Abstract
Secondary osteoporosis resulting from specific clinical disorders may be potentially reversible, and thus continuous efforts to find and adequately treat the secondary causes of skeletal fragility are critical to ameliorate fracture risk and to avoid unnecessary treatment with anti-osteoporotic drugs. Among the hyperfunctional adrenal masses, Cushing's syndrome, pheochromocytoma, and primary aldosteronism are receiving particularly great attention due to their high morbidity and mortality mainly by increasing cardiovascular risk. Interestingly, there is accumulating experimental and clinical evidence that adrenal hormones may have direct detrimental effects on bone metabolism as well. Thus, the present review discusses the possibility of adrenal disorders, especially focusing on pheochromocytoma and primary aldosteronism, as secondary causes of osteoporosis.
Keywords: Osteoporosis, Fracture, Pheochromocytoma, Hyperaldosteronism, Cushing syndrome
The Namgok Award is the highest scientific award of the Korean Endocrine Society, and is given to honor an individual who has made excellent contributions to progress in the field of endocrinology and metabolism. The Namgok Award is named after the pen name of Professor Hun Ki Min, who founded the Korean Endocrine Society in 1982.
Professor Jung-Min Koh received the Namgok Award at the Autumn Symposium of the Korean Endocrine Society in November 2017.
INTRODUCTION
A decrease in bone strength is an inevitable consequence of aging, starting some years before menopause, accelerating after its onset, and continuing throughout one's remaining years in both men and women. A very large number of heterogeneous causes, collectively defined as “secondary causes of osteoporosis,” may also result in bone fragility through a number of mechanisms, independently of age or estrogen deficiency [1]. Actually, secondary osteoporosis affects about two-thirds of men, more than half of premenopausal women, and about one-fifths of postmenopausal women [2,3]. Importantly, secondary osteoporosis resulting from specific clinical disorders may be potentially reversible [4]. Therefore, continuous efforts to find and correctly treat secondary causes of skeletal fragility are critical to ameliorate fracture risk and to avoid unnecessary treatment with antiosteoporotic drugs.
An adrenal incidentaloma (AI) is an adrenal lesion discovered unexpectedly by radiologic examination performed for reasons other than to evaluate the adrenal gland. Given the widespread availability and use of highly sensitive imaging techniques, the detection rate of AIs is increasing [5]. Although the majority of AIs are nonfunctional benign cortical adenomas, their differential diagnosis must include a wide range of pathologies, including those that are hyperfunctional (hormonally-active) and/or malignant [6]. Among the hyperfunctional adrenal masses, Cushing's syndrome, pheochromocytoma, and primary aldosteronism (PA) are receiving particularly great attention due to their high morbidity and mortality mainly by increasing cardiovascular risk [7,8,9,10,11]. Interestingly, there is accumulating experimental and clinical evidence that adrenal hormones may have direct detrimental effects on bone metabolism as well. Thus, the present review discusses the possibility of adrenal disorders as secondary causes of osteoporosis.
POOR BONE HEALTH IN CUSHING'S SYNDROME
Cushing's syndrome reflects a constellation of clinical features that result from chronic exposure to excess glucocorticoids of any etiology. The disorder can be adrenocorticotropic hormone (ACTH)-dependent or ACTH-independent, as well as iatrogenic (e.g., administration of exogenous glucocorticoids to treat various inflammatory conditions). Among these, adrenal Cushing's syndrome related to adrenocortical adenoma, adrenocortical carcinoma, or nodular adrenal hyperplasia is classified in the ACTH-independent category. Because the adverse effects of cortisol excess on bone are consistent regardless of an endogenous or exogenous etiology [12,13], there is no need to differently consider its impact on bone metabolism depending on the causes of Cushing's syndrome. In fact, glucocorticoid-induced osteoporosis (GIO) including all related etiologies is the most common form of secondary osteoporosis [12,14]. GIO has extensively been discussed in other review articles due to its clinical importance [13,15,16]; our paper focuses on the roles of pheochromocytoma and PA in bone health.
IMPORTANCE OF SYMPATHETIC ACTIVITY ON HUMAN BONE: LESSONS FROM PHEOCHROMOCYTOMA
The sympathetic nervous system (SNS) has been identified as an important regulator of bone metabolism in mice, primarily via β2-adrenergic receptors (β2ARs). Disruption of sympathetic signaling by knockout of the β2AR in osteoblasts has led to a high bone mass phenotype by increasing bone formation and decreasing bone resorption [17]. Consistently, pharmacological inhibition and stimulation of β-adrenergic receptors in rodents increased and decreased bone mass, respectively [18,19]. Another study has shown that deletion of the gene responsible for the production of dopamine β-hydroxylase, an enzyme required for the synthesis of catecholamines, causes a high bone mass phenotype in 6-month-old mice [20].
In spite of the biological relevance of sympathetic activity in rodents, its role in human bone metabolism remains a question. A recent meta-analysis reported that β-blockers were associated with a reduced risk of fracture, with a relatively small effect size [21]. In detail, the risk of fracture was 15% lower in patients treated with β-adrenergic blockers compared to controls independent of gender and fracture site. However, other clinical studies about the association between β-blocker use and bone health have yielded conflicting results showing positive, adverse, or no effects [22,23,24,25,26]. On the other hand, only three cross-sectional studies have investigated the effects of β-agonists on fracture risk, but the results of these studies in chronic obstructive pulmonary disease were all negative [27,28,29]. A prospective study reported that selective β2-adrenergic agonists did not affect human bone turnover, either [26]. These inconsistent findings in humans could be explained by the highly heterogeneous characteristics of the included populations in terms of the selectivity, dose, duration, and delivery route of β-adrenergic receptor inhibitors or stimulators. Considering the fundamental difficulty in controlling for diverse confounding factors in clinical studies with β-adrenergic receptor modulators, a disease model simulating sympathetic activity is necessary to adequately assess the role of the SNS in human bone metabolism.
Pheochromocytoma is a catecholamine-secreting neuroendocrine tumor that arises from the chromaffin cells of the adrenal medulla [30]. Because catecholamines are capable of activating the β2AR and the main neurotransmitters of the SNS, pheochromocytoma could be an ideal human model for elucidating the role of sympathetic overstimulation in the pathogenesis of diverse diseases including osteoporosis. Based on these backgrounds, Veldhuis-Vlug et al. [31] hypothesized that catecholamine excess in patients with pheochromocytoma may contribute to increased bone resorption. Specifically, in this retrospective case-control study, cases with pheochromocytoma (n=21) had higher blood levels of the bone resorption marker, C-terminal telopeptide of type I collagen (CTX), than controls (n=126), and adrenalectomy, the surgical removal of the catecholamine-producing tumor, resulted in normalization. In spite of the lack of information regarding the effects on bone mass, this study has an important implication in that it provided evidence supporting the concept of regulation of bone remodeling by the SNS in humans.
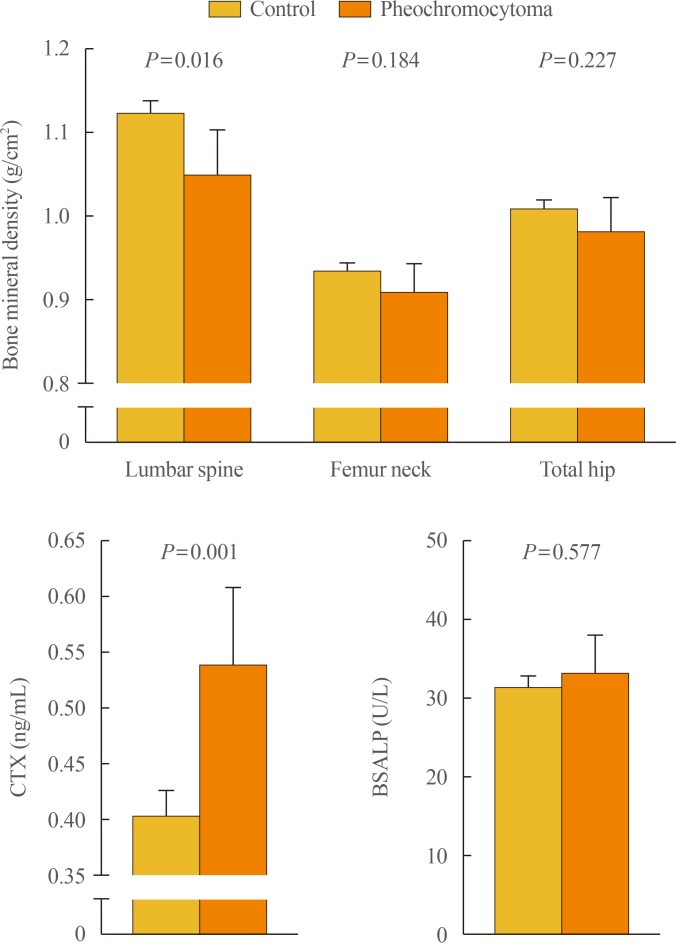
To further clarify the role of sympathetic activity in human bone health, we recently investigated the association of catecholamine excess with osteoporosis-related phenotypes, especially focusing on bone mineral density (BMD), in a Korean cohort consisting of patients with pheochromocytoma (n=31) and controls (n=280) [32]. After adjustment for potential confounders including age, sex, menopausal status, body mass index, current smoking, alcohol intake, regular outdoor exercise, diabetes, and medication use, patients with pheochromocytoma had 7.2% lower bone mass at the lumbar spine and 33.5% higher serum CTX than those without pheochromocytoma, whereas there were no statistical differences between groups in BMD at the femur neck and total hip and in serum bone-specific alkaline phosphatase (BSALP) level (Fig. 1). Consistently, both urinary metanephrine and normetanephrine levels were inversely correlated with lumbar spine BMD and positively correlated with serum CTX level, but neither with proximal femur BMD nor serum BSALP. The odds ratio (OR) for lower BMD at the lumbar spine in the presence of pheochromocytoma was 3.31 (95% confidence interval, 1.23 to 8.56) (Table 1). However, the ORs for lower BMD at the femur neck and total hip did not differ according to the presence of pheochromocytoma. Finally, as observed in the referenced study above [31], serum CTX level markedly decreased after adrenalectomy. These findings of ours provide the first clinical evidence that catecholamine excess and the resultant sympathetic overstimulation in pheochromocytoma could contribute to adverse effects on human bone through the increase of bone loss, especially in trabecular bone, as well as bone resorption.
Fig. 1. Differences in bone mineral density and bone turnover markers between subjects without and with pheochromocytoma. Values are presented as the estimated means with 95% confidence intervals, from analysis of covariance after adjustment for age, sex, menopausal status, body mass index, current smoking, alcohol intake, regular outdoor exercise, diabetes, and medication use including corticosteroids, antihypertensive agents, bisphosphonates, and hormone replacement. Adapted from Kim et al., with permission from Oxford University Press [32]. CTX, C-terminal telopeptide of type I collagen; BSALP, bone-specific alkaline phosphatase.
Table 1. The Determination of the Risk for Lower BMDa According to the Presence of Pheochromocytoma.
| Patients with pheochromocytoma | ||
|---|---|---|
| Odds ratio (95% CI) | P value | |
| Lower BMD at any siteb | 2.54 (0.98–6.60) | 0.056 |
| Lower BMD at the lumbar spine | 3.31 (1.23–8.56)c | 0.014c |
| Lower BMD at the femur neck | 1.18 (0.31–4.45) | 0.806 |
| Lower BMD at the total hip | 0.80 (0.17–3.75) | 0.772 |
Adapted from Kim et al., with permission from Oxford University Press [32].
BMD, bone mineral density; CI, confidence interval.
aLower BMD was defined by Z-score ≤−2.0 for premenopausal women and men aged <50 years, or T-score ≤−1.0 for postmenopausal women and men aged ≥50 years; b“Any site” includes the lumbar spine, femur neck, and/or total hip; cNumbers indicate statistically significant values. The multiple logistic regression analyses were performed after adjustment for age, sex, menopausal status, body mass index, current smoking, alcohol intake, regular outdoor exercise, diabetes, and medication use including corticosteroids, antihypertensive agents, bisphosphonates, and hormone replacement.
A particularly important observation in both the study by Veldhuis-Vlug et al. [31] and our study [32] is that patients with pheochromocytoma showed a markedly higher bone resorption rate without affecting the bone formation rate. This uncoupling in pheochromocytoma can be adequately explained by an animal study with β2AR-deficient mice showing that sympathetic signaling in osteoblasts triggers an increase in the circulating levels of the osteoclast differentiation factor RANKL (receptor activator of nuclear factor kappa-B ligand) [33]. Furthermore, continuous treatment with a β-agonist in mice reduces bone mass by increasing bone resorption without suppressing bone formation [19]. All these data indicate that bone fragility associated with sympathetic overstimulation could result from an uncoupling between excessive bone degradation and inadequately balanced bone formation, and subsequent ongoing bone loss.
Although the ultimate goal of bone biology research is to prevent osteoporotic fractures (OFs) associated with high morbidity and mortality, no clinical studies have been performed about OFs related to pheochromocytoma due to the rare nature of the disease. However, both lower BMD as a static marker and higher bone resorption rate as a dynamic indicator are well-established predictors of OFs [34,35], and the risk of fracture is much higher when these conditions occur concurrently [36]. Therefore, there is no shortage of current evidence to suggest that pheochromocytoma with sympathetic overstimulation could be a potential risk factor for osteoporosis and related fractures, and should be effectively treated to maintain bone health.
HUMAN SKELETAL DETERIORATION BY ALDOSTERONE EXCESS
PA is a disorder of the adrenal gland characterized by the autonomous hypersecretion of aldosterone and is the commonest cause of secondary hypertension, accounting for 5% to 10% of all hypertensive patients [37,38]. PA is known to be associated with end-organ damage, particularly affecting the heart, carotid arteries and kidneys, beyond its effects on intravascular volume and blood pressure [39]. Several lines of evidence now point to the important role of aldosterone excess in human bone health as well. By literature search, we could identify three clinical studies assessing the relationship between PA and fracture. Salcuni et al. [40] showed that vertebral fractures tended to become more prevalent among 11 patients with PA than in 15 patients belonging to the non-PA group. A similar result was obtained in the longitudinal population-based National Health Insurance Research Database of Taiwan [41]. In the only study that separately considered men and women in terms of PA and fracture, Wu et al. [41] showed that patients with PA were confronted with a higher risk for all areal bone fractures, especially among female patients. A recent study also reported a higher prevalence of vertebral fractures in 56 patients with PA than in 56 age- and sex-matched controls [42]. Collectively, the existing clinical studies have shown that PA is associated with an increased risk of bone fracture, particularly of the vertebrae.
Despite these consistently adverse outcomes of PA in terms of fracture, studies assessing the association between aldosterone excess and BMD have yielded conflicting results. For example, one study showed that BMD expressed as Z-value at the lumbar spine, femur neck, and total neck was lower in PA patients than in those without PA [40], whereas there was no difference between plasma aldosterone concentration (PAC) and BMD in another study [42]. Consequently, in spite of the possible detrimental effects of aldosterone excess on human bone metabolism, there are discrepancies between fracture risk and bone mass in patients with and without PA, raising the possibility that aldosterone excess may mainly affect bone quality, another important component of bone strength, besides bone mass.
The trabecular bone score (TBS), which is determined by quantifying pixel gray-level variations on lumbar spine dual-energy X-ray absorptiometry scans, has been introduced as a parameter representing bone microarchitecture [43,44]. Low TBS values reflected deteriorated microarchitecture and predicted OFs, independent of BMD [45,46,47]. Therefore, TBS is regarded as a valuable noninvasive clinical tool in fracture risk assessment as it provides skeletal information that is not captured during the standard BMD measurement [43]. Importantly, TBS can be particularly useful in case of a paradox between fracture risk and BMD, such as occurs in diabetes [48,49,50].
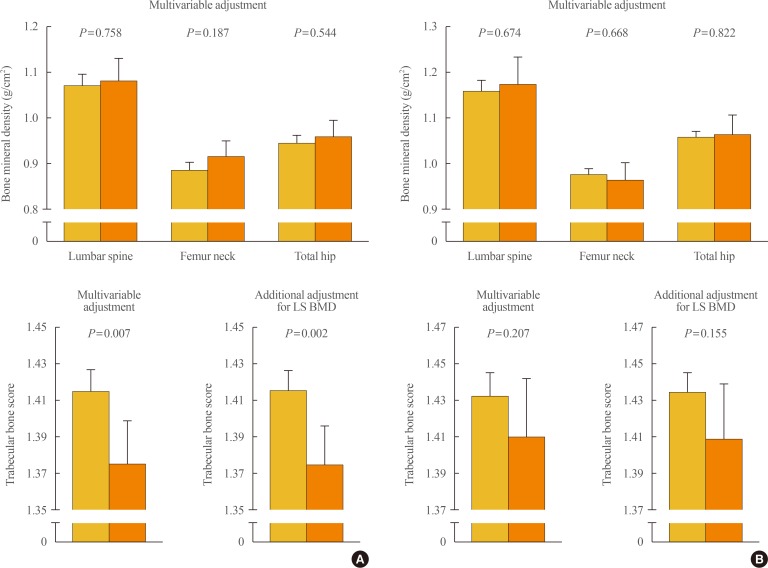
To clarify the potential effects of aldosterone excess on bone properties which are not explained by BMD, we employed this method as a skeletal fragility index in our recent study [51]. In a Korean cohort consisting of patients with PA (n=72) and controls (n=335), women with PA had significantly lower lumbar spine TBS (Fig. 2A), although there was no difference in BMD, and PAC was inversely correlated with lumbar spine TBS in women, after adjustment for potential confounders. Compared with women in the lowest PAC quartile, those in the highest PAC quartile had significantly lower lumbar TBS. Importantly, all these observations in women remained statistically significant following the additional adjustment for lumbar spine BMD in the multivariable model. However, BMD at any site of measurement and lumbar spine TBS did not differ according to the presence of PA in men (Fig. 2B). These findings provide the first clinical evidence that aldosterone excess in PA may contribute to the deterioration of bone quality through weak microarchitecture, regardless of bone mass, especially in women.
Fig. 2. Differences in bone mineral density and trabecular bone score between subjects without and with primary aldosteronism in (A) women and (B) men. Values are presented as the estimated means with 95% confidence intervals, from analysis of covariance after adjustment for age, menopausal status in women, body mass index, current smoking, alcohol intake, regular outdoor exercise, systolic and diastolic blood pressure, and glomerular filtration rate. Adapted from Kim et al., with permission from Oxford University Press [51]. LS BMD, lumbar spine bone mineral density.
The activation of angiotensin II could induce osteoporosis via increased bone resorption [52,53]. However, PA is a condition that involves the suppression of angiotensin II through a negative feedback due to elevated PAC, indicating that skeletal deterioration in patients with PA may result from the effects of aldosterone excess per se. Several possible mechanisms may explain the association between aldosterone and bone health. First, mineralocorticoid receptors have been identified in human bone cells including osteoblasts, osteoclasts, and osteocytes [54], suggesting, although still unknown, the possible direct effects on bone metabolism. Second, hyperaldosteronism could increase urinary calcium excretion and consequent secondary hyperparathyroidism, resulting in a negative balance on bone homeostasis [55,56,57]. In fact, in some animal studies, the continuous administration of aldosterone to rats induced persistent hypercalciuria and hypersecretion of parathyroid hormone, with a reduction in bone strength [58,59]. Third, a genome-wide association study showed that genes belonging to mineralocorticoid pathways were strongly associated with phenotypes of bone strength [60], supporting the potential cross-talk between aldosterone and bone. Fourth, chronic inflammation could be involved in the pathophysiological mechanisms underlying osteoporosis and fracture [61,62], and thus the proinflammatory property of aldosterone [63] may indirectly and adversely affect human bone.
CONCLUSIONS
Based on the existing literature, sympathetic overstimulation in pheochromocytoma can contribute to detrimental effects on human bone through the increase of bone loss as well as bone resorption. Furthermore, PA was consistently associated with an increased risk of bone fracture, particularly of the vertebrae, and these adverse outcomes by aldosterone excess may be, at least in part, attributable to poor bone quality in these patients. The data discussed here may have clinical implications in that they provide an important background, justifying aggressive treatment for pheochromocytoma and PA in terms of bone metabolism in addition to cardiovascular risks. If additional evidence that poor bone health in pheochromocytoma and PA can be reversed by adrenalectomy is provided, pheochromocytoma and PA would be regarded as important causes of secondary osteoporosis in cases of Cushing's syndrome.
ACKNOWLEDGMENTS
I, Jung-Min Koh, would like to express my sincere gratitude to Emeritus Professor Hun Ki Min (Namgok) for giving me the prestigious Namgok Award. I would like to thank Prof. Ghi Su Kim, Prof. Ki-Up Lee, Prof. Seung Hun Lee, and Prof. Beom-Jun Kim at Asan Medical Center, University of Ulsan College of Medicine, and all the professor-mentors who guided and taught me. This work was supported by the Korean Endocrine Society for Namgok Award 2017.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Diab DL, Watts NB. Secondary osteoporosis: differential diagnosis and workup. Clin Obstet Gynecol. 2013;56:686–693. doi: 10.1097/GRF.0b013e3182a9b5f9. [DOI] [PubMed] [Google Scholar]
- 2.Painter SE, Kleerekoper M, Camacho PM. Secondary osteoporosis: a review of the recent evidence. Endocr Pract. 2006;12:436–445. doi: 10.4158/EP.12.4.436. [DOI] [PubMed] [Google Scholar]
- 3.Cerda Gabaroi D, Peris P, Monegal A, Albaladejo C, Martinez MA, Muxi A, et al. Search for hidden secondary causes in postmenopausal women with osteoporosis. Menopause. 2010;17:135–139. doi: 10.1097/gme.0b013e3181ade8e5. [DOI] [PubMed] [Google Scholar]
- 4.Emkey GR, Epstein S. Secondary osteoporosis: pathophysiology & diagnosis. Best Pract Res Clin Endocrinol Metab. 2014;28:911–935. doi: 10.1016/j.beem.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab. 2010;95:4106–4113. doi: 10.1210/jc.2010-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnaldi G, Boscaro M. Adrenal incidentaloma. Best Pract Res Clin Endocrinol Metab. 2012;26:405–419. doi: 10.1016/j.beem.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Favier J, Amar L, Gimenez-Roqueplo AP. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol. 2015;11:101–111. doi: 10.1038/nrendo.2014.188. [DOI] [PubMed] [Google Scholar]
- 8.Prejbisz A, Lenders JW, Eisenhofer G, Januszewicz A. Mortality associated with phaeochromocytoma. Horm Metab Res. 2013;45:154–158. doi: 10.1055/s-0032-1331217. [DOI] [PubMed] [Google Scholar]
- 9.Vilela LAP, Almeida MQ. Diagnosis and management of primary aldosteronism. Arch Endocrinol Metab. 2017;61:305–312. doi: 10.1590/2359-3997000000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prejbisz A, Warchol-Celinska E, Lenders JW, Januszewicz A. Cardiovascular risk in primary hyperaldosteronism. Horm Metab Res. 2015;47:973–980. doi: 10.1055/s-0035-1565124. [DOI] [PubMed] [Google Scholar]
- 11.De Leo M, Pivonello R, Auriemma RS, Cozzolino A, Vitale P, Simeoli C, et al. Cardiovascular disease in Cushing's syndrome: heart versus vasculature. Neuroendocrinology. 2010;92(Suppl 1):50–54. doi: 10.1159/000318566. [DOI] [PubMed] [Google Scholar]
- 12.Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319–1328. doi: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 13.Rizzoli R, Biver E. Glucocorticoid-induced osteoporosis: who to treat with what agent? Nat Rev Rheumatol. 2015;11:98–109. doi: 10.1038/nrrheum.2014.188. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62–70. doi: 10.1056/NEJMcp1012926. [DOI] [PubMed] [Google Scholar]
- 15.Compston J. Management of glucocorticoid-induced osteoporosis. Nat Rev Rheumatol. 2010;6:82–88. doi: 10.1038/nrrheum.2009.259. [DOI] [PubMed] [Google Scholar]
- 16.Rizzoli R, Adachi JD, Cooper C, Dere W, Devogelaer JP, Diez-Perez A, et al. Management of glucocorticoid-induced osteoporosis. Calcif Tissue Int. 2012;91:225–243. doi: 10.1007/s00223-012-9630-5. [DOI] [PubMed] [Google Scholar]
- 17.Kajimura D, Hinoi E, Ferron M, Kode A, Riley KJ, Zhou B, et al. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J Exp Med. 2011;208:841–851. doi: 10.1084/jem.20102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonnet N, Laroche N, Vico L, Dolleans E, Benhamou CL, Courteix D. Dose effects of propranolol on cancellous and cortical bone in ovariectomized adult rats. J Pharmacol Exp Ther. 2006;318:1118–1127. doi: 10.1124/jpet.106.105437. [DOI] [PubMed] [Google Scholar]
- 19.Kondo H, Togari A. Continuous treatment with a low-dose β-agonist reduces bone mass by increasing bone resorption without suppressing bone formation. Calcif Tissue Int. 2011;88:23–32. doi: 10.1007/s00223-010-9421-9. [DOI] [PubMed] [Google Scholar]
- 20.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 21.Toulis KA, Hemming K, Stergianos S, Nirantharakumar K, Bilezikian JP. β-Adrenergic receptor antagonists and fracture risk: a meta-analysis of selectivity, gender, and site-specific effects. Osteoporos Int. 2014;25:121–129. doi: 10.1007/s00198-013-2498-z. [DOI] [PubMed] [Google Scholar]
- 22.Schlienger RG, Kraenzlin ME, Jick SS, Meier CR. Use of beta-blockers and risk of fractures. JAMA. 2004;292:1326–1332. doi: 10.1001/jama.292.11.1326. [DOI] [PubMed] [Google Scholar]
- 23.Reid IR, Lucas J, Wattie D, Horne A, Bolland M, Gamble GD, et al. Effects of a beta-blocker on bone turnover in normal postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab. 2005;90:5212–5216. doi: 10.1210/jc.2005-0573. [DOI] [PubMed] [Google Scholar]
- 24.Bonnet N, Gadois C, McCloskey E, Lemineur G, Lespessailles E, Courteix D, et al. Protective effect of beta blockers in postmenopausal women: influence on fractures, bone density, micro and macroarchitecture. Bone. 2007;40:1209–1216. doi: 10.1016/j.bone.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 25.de Vries F, Souverein PC, Cooper C, Leufkens HG, van Staa TP. Use of beta-blockers and the risk of hip/femur fracture in the United Kingdom and The Netherlands. Calcif Tissue Int. 2007;80:69–75. doi: 10.1007/s00223-006-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veldhuis-Vlug AG, Tanck MW, Limonard EJ, Endert E, Heijboer AC, Lips P, et al. The effects of beta-2 adrenergic agonist and antagonist on human bone metabolism: a randomized controlled trial. Bone. 2015;71:196–200. doi: 10.1016/j.bone.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 27.de Vries F, Pouwels S, Bracke M, Leufkens HG, Cooper C, Lammers JW, et al. Use of beta-2 agonists and risk of hip/femur fracture: a population-based case-control study. Pharmacoepidemiol Drug Saf. 2007;16:612–619. doi: 10.1002/pds.1318. [DOI] [PubMed] [Google Scholar]
- 28.Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk in patients with chronic lung diseases treated with bronchodilator drugs and inhaled and oral corticosteroids. Chest. 2007;132:1599–1607. doi: 10.1378/chest.07-1092. [DOI] [PubMed] [Google Scholar]
- 29.Gonnelli S, Caffarelli C, Maggi S, Guglielmi G, Siviero P, Rossi S, et al. Effect of inhaled glucocorticoids and beta(2) agonists on vertebral fracture risk in COPD patients: the EOLO study. Calcif Tissue Int. 2010;87:137–143. doi: 10.1007/s00223-010-9392-x. [DOI] [PubMed] [Google Scholar]
- 30.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–675. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 31.Veldhuis-Vlug AG, El Mahdiui M, Endert E, Heijboer AC, Fliers E, Bisschop PH. Bone resorption is increased in pheochromocytoma patients and normalizes following adrenalectomy. J Clin Endocrinol Metab. 2012;97:E2093–E2097. doi: 10.1210/jc.2012-2823. [DOI] [PubMed] [Google Scholar]
- 32.Kim BJ, Kwak MK, Ahn SH, Kim H, Lee SH, Song KH, et al. Lower bone mass and higher bone resorption in pheochromocytoma: importance of sympathetic activity on human bone. J Clin Endocrinol Metab. 2017;102:2711–2718. doi: 10.1210/jc.2017-00169. [DOI] [PubMed] [Google Scholar]
- 33.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 34.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnell O, Oden A, De Laet C, Garnero P, Delmas PD, Kanis JA. Biochemical indices of bone turnover and the assessment of fracture probability. Osteoporos Int. 2002;13:523–526. doi: 10.1007/s001980200068. [DOI] [PubMed] [Google Scholar]
- 36.Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996;11:1531–1538. doi: 10.1002/jbmr.5650111021. [DOI] [PubMed] [Google Scholar]
- 37.Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf) 2007;66:607–618. doi: 10.1111/j.1365-2265.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 38.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101:1889–1916. doi: 10.1210/jc.2015-4061. [DOI] [PubMed] [Google Scholar]
- 39.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 40.Salcuni AS, Palmieri S, Carnevale V, Morelli V, Battista C, Guarnieri V, et al. Bone involvement in aldosteronism. J Bone Miner Res. 2012;27:2217–2222. doi: 10.1002/jbmr.1660. [DOI] [PubMed] [Google Scholar]
- 41.Wu VC, Chang CH, Wang CY, Lin YH, Kao TW, Lin PC, et al. Risk of fracture in primary aldosteronism: a population-based cohort study. J Bone Miner Res. 2017;32:743–752. doi: 10.1002/jbmr.3033. [DOI] [PubMed] [Google Scholar]
- 42.Notsu M, Yamauchi M, Yamamoto M, Nawata K, Sugimoto T. Primary aldosteronism as a risk factor for vertebral fracture. J Clin Endocrinol Metab. 2017;102:1237–1243. doi: 10.1210/jc.2016-3206. [DOI] [PubMed] [Google Scholar]
- 43.Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518–530. doi: 10.1002/jbmr.2176. [DOI] [PubMed] [Google Scholar]
- 44.Bousson V, Bergot C, Sutter B, Levitz P, Cortet B Scientific Committee of the Groupe de Recherche et d'Information sur les Osteoporoses. Trabecular bone score (TBS): available knowledge, clinical relevance, and future prospects. Osteoporos Int. 2012;23:1489–1501. doi: 10.1007/s00198-011-1824-6. [DOI] [PubMed] [Google Scholar]
- 45.Boutroy S, Hans D, Sornay-Rendu E, Vilayphiou N, Winzenrieth R, Chapurlat R. Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos Int. 2013;24:77–85. doi: 10.1007/s00198-012-2188-2. [DOI] [PubMed] [Google Scholar]
- 46.Iki M, Tamaki J, Kadowaki E, Sato Y, Dongmei N, Winzenrieth R, et al. Trabecular bone score (TBS) predicts vertebral fractures in Japanese women over 10 years independently of bone density and prevalent vertebral deformity: the Japanese Population-Based Osteoporosis (JPOS) cohort study. J Bone Miner Res. 2014;29:399–407. doi: 10.1002/jbmr.2048. [DOI] [PubMed] [Google Scholar]
- 47.Silva BC, Broy SB, Boutroy S, Schousboe JT, Shepherd JA, Leslie WD. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD Official Positions Part 2: trabecular bone score. J Clin Densitom. 2015;18:309–330. doi: 10.1016/j.jocd.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 48.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes: a meta-analysis. Osteoporos Int. 2007;18:427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 49.Kim JH, Choi HJ, Ku EJ, Kim KM, Kim SW, Cho NH, et al. Trabecular bone score as an indicator for skeletal deterioration in diabetes. J Clin Endocrinol Metab. 2015;100:475–482. doi: 10.1210/jc.2014-2047. [DOI] [PubMed] [Google Scholar]
- 50.Leslie WD, Aubry-Rozier B, Lamy O, Hans D Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602–609. doi: 10.1210/jc.2012-3118. [DOI] [PubMed] [Google Scholar]
- 51.Kim BJ, Kwak MK, Ahn SH, Kim H, Lee SH, Koh JM. Lower trabecular bone score in patients with primary aldosteronism: human skeletal deterioration by aldosterone excess. J Clin Endocrinol Metab. 2018;103:615–621. doi: 10.1210/jc.2017-02043. [DOI] [PubMed] [Google Scholar]
- 52.Asaba Y, Ito M, Fumoto T, Watanabe K, Fukuhara R, Takeshita S, et al. Activation of renin-angiotensin system induces osteoporosis independently of hypertension. J Bone Miner Res. 2009;24:241–250. doi: 10.1359/jbmr.081006. [DOI] [PubMed] [Google Scholar]
- 53.Shimizu H, Nakagami H, Osako MK, Hanayama R, Kunugiza Y, Kizawa T, et al. Angiotensin II accelerates osteoporosis by activating osteoclasts. FASEB J. 2008;22:2465–2475. doi: 10.1096/fj.07-098954. [DOI] [PubMed] [Google Scholar]
- 54.Beavan S, Horner A, Bord S, Ireland D, Compston J. Colocalization of glucocorticoid and mineralocorticoid receptors in human bone. J Bone Miner Res. 2001;16:1496–1504. doi: 10.1359/jbmr.2001.16.8.1496. [DOI] [PubMed] [Google Scholar]
- 55.Pilz S, Kienreich K, Drechsler C, Ritz E, Fahrleitner-Pammer A, Gaksch M, et al. Hyperparathyroidism in patients with primary aldosteronism: cross-sectional and interventional data from the GECOH study. J Clin Endocrinol Metab. 2012;97:E75–E79. doi: 10.1210/jc.2011-2183. [DOI] [PubMed] [Google Scholar]
- 56.Petramala L, Zinnamosca L, Settevendemmie A, Marinelli C, Nardi M, Concistre A, et al. Bone and mineral metabolism in patients with primary aldosteronism. Int J Endocrinol. 2014;2014:836529. doi: 10.1155/2014/836529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ceccoli L, Ronconi V, Giovannini L, Marcheggiani M, Turchi F, Boscaro M, et al. Bone health and aldosterone excess. Osteoporos Int. 2013;24:2801–2807. doi: 10.1007/s00198-013-2399-1. [DOI] [PubMed] [Google Scholar]
- 58.Chhokar VS, Sun Y, Bhattacharya SK, Ahokas RA, Myers LK, Xing Z, et al. Loss of bone minerals and strength in rats with aldosteronism. Am J Physiol Heart Circ Physiol. 2004;287:H2023–H2026. doi: 10.1152/ajpheart.00477.2004. [DOI] [PubMed] [Google Scholar]
- 59.Chhokar VS, Sun Y, Bhattacharya SK, Ahokas RA, Myers LK, Xing Z, et al. Hyperparathyroidism and the calcium paradox of aldosteronism. Circulation. 2005;111:871–878. doi: 10.1161/01.CIR.0000155621.10213.06. [DOI] [PubMed] [Google Scholar]
- 60.Gupta M, Cheung CL, Hsu YH, Demissie S, Cupples LA, Kiel DP, et al. Identification of homogeneous genetic architecture of multiple genetically correlated traits by block clustering of genome-wide associations. J Bone Miner Res. 2011;26:1261–1271. doi: 10.1002/jbmr.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koh JM, Khang YH, Jung CH, Bae S, Kim DJ, Chung YE, et al. Higher circulating hsCRP levels are associated with lower bone mineral density in healthy pre- and postmenopausal women: evidence for a link between systemic inflammation and osteoporosis. Osteoporos Int. 2005;16:1263–1271. doi: 10.1007/s00198-005-1840-5. [DOI] [PubMed] [Google Scholar]
- 62.Cauley JA, Danielson ME, Boudreau RM, Forrest KY, Zmuda JM, Pahor M, et al. Inflammatory markers and incident fracture risk in older men and women: the Health Aging and Body Composition Study. J Bone Miner Res. 2007;22:1088–1095. doi: 10.1359/jbmr.070409. [DOI] [PubMed] [Google Scholar]
- 63.Stehr CB, Mellado R, Ocaranza MP, Carvajal CA, Mosso L, Becerra E, et al. Increased levels of oxidative stress, subclinical inflammation, and myocardial fibrosis markers in primary aldosteronism patients. J Hypertens. 2010;28:2120–2126. doi: 10.1097/HJH.0b013e32833d0177. [DOI] [PubMed] [Google Scholar]