y, Benediktsson R, Bennett AJ, Bergmann S, Bochud M, Boerwinkle E, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Böttcher Y, Brunner E, Bumpstead SJ, Charpentier G, Chen Y-DI, Chines P, Clarke R, Coin LJM, Cooper MN, Cornelis M, Crawford G, Crisponi L, Day INM, de Geus EJC, Delplanque J, Dina C, Erdos MR, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Fox CS, Frants R, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Groves CJ, Grundy S, Gwilliam R, Gyllensten U, Hadjadj S, Hallmans G, Hammond N, Han X, Hartikainen A-L, Hassanali N, Hayward C, Heath SC, Hercberg S, Herder C, Hicks AA, Hillman DR, Hingorani AD, Hofman A, Hui J, Hung J, Isomaa B, Johnson PRV, J⊘rgensen T, Jula A, Kaakinen M, Kaprio J, Kesaniemi YA, Kivimaki M, Knight B, Koskinen S, Kovacs P, Kyvik KO, Lathrop GM, Lawlor DA, Le Bacquer O, Lecoeur C, Li Y, Lyssenko V, Mahley R, Mangino M, Manning AK, Martínez-Larrad MT, McAteer JB, McCulloch LJ, McPherson R, Meisinger C, Melzer D, Meyre D, Mitchell BD, Morken MA, Mukherjee S, Naitza S, Narisu N, Neville MJ, Oostra BA, Orrù M, Pakyz R, Palmer CNA, Paolisso G, Pattaro C, Pearson D, Peden JF, Pedersen NL, Perola M, Pfeiffer AFH, Pichler I, Polasek O, Posthuma D, Potter SC, Pouta A, Province MA, Psaty BM, Rathmann W, Rayner NW, Rice K, Ripatti S, Rivadeneira F, Roden M, Rolandsson O, Sandbaek A, Sandhu M, Sanna S, Sayer AA, Scheet P, Scott LJ, Seedorf U, Sharp SJ, Shields B, Sigurethsson G, Sijbrands EJG, Silveira A, Simpson L, Singleton A, Smith NL, Sovio U, Swift A, Syddall H, Syvänen A-C, Tanaka T, Thorand B, Tichet J, Tönjes A, Tuomi T, Uitterlinden AG, van Dijk KW, van Hoek M, Varma D, Visvikis-Siest S, Vitart V, Vogelzangs N, Waeber G, Wagner PJ, Walley A, Walters GB, Ward KL, Watkins H, Weedon MN, Wild SH, Willemsen G, Witteman JCM, Yarnell JWG, Zeggini E, Zelenika D, Zethelius B, Zhai G, Zhao JH, Zillikens MC, Loos RJF, Meneton P, Magnusson PKE, Nathan DM, Williams GH, Hattersley AT, Silander K, Salomaa V, Smith GD, Bornstein SR, Schwarz P, Spranger J, Karpe F, Shuldiner AR, Cooper C, Dedoussis GV, Serrano-Ríos M, Morris AD, Lind L, Palmer LJ, Hu FB, Franks PW, Ebrahim S, Marmot M, Kao WHL, Pankow JS, Sampson MJ, Kuusisto J, Laakso M, Hansen T, Pedersen O, Pramstaller PP, Wichmann HE, Illig T, Rudan I, Wright AF, Stumvoll M, Campbell H, Wilson JF, Bergman RN, Buchanan TA, Collins FS, Mohlke KL, Tuomilehto J, Valle TT, Altshuler D, Rotter JI, Siscovick DS, Penninx BWJH, Boomsma DI, Deloukas P, Spector TD, Frayling TM, Ferrucci L, Kong A, Thorsteinsdottir U, Stefansson K, van Duijn CM, Aulchenko YS, Cao A, Scuteri A, Schlessinger D, Uda M, Ruokonen A, Jarvelin M-R, Waterworth DM, Vollenweider P, Peltonen L, Mooser V, Abecasis GR, Wareham NJ, Sladek R, Froguel P, Watanabe RM, Meigs JB, Groop L, Boehnke M, McCarthy MI, Florez JC, Barroso I DIAGRAM Consortium GIANT Consortium Global BPgen Consortium Borecki IB, Anders Hamsten on behalf of Procardis Consortium MAGIC investigators. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdin CA, Anderson SG, Salimi-Khorshidi G, Woodward M, MacMahon S, Dwyer T, Rahimi K. Usual blood pressure, atrial fibrillation and vascular risk: evidence from 4.3 million adults. Int J Epidemiol. 2016:dyw053. doi: 10.1093/ije/dyw053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ference BA, Yoo W, Alesh I, Mahajan N, Mirowska KK, Mewada A, Kahn J, Afonso L, Williams KA, Flack JM. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J Am Coll Cardiol. 2012;60:2631–2639. doi: 10.1016/j.jacc.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Garber AJ. Obesity and type 2 diabetes: which patients are at risk? Diabetes Obes Metab. 2012;14:399–408. doi: 10.1111/j.1463-1326.2011.01536.x. [DOI] [PubMed] [Google Scholar]
- Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/S0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- Govindaraju DR, Cupples LA, Kannel WB, O’Donnell CJ, Atwood LD, D’Agostino RB, Fox CS, Larson M, Levy D, Murabito J, Vasan RS, Splansky GL, Wolf PA, Benjamin EJ. Genetics of the Framingham Heart Study population. Adv Genet. 2008;62:33–65. doi: 10.1016/S0065-2660(08)00602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägg S, Fall T, Ploner A, Mägi R, Fischer K, Draisma HH, Kals M, Vries PS, de Dehghan A, Willems SM, Sarin A-P, Kristiansson K, Nuotio M-L, Havulinna AS, Bruijn RF, de Ikram MA, Kuningas M, Stricker BH, Franco OH, Benyamin B, Gieger C, Hall AS, Huikari V, Jula A, Järvelin M-R, Kaakinen M, Kaprio J, Kobl M, Mangino M, Nelson CP, Palotie A, Samani NJ, Spector TD, Strachan DP, Tobin MD, Whitfield JB, Uitterlinden AG, Salomaa V, Syvänen A-C, Kuulasmaa K, Magnusson PK, Esko T, Hofman A, Geus EJ, de Lind L, Giedraitis V, Perola M, Evans A, Ferrières J, Virtamo J, Kee F, Tregouet D-A, Arveiler D, Amouyel P, Gianfagna F, Brambilla P, Ripatti S, Duijn CM, van Metspalu A, Prokopenko I, McCarthy MI, Pedersen NL, Ingelsson E Consortium, for the E.N. for G. and G.E. (ENGAGE) Adiposity as a cause of cardiovascular disease: a Mendelian randomization study. Int J Epidemiol. 2015:dyv094. doi: 10.1093/ije/dyv094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Tuomilehto J, Qiao Q, Söderberg S, Daimon M, Chambers J, Pitkäniemi J DECODA study group. Impact of classical risk factors of type 2 diabetes among Asian Indian, Chinese and Japanese populations. Diabetes Metab. 2015;41:401–409. doi: 10.1016/j.diabet.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Hjellvik V, Sakshaug S, Str⊘m H. Body mass index, triglycerides, glucose, and blood pressure as predictors of type 2 diabetes in a middle-aged Norwegian cohort of men and women. Clin Epidemiol. 2012;4:213–224. doi: 10.2147/CLEP.S31830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MV, Asselbergs FW, Palmer TM, Drenos F, Lanktree MB, Nelson CP, Dale CE, Padmanabhan S, Finan C, Swerdlow DI, Tragante V, van Iperen EPA, Sivapalaratnam S, Shah S, Elbers CC, Shah T, Engmann J, Giambartolomei C, White J, Zabaneh D, Sofat R, McLachlan S, Doevendans PA, Balmforth AJ, Hall AS, North KE, Almoguera B, Hoogeveen RC, Cushman M, Fornage M, Patel SR, Redline S, Siscovick DS, Tsai MY, Karczewski KJ, Hofker MH, Verschuren WM, Bots ML, van der Schouw YT, Melander O, Dominiczak AF, Morris R, Ben-Shlomo Y, Price J, Kumari M, Baumert J, Peters A, Thorand B, Koenig W, Gaunt TR, Humphries SE, Clarke R, Watkins H, Farrall M, Wilson JG, Rich SS, de Bakker PIW, Lange LA, Davey Smith G, Reiner AP, Talmud PJ, Kivimäki M, Lawlor DA, Dudbridge F, Samani NJ, Keating BJ, Hingorani AD, Casas JP UCLEB consortium. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 2015;36:539–550. doi: 10.1093/eurheartj/eht571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MV, Lange LA, Palmer T, Lanktree MB, North KE, Almoguera B, Buxbaum S, Chandrupatla HR, Elbers CC, Guo Y, Hoogeveen RC, Li J, Li YR, Swerdlow DI, Cushman M, Price TS, Curtis SP, Fornage M, Hakonarson H, Patel SR, Redline S, Siscovick DS, Tsai MY, Wilson JG, van der Schouw YT, FitzGerald GA, Hingorani AD, Casas JP, de Bakker PIW, Rich SS, Schadt EE, Asselbergs FW, Reiner AP, Keating BJ. Causal Effects of Body Mass Index on Cardiometabolic Traits and Events: A Mendelian Randomization Analysis. Am J Hum Genet. 2014;94:198–208. doi: 10.1016/j.ajhg.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi Y, Snieder H, Ge D, Spector TD, O’Dell SD. The SH2B gene is associated with serum leptin and body fat in normal female twins. Obes Silver Spring Md. 2007;15:5–9. doi: 10.1038/oby.2007.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukema JW, Cannon CP, de Craen AJM, Westendorp RGJ, Trompet S. The controversies of statin therapy: weighing the evidence. J Am Coll Cardiol. 2012;60:875–881. doi: 10.1016/j.jacc.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Rabinovitch PS, Martin GM. Healthy aging: The ultimate preventative medicine. Science. 2015;350:1191–1193. doi: 10.1126/science.aad3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenchaiah S, Evans JC, Levy D, Wilson PWF, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the Risk of Heart Failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Lee S-H, Ryu W-S, Kim CK, Yoon B-W. Adipocytokines and ischemic stroke: differential associations between stroke subtypes. J Neurol Sci. 2012;312:117–122. doi: 10.1016/j.jns.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Klimentidis YC, Arora A. Interaction of Insulin Resistance and Related Genetic Variants With Triglyceride-Associated Genetic Variants. Circ Cardiovasc Genet. 2016;9:154–161. doi: 10.1161/CIRCGENETICS.115.001246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimentidis YC, Chougule A, Arora A, Frazier-Wood AC, Hsu C-H. Triglyceride-Increasing Alleles Associated with Protection against Type-2 Diabetes. PLOS Genet. 2015;11:e1005204. doi: 10.1371/journal.pgen.1005204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarca R, Alonso J, Gómez G, Muñoz A. Left-truncated data with age as time scale: an alternative for survival analysis in the elderly population. J Gerontol A Biol Sci Med Sci. 1998;53:M337–343. doi: 10.1093/gerona/53a.5.m337. [DOI] [PubMed] [Google Scholar]
- Lavie CJ, Cahalin LP, Chase P, Myers J, Bensimhon D, Peberdy MA, Ashley E, West E, Forman DE, Guazzi M, Arena R. Impact of cardiorespiratory fitness on the obesity paradox in patients with heart failure. Mayo Clin Proc. 2013;88:251–258. doi: 10.1016/j.mayocp.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne PM, Jafri H, Karas R. The association between lower levels of low-density lipoprotein cholesterol and cancer predates the diagnosis of cancer by 18 years. J Am Coll Cardiol. 2012;59:E1622–E1622. doi: 10.1016/S0735-1097(12)61623-4. [DOI] [Google Scholar]
- Li J, Fine J, Brookhart A. Instrumental variable additive hazards models. Biometrics. 2015;71:122–130. doi: 10.1111/biom.12244. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhou Y, Carter-Su C, Myers MG, Rui L. SH2B1 enhances leptin signaling by both Janus kinase 2 Tyr813 phosphorylation-dependent and -independent mechanisms. Mol Endocrinol Baltim Md. 2007;21:2270–2281. doi: 10.1210/me.2007-0111. [DOI] [PubMed] [Google Scholar]
- Linsel-Nitschke P, Götz A, Erdmann J, Braenne I, Braund P, Hengstenberg C, Stark K, Fischer M, Schreiber S, Mokhtari NEE, Schaefer A, Schrezenmeier J, Rubin D, Hinney A, Reinehr T, Roth C, Ortlepp J, Hanrath P, Hall AS, Mangino M, Lieb W, Lamina C, Heid IM, Doering A, Gieger C, Peters A, Meitinger T, Wichmann H-E, König IR, Ziegler A, Kronenberg F, Samani NJ, Schunkert H Consortium for the WTCCC(WTCCC), the C. Lifelong Reduction of LDL-Cholesterol Related to a Common Variant in the LDL-Receptor Gene Decreases the Risk of Coronary Artery Disease—A Mendelian Randomisation Study. PLOS ONE. 2008;3:e2986. doi: 10.1371/journal.pone.0002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Butler KR, Buxbaum SG, Sung JH, Campbell BW, Taylor HA. Leptinemia and its association with stroke and coronary heart disease in the Jackson Heart Study. Clin Endocrinol (Oxf) 2010;72:32–37. doi: 10.1111/j.1365-2265.2009.03627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall DM, Celis-Morales C, Ward J, Iliodromiti S, Anderson JJ, Gill JMR, Smith DJ, Ntuk UE, Mackay DF, Holmes MV, Sattar N, Pell JP. Association of Body Mass Index With Cardiometabolic Disease in the UK Biobank: A Mendelian Randomization Study. JAMA Cardiol. 2017;2:882–889. doi: 10.1001/jamacardio.2016.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolis AJ, Rosei EA, Coca A, Cifkova R, Erdine SE, Kjeldsen S, Lip GYH, Narkiewicz K, Parati G, Redon J, Schmieder R, Tsioufis C, Mancia G. Hypertension and atrial fibrillation: diagnostic approach, prevention and treatment. Position paper of the Working Group “Hypertension Arrhythmias and Thrombosis” of the European Society of Hypertension. J Hypertens. 2012;30:239–252. doi: 10.1097/HJH.0b013e32834f03bf. [DOI] [PubMed] [Google Scholar]
- Martinussen T, Scheike TH. Dynamic regression models for survival data. Springer Science & Business Media; 2007. [Google Scholar]
- Martinussen T, Vansteelandt S. On collapsibility and confounding bias in Cox and Aalen regression models. Lifetime Data Anal. 2013;19:279–296. doi: 10.1007/s10985-013-9242-z. [DOI] [PubMed] [Google Scholar]
- Maures TJ, Kurzer JH, Carter-Su C. SH2B1 (SH2-B) and JAK2: a multifunctional adaptor protein and kinase made for each other. Trends Endocrinol Metab TEM. 2007;18:38–45. doi: 10.1016/j.tem.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Meisinger C, Döring A, Thorand B, Heier M, Löwel H. Body fat distribution and risk of type 2 diabetes in the general population: are there differences between men and women? The MONICA/KORA Augsburg cohort study. Am J Clin Nutr. 2006;84:483–489. doi: 10.1093/ajcn/84.3.483. [DOI] [PubMed] [Google Scholar]
- Nakhjavani M, Khalilzadeh O, Khajeali L, Esteghamati A, Morteza A, Jamali A, Dadkhahipour S. Serum oxidized-LDL is associated with diabetes duration independent of maintaining optimized levels of LDL-cholesterol. Lipids. 2010;45:321–327. doi: 10.1007/s11745-010-3401-8. [DOI] [PubMed] [Google Scholar]
- Njajou OT, Kanaya AM, Holvoet P, Connelly S, Strotmeyer ES, Harris TB, Cummings SR, Hsueh W-C Health ABC Study. Association between oxidized LDL, obesity and type 2 diabetes in a population-based cohort, the Health, Aging and Body Composition Study. Diabetes Metab Res Rev. 2009;25:733–739. doi: 10.1002/dmrr.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156:13–22. doi: 10.1016/j.ahj.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Orho-Melander M, Melander O, Guiducci C, Perez-Martinez P, Corella D, Roos C, Tewhey R, Rieder MJ, Hall J, Abecasis G, Tai ES, Welch C, Arnett DK, Lyssenko V, Lindholm E, Saxena R, de Bakker PIW, Burtt N, Voight BF, Hirschhorn JN, Tucker KL, Hedner T, Tuomi T, Isomaa B, Eriksson K-F, Taskinen M-R, Wahlstrand B, Hughes TE, Parnell LD, Lai C-Q, Berglund G, Peltonen L, Vartiainen E, Jousilahti P, Havulinna AS, Salomaa V, Nilsson P, Groop L, Altshuler D, Ordovas JM, Kathiresan S. Common missense variant in the glucokinase regulatory protein gene is associated with increased plasma triglyceride and C-reactive protein but lower fasting glucose concentrations. Diabetes. 2008;57:3112–3121. doi: 10.2337/db08-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østergaard SD, Mukherjee S, Sharp SJ, Proitsi P, Lotta LA, Day F, Perry JRB, Boehme KL, Walter S, Kauwe JS, Gibbons LE, Larson EB, Powell JF, Langenberg C, Crane PK, Wareham NJ, Scott RA Consortium ADG, Consortium TG. Associations between Potentially Modifiable Risk Factors and Alzheimer Disease: A Mendelian Randomization Study. PLOS Med. 2015;12:e1001841. doi: 10.1371/journal.pmed.1001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl J Consortium E-I. Causality: Models, Reasoning and Inference. 2. Cambridge University Press; Cambridge, U.K.; New York: 2000. [Google Scholar]
- Proitsi P, Lupton MK, Velayudhan L, Newhouse S, Fogh I, Tsolaki M, Daniilidou M, Pritchard M, Kloszewska I, Soininen H, Mecocci P, Vellas B, Williams J, Stewart R, Sham P, Lovestone S, Powell JF. Genetic Predisposition to Increased Blood Cholesterol and Triglyceride Lipid Levels and Risk of Alzheimer Disease: A Mendelian Randomization Analysis. PLOS Med. 2014;11:e1001713. doi: 10.1371/journal.pmed.1001713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondo A, Rees MG, Gloyn AL. Glucokinase regulatory protein: complexity at the crossroads of triglyceride and glucose metabolism. Curr Opin Lipidol. 2015;26:88–95. doi: 10.1097/MOL.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond RC, Smith GD, Ness AR, Hoed Mden McMahon G, Timpson NJ. Assessing Causality in the Association between Child Adiposity and Physical Activity Levels: A Mendelian Randomization Analysis. PLOS Med. 2014;11:e1001618. doi: 10.1371/journal.pmed.1001618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues dos Santos C, Domingues G, Matias I, Matos J, Fonseca I, de Almeida JM, Dias S. LDL-cholesterol signaling induces breast cancer proliferation and invasion. Lipids Health Dis. 2014;13:16. doi: 10.1186/1476-511X-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun NR, Lentzner H, Hoyert D, Robinson KN. Trends in causes of death among the elderly. Aging Trends. 2001;1:1–10. doi: 10.1037/e620692007-001. [DOI] [PubMed] [Google Scholar]
- Sharrett AR. The Atherosclerosis Risk in Communities (ARIC) Study. Introduction and objectives of the hemostasis component. Ann Epidemiol. 1992;2:467–469. doi: 10.1016/1047-2797(92)90096-9. [DOI] [PubMed] [Google Scholar]
- Sniderman AD, Scantlebury T, Cianflone K. Hypertriglyceridemic hyperapob: the unappreciated atherogenic dyslipoproteinemia in type 2 diabetes mellitus. Ann Intern Med. 2001;135:447–459. doi: 10.7326/0003-4819-135-6-200109180-00014. [DOI] [PubMed] [Google Scholar]
- Söderberg S, Stegmayr B, Ahlbeck-Glader C, Slunga-Birgander L, Ahrén B, Olsson T. High leptin levels are associated with stroke. Cerebrovasc Dis Basel Switz. 2003;15:63–69. doi: 10.1159/000067128. 67128. [DOI] [PubMed] [Google Scholar]
- Solovieff N, Cotsapas C, Lee PH, Purcell SM, Smoller JW. Pleiotropy in complex traits: challenges and strategies. Nat Rev Genet. 2013;14:483–495. doi: 10.1038/nrg3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spars⊘ T, Andersen G, Nielsen T, Burgdorf KS, Gjesing AP, Nielsen AL, Albrechtsen A, Rasmussen SS, J⊘rgensen T, Borch-Johnsen K, Sandbaek A, Lauritzen T, Madsbad S, Hansen T, Pedersen O. The GCKR rs780094 polymorphism is associated with elevated fasting serum triacylglycerol, reduced fasting and OGTT-related insulinaemia, and reduced risk of type 2 diabetes. Diabetologia. 2008;51:70–75. doi: 10.1007/s00125-007-0865-z. [DOI] [PubMed] [Google Scholar]
- Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, Allen HL, Lindgren CM, Luan J, Mägi R, Randall JC, Vedantam S, Winkler TW, Qi L, Workalemahu T, Heid IM, Steinthorsdottir V, Stringham HM, Weedon MN, Wheeler E, Wood AR, Ferreira T, Weyant RJ, Segrè AV, Estrada K, Liang L, Nemesh J, Park J-H, Gustafsson S, Kilpeläinen TO, Yang J, Bouatia-Naji N, Esko T, Feitosa MF, Kutalik Z, Mangino M, Raychaudhuri S, Scherag A, Smith AV, Welch R, Zhao JH, Aben KK, Absher DM, Amin N, Dixon AL, Fisher E, Glazer NL, Goddard ME, Heard-Costa NL, Hoesel V, Hottenga J-J, Johansson Å, Johnson T, Ketkar S, Lamina C, Li S, Moffatt MF, Myers RH, Narisu N, Perry JRB, Peters MJ, Preuss M, Ripatti S, Rivadeneira F, Sandholt C, Scott LJ, Timpson NJ, Tyrer JP, van Wingerden S, Watanabe RM, White CC, Wiklund F, Barlassina C, Chasman DI, Cooper MN, Jansson J-O, Lawrence RW, Pellikka N, Prokopenko I, Shi J, Thiering E, Alavere H, Alibrandi MTS, Almgren P, Arnold AM, Aspelund T, Atwood LD, Balkau B, Balmforth AJ, Bennett AJ, Ben-Shlomo Y, Bergman RN, Bergmann S, Biebermann H, Blakemore AIF, Boes T, Bonnycastle LL, Bornstein SR, Brown MJ, Buchanan TA, Busonero F, Campbell H, Cappuccio FP, Cavalcanti-Proença C, Chen Y-DI, Chen C-M, Chines PS, Clarke R, Coin L, Connell J, Day INM, Heijer Mden Duan J, Ebrahim S, Elliott P, Elosua R, Eiriksdottir G, Erdos MR, Eriksson JG, Facheris MF, Felix SB, Fischer-Posovszky P, Folsom AR, Friedrich N, Freimer NB, Fu M, Gaget S, Gejman PV, Geus EJC, Gieger C, Gjesing AP, Goel A, Goyette P, Grallert H, Gräßler J, Greenawalt DM, Groves CJ, Gudnason V, Guiducci C, Hartikainen A-L, Hassanali N, Hall AS, Havulinna AS, Hayward C, Heath AC, Hengstenberg C, Hicks AA, Hinney A, Hofman A, Homuth G, Hui J, Igl W, Iribarren C, Isomaa B, Jacobs KB, Jarick I, Jewell E, John U, J⊘rgensen T, Jousilahti P, Jula A, Kaakinen M, Kajantie E, Kaplan LM, Kathiresan S, Kettunen J, Kinnunen L, Knowles JW, Kolcic I, König IR, Koskinen S, Kovacs P, Kuusisto J, Kraft P, Kval⊘y K, Laitinen J, Lantieri O, Lanzani C, Launer LJ, Lecoeur C, Lehtimäki T, Lettre G, Liu J, Lokki M-L, Lorentzon M, Luben RN, Ludwig B, Magic Manunta P, Marek D, Marre M, Martin NG, McArdle WL, McCarthy A, McKnight B, Meitinger T, Melander O, Meyre D, Midthjell K, Montgomery GW, Morken MA, Morris AP, Mulic R, Ngwa JS, Nelis M, Neville MJ, Nyholt DR, O’Donnell CJ, O’Rahilly S, Ong KK, Oostra B, Paré G, Parker AN, Perola M, Pichler I, Pietiläinen KH, Platou CGP, Polasek O, Pouta A, Rafelt S, Raitakari O, Rayner NW, Ridderstråle M, Rief W, Ruokonen A, Robertson NR, Rzehak P, Salomaa V, Sanders AR, Sandhu MS, Sanna S, Saramies J, Savolainen MJ, Scherag S, Schipf S, Schreiber S, Schunkert H, Silander K, Sinisalo J, Siscovick DS, Smit JH, Soranzo N, Sovio U, Stephens J, Surakka I, Swift AJ, Tammesoo M-L, Tardif J-C, Teder-Laving M, Teslovich TM, Thompson JR, Thomson B, Tönjes A, Tuomi T, van Meurs JBJ, van Ommen G-J, Vatin V, Viikari J, Visvikis-Siest S, Vitart V, Vogel CIG, Voight BF, Waite LL, Wallaschofski H, Walters GB, Widen E, Wiegand S, Wild SH, Willemsen G, Witte DR, Witteman JC, Xu J, Zhang Q, Zgaga L, Ziegler A, Zitting P, Beilby JP, Farooqi IS, Hebebrand J, Huikuri HV, James AL, Kähönen M, Levinson DF, Macciardi F, Nieminen MS, Ohlsson C, Palmer LJ, Ridker PM, Stumvoll M, Beckmann JS, Boeing H, Boerwinkle E, Boomsma DI, Caulfield MJ, Chanock SJ, Collins FS, Cupples LA, Smith GD, Erdmann J, Froguel P, Grönberg H, Gyllensten U, Hall P, Hansen T, Harris TB, Hattersley AT, Hayes RB, Heinrich J, Hu FB, Hveem K, Illig T, Jarvelin M-R, Kaprio J, Karpe F, Khaw K-T, Kiemeney LA, Krude H, Laakso M, Lawlor DA, Metspalu A, Munroe PB, Ouwehand WH, Pedersen O, Penninx BW, Peters A, Pramstaller PP, Quertermous T, Reinehr T, Rissanen A, Rudan I, Samani NJ, Schwarz PEH, Shuldiner AR, Spector TD, Tuomilehto J, Uda M, Uitterlinden A, Valle TT, Wabitsch M, Waeber G, Wareham NJ, Watkins H, Wilson JF, Wright AF, Zillikens MC, Chatterjee N, McCarroll SA, Purcell S, Schadt EE, Visscher PM, Assimes TL, Borecki IB, Deloukas P, Fox CS, Groop LC, Haritunians T, Hunter DJ, Kaplan RC, Mohlke KL, O’Connell JR, Peltonen L, Schlessinger D, Strachan DP, van Duijn CM, Wichmann H-E, Frayling TM, Thorsteinsdottir U, Abecasis GR, Barroso I, Boehnke M, Stefansson K, North KE, McCarthy MI, Hirschhorn JN, Ingelsson E, Loos RJF. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D’Agostino RB, Fox CS, Larson MG, Murabito JM, O’Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Davies NM, Ware JJ, VanderWeele T, Smith GD, Munafò MR. Mendelian randomization in health research: Using appropriate genetic variants and avoiding biased estimates. Econ Hum Biol. 2014;13:99–106. doi: 10.1016/j.ehb.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchetgen Tchetgen EJ, Walter S, Vansteelandt S, Martinussen T, Glymour M. Instrumental variable estimation in a survival context. Epidemiol Camb Mass. 2015;26:402–410. doi: 10.1097/EDE.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The ARIC investigators. The atherosclerosis risk in communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- The International Consortium for Blood Pressure Genome-Wide Association Studies. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- Tirosh A, Shai I, Bitzur R, Kochba I, Tekes-Manova D, Israeli E, Shochat T, Rudich A. Changes in Triglyceride Levels Over Time and Risk of Type 2 Diabetes in Young Men. Diabetes Care. 2008;31:2032–2037. doi: 10.2337/dc08-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompet S, Jukema JW, Katan MB, Blauw GJ, Sattar N, Buckley B, Caslake M, Ford I, Shepherd J, Westendorp RGJ, de Craen AJM. Apolipoprotein e genotype, plasma cholesterol, and cancer: a Mendelian randomization study. Am J Epidemiol. 2009;170:1415–1421. doi: 10.1093/aje/kwp294. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E, Detheux M, Veiga da Cunha M. Short-term control of glucokinase activity: role of a regulatory protein. FASEB J Off Publ Fed Am Soc Exp Biol. 1994;8:414–419. doi: 10.1096/fasebj.8.6.8168691. [DOI] [PubMed] [Google Scholar]
- Vergès B. Lipid modification in type 2 diabetes: the role of LDL and HDL. Fundam Clin Pharmacol. 2009;23:681–685. doi: 10.1111/j.1472-8206.2009.00739.x. [DOI] [PubMed] [Google Scholar]
- Voight BF, Kang HM, Ding J, Palmer CD, Sidore C, Chines PS, Burtt NP, Fuchsberger C, Li Y, Erdmann J, Frayling TM, Heid IM, Jackson AU, Johnson T, Kilpeläinen TO, Lindgren CM, Morris AP, Prokopenko I, Randall JC, Saxena R, Soranzo N, Speliotes EK, Teslovich TM, Wheeler E, Maguire J, Parkin M, Potter S, Rayner NW, Robertson N, Stirrups K, Winckler W, Sanna S, Mulas A, Nagaraja R, Cucca F, Barroso I, Deloukas P, Loos RJF, Kathiresan S, Munroe PB, Newton-Cheh C, Pfeufer A, Samani NJ, Schunkert H, Hirschhorn JN, Altshuler D, McCarthy MI, Abecasis GR, Boehnke M. The Metabochip, a Custom Genotyping Array for Genetic Studies of Metabolic, Cardiovascular, and Anthropometric Traits. PLOS Genet. 2012;8:e1002793. doi: 10.1371/journal.pgen.1002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Hólm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart AF, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett M-S, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki M-L, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PIW, Klungel OH, Maitland-van der Zee A-H, Peters BJM, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VHM, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, König IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schäfer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O’Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voulgari C, Tentolouris N, Dilaveris P, Tousoulis D, Katsilambros N, Stefanadis C. Increased heart failure risk in normal-weight people with metabolic syndrome compared with metabolically healthy obese individuals. J Am Coll Cardiol. 2011;58:1343–1350. doi: 10.1016/j.jacc.2011.04.047. [DOI] [PubMed] [Google Scholar]
- White J, Swerdlow DI, Preiss D, et al. Initiative for the ADN, Consortium for the G, on behalf of Procardis Consortium. Association of lipid fractions with risks for coronary artery disease and diabetes. JAMA Cardiol. 2016 doi: 10.1001/jamacardio.2016.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: The density plot of years from age at the baseline to the onset of the age-related diseases in each cohort.
Text S1: The detailed description of the derivation of the MR-Egger method in the survival context.
Table S1. The detailed information of each SNP included in the MR analyses for the construction of polygenic score for BMI. SNP: rs ID. Gene: the gene associated with the SNP. Chr: the chromosome on which the SNP is located. Pos: the position of the SNP on that chromosome. Weight: the weight used for calculation of the polygenic score for BMI. The weight is obtained from the effect size estimated from the previous reported meta-analysis (Speliotes et al., 2010). Increasing/other allele: The allele corresponding to the positive effect size/the other allele. MAF: the MAF estimated from the samples in a specific cohort. Info score: The imputation quality score from IMPUTE2.
Table S10. The results for checking the assumption of conditional independence required for the MR analyses. ES/SD/P (POLY): the effect sizes/standard errors/p-values of the polygenic score on the age-at-onset of the disease based on the additive hazards model (described in the Materials and Methods section) with the adjustment of the risk factor. ES/SD/P (RF): the effect sizes/standard errors/p-values of the risk factor on the age-at-onset of the disease based on the additive hazards model (described in the Materials and Methods section) without other adjustment.
Table S11. The results from the MR-egger sensitivity analysis. The analysis adjusted for all covariates. The meta-analysis combining the results from the five cohorts was performed by using a fixed-effects model. Risk factor: the risk factor of which the causal effect is estimated. Disease: the disease on which the causal effect is estimated. Coef_intercept: the estimate of the intercept (directional pleiotropy) in MR-Egger. Sd_intercept: the standard error of the intercept estimate in MR-Egger. P_intercept: the p-value for the intercept. Coef_slope: the estimate of the slope (causal effect) in MR-Egger. Sd_slope: the standard error of the slope estimate in MR-Egger. P_slope: the p-value for the slope.
Table S12. The detailed results from the main MR analysis and the sensitivity analysis adjusted for the covariates.
Table S2. The detailed information of each SNP included in the MR analyses for the construction of polygenic score for SBP. SNP: rs ID. Gene: the gene associated with the SNP. Chr: the chromosome on which the SNP is located. Pos: the position of the SNP on that chromosome. Weight: the weight used for calculation of the polygenic score for SBP. The weight is obtained from the effect size estimated from the previous reported meta-analysis (The International Consortium for Blood Pressure Genome-Wide Association Studies, 2011). Increasing/other allele: The allele corresponding to the positive effect size/the other allele. MAF: the MAF estimated from the samples in a specific cohort. Info score: The imputation quality score from IMPUTE2.
Table S3. The detailed information of each SNP included in the MR analyses for the construction of polygenic score for LDL-C. SNP: rs ID. Gene: the gene associated with the SNP. Chr: the chromosome on which the SNP is located. Pos: the position of the SNP on that chromosome. Weight: the weight used for calculation of the polygenic score for LDL-C. The weight is obtained from the effect size estimated from the previous reported meta-analysis (Benjamin F. Voight et al., 2012). Increasing/other allele: The allele corresponding to the positive effect size/the other allele. MAF: the MAF estimated from the samples in a specific cohort. Info score: The imputation quality score from IMPUTE2.
Table S4. The detailed information of each SNP included in the MR analyses for the construction of polygenic score for HDL-C. SNP: rs ID. Gene: the gene associated with the SNP. Chr: the chromosome on which the SNP is located. Pos: the position of the SNP on that chromosome. Weight: the weight used for calculation of the polygenic score for HDL-C. The weight is obtained from the effect size estimated from the previous reported meta-analysis (Benjamin F. Voight et al., 2012). Increasing/other allele: The allele corresponding to the positive effect size/the other allele. MAF: the MAF estimated from the samples in a specific cohort. Info score: The imputation quality score from IMPUTE2.
Table S5. The detailed information of each SNP included in the MR analyses for the construction of polygenic score for triglycerides. SNP: rs ID. Gene: the gene associated with the SNP. Chr: the chromosome on which the SNP is located. Pos: the position of the SNP on that chromosome. Weight: the weight used for calculation of the polygenic score for triglycerides. The weight is obtained from the effect size estimated from the previous reported meta-analysis (Benjamin F. Voight et al., 2012). Increasing/other allele: The allele corresponding to the positive effect size/the other allele. MAF: the MAF estimated from the samples in a specific cohort. Info score: The imputation quality score from IMPUTE2.
Table S6. The results of testing age-invariant causal effects from a time-varying additive hazards model based on a Kolmogorov–Smirnov test. P(B=0): The p-value from the test of no effects. P(B=C): The p-value from the test of an age-invariant effect.
Table S7. The detailed results in each cohort from the discovery meta-analysis and the sensitivity meta-analyses adjusted for sex, birth cohort, education level, smoking and alcohol consumption history for the association between genetically predicted risk factors and onset of age-related diseases. Coef: the effect size from the additive hazards model. SD_boot: the SD calculated from the bootstrap method. P_boot: the p-value calculated based on the effect size and SD. P_polygenic: the p-value for the association between the polygenic score and the risk factor in that specific analysis.
Table S8. The results from the leave-one-out sensitivity analysis, in which each SNP is excluded from the polygenic score. p_meta: the p-values from the meta-analysis. p_ARIC: the p-values from the ARIC (the same for FHS, MESA, CHS and WHI).
Table S9. Details of the construction of the CVD variable in each cohort.