Abstract
Background
The nuclear receptor peroxisome proliferator-activator gamma (PPARγ) is a useful therapeutic target for obesity and diabetes, but its role in protecting β-cell function and viability is unclear.
Methods
To identify the potential functions of PPARγ in β-cells, we treated mouse insulinoma 6 (MIN6) cells with the PPARγ agonist pioglitazone in conditions of lipotoxicity, endoplasmic reticulum (ER) stress, and inflammation.
Results
Palmitate-treated cells incubated with pioglitazone exhibited significant improvements in glucose-stimulated insulin secretion and the repression of apoptosis, as shown by decreased caspase-3 cleavage and poly (adenosine diphosphate [ADP]-ribose) polymerase activity. Pioglitazone also reversed the palmitate-induced expression of inflammatory cytokines (tumor necrosis factor α, interleukin 6 [IL-6], and IL-1β) and ER stress markers (phosphor-eukaryotic translation initiation factor 2α, glucose-regulated protein 78 [GRP78], cleaved-activating transcription factor 6 [ATF6], and C/EBP homologous protein [CHOP]), and pioglitazone significantly attenuated inflammation and ER stress in lipopolysaccharide- or tunicamycin-treated MIN6 cells. The protective effect of pioglitazone was also tested in pancreatic islets from high-fat-fed KK-Ay mice administered 0.02% (wt/wt) pioglitazone or vehicle for 6 weeks. Pioglitazone remarkably reduced the expression of ATF6α, GRP78, and monocyte chemoattractant protein-1, prevented α-cell infiltration into the pancreatic islets, and upregulated glucose transporter 2 (Glut2) expression in β-cells. Moreover, the preservation of β-cells by pioglitazone was accompanied by a significant reduction of blood glucose levels.
Conclusion
Altogether, these results support the proposal that PPARγ agonists not only suppress insulin resistance, but also prevent β-cell impairment via protection against ER stress and inflammation. The activation of PPARγ might be a new therapeutic approach for improving β-cell survival and insulin secretion in patients with diabetes mellitus
Keywords: Pioglitazone, Insulin-secreting cells, Glucolipotoxicity, Inflammation, Endoplasmic reticulum stress
INTRODUCTION
Chronic exposure to high levels of glucose and non-esterified fatty acids results in pancreatic β-cell dysfunction and death, thereby leading to the onset of type 2 diabetes [1]. Thiazolidinediones (TZDs) are specific agonists of peroxisome proliferator-activator gamma (PPARγ), a nuclear transcription factor, and several TZDs are prescribed throughout the world to treat type 2 diabetes. The main goal of TZD therapy is to improve the insulin sensitivity of peripheral tissues, but many studies in human and animal models have reported that these agents could protect pancreatic β-cells [2,3]. PPARγ agonists were found to protect the viability and function of β-cells against human islet amyloid polypeptide (IAPP)-induced toxicity [3]. Moreover, PPARγ expression has been detected in the pancreatic islet, and treatment with a PPARγ agonist or overexpression of PPARG improved glucose-stimulated insulin secretion, with an increased expression of genes involved in β-cell function, including glucose transporter 2 (GLUT2), insulin receptor substrate 2 (IRS2), and pancreatic duodenal homeobox 1 (PDX1) [4].
In type 2 diabetes and/or obesity, numerous etiological factors lead to dysfunction and apoptosis of pancreatic β-cells, such as inflammation, oxidative stress, and endoplasmic reticulum (ER) stress [5]. Several clinical studies have suggested that anti-inflammatory therapy in patients with type 2 diabetes may improve hyperglycemia and insulin secretion by β-cells [6,7], and inflammation is known to be a pivotal pathway in the pathogenesis of various forms of cellular dysfunction and apoptosis. Pancreatic β-cells are exposed to circulating cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), and interferon gamma, which inhibit insulin secretion by interrupting calcium flow. Moreover, TNF-α induces β-cell death via the expression and cellular deposition of IAPP [7], and increased cellular deposition of IAPP causes β-cell inflammation [8,9,10]. The inflammation of β-cells can also be caused by lipotoxicity, which is induced by the irregular increase of free fatty acids, including palmitic acid. This causes oxidative stress and jun N-terminal kinase (JNK) activation [10], further increases the expression of TNF-α, IL-1β, IL-6, and IL-8, and activates nuclear factor-kappa B (NF-κB) in pancreatic β-cells, leading to β-cell dysfunction and apoptosis [11].
Although ER stress is linked to cellular damage in all tissues involved in diabetes [12], it is a factor to which insulin-releasing β-cells are particularly susceptible. Abnormal expansion of the ER was observed in β-cells from patients with type 2 diabetes, and ER stress markers were increased in islets from db/db mice and β-cells of type 2 diabetes patients [13,14]. These studies suggest that ER stress is a strong etiological factor inducing β-cell impairment. Exposure to high levels of glucose and lipids is known to induce the overproduction of insulin, which leads to β-cell dysfunction and apoptosis through ER stress. In the early stages of ER stress, the unfolded protein response (UPR) is activated to maintain the ER function through an increase in UPR proteins, such as glucose-regulated protein 78 (GRP78), phosphor-eukaryotic translation initiation factor 2-α (p-eIF2α), and activating transcription factor 6 (ATF6); however, chronic activation of UPR increases the expression of C/EBP homologous protein (CHOP) and results in cell death. CHOP is a transcriptional regulator that stimulates cell death by downregulating the anti-apoptotic protein Bcl-2 and upregulating cellular reactive oxygen species and ER oxidoreductase 1, leading to hyperoxidizing conditions in the ER [15].
Although growing evidence suggests that the activation of PPARγ preserves pancreatic islets, the mechanism through which PPARγ acts to regulate molecular abnormalities is poorly understood. In the present study, therefore, we examined the effects of a PPARγ agonist on the regulation of glucolipotoxicity-induced pathophysiological events, focusing on the inflammatory response and ER stress in pancreatic β-cells.
METHODS
Cell culture and treatment
Mouse insulinoma 6 (MIN6) cell lines (passages 15 to 25) were cultured at 37℃ in a humidified incubator with 5% CO2 in Dulbecco's Modified Eagle's medium (DMEM) containing 4.5 g/L glucose supplemented with 10% heat-inactivated fetal bovine serum, 100 IU/mL penicillin, 50 µg/mL streptomycin, and 50 µM 2-mercaptoethanol (all from Life Technologies, Paisley, UK). At 50% to 70% confluence, the cells were incubated with or without 10 µM pioglitazone (Takara Pharmaceuticals, Kusatsu, Japan) and/or 0.5 mM palmitic acid (R&D Systems Inc., Minneapolis, MN, USA) in different glucose concentrations (1 or 4.5 g/L) for 48 hours. A group of cells was treated with 10 µg/mL lipopolysaccharide (LPS) and/or 10 µM pioglitazone, and another group was treated with 2 µg/mL tunicamycin with or without pioglitazone for 48 hours.
Mice and treatment protocols
Male KK-Ay mice 8 weeks of age were obtained from Oriental Bio Laboratory (Seoul, Korea) and bred in standard conditions under a 12-hour light/dark cycle. All experiments using animals were performed in accordance with the Ethics Committee for Animal Experiments of the Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine. The experimental mice were placed on a high-fat (HF) diet (fat: 45% of energy content [39.4% from lard and 4.4 from soybean oil]; protein: 20% of energy content; and carbohydrate: 35% of energy content) or a HF diet containing 0.02% (wt/wt) pioglitazone for 6 weeks. Body weight and blood glucose levels were checked every week.
Palmitate-bovine serum albumin solution preparation
A stock solution was made by dissolving palmitate (R&D Systems Inc.) with 50% ethanol to 100 mM. This was diluted in serum-free DMEM containing 1% immunoglobulin G and fatty acid-free bovine serum albumin (BSA) fraction V (GIBCO, Grand Island, NY, USA) to 500 µM and then mixed at 40℃. Stock solutions were kept at −20℃ and used for treatment until they had been stored for 6 months. BSA solution in serum-free DMEM medium was used as the vehicle control.
Glucose-stimulated insulin secretion test
At 60% to 70% confluence, MIN6 cells were pre-incubated in glucose-free Krebs-Ringer bicarbonate HEPES (KRPH) buffer for 60 minutes and treated with 1 or 4.5 g/L glucose in DMEM for 60 minutes. The dose of insulin released in the medium was measured with a mouse insulin enzyme-linked immunosorbent assay (Linco Research, St Charles, MO, USA).
Protein isolation and immunoblotting
Cellular proteins were prepared by a RIPA lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA) with protease and phosphatase inhibitors. The concentration of protein was measured by the Bradford assay (Amersham Life Science, Arlington Height, IL, USA), and 20 µg of protein from each group was separated in 4% to 12% SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) gels and transferred to polyvinylidene difluoride membranes. Proteins in the membranes were probed with primary antibodies for p-eIF2α (Ser51), IRE1α (14C10), CHOP (L63F7), phosphor-NF-κB p65 (S536), NF-κB, phosphor-stress-activated protein kinase (SAPK)/JNK (T183/Y185), SAPK/JNK, cleaved caspase-3 (D175), total caspase-3, β-actin (Cell Signaling Technology, Danvers, MA, USA), ATF6 (Abcam, Cambridge, MA, USA), and GRP78 (Santa Cruz Biotechnology) for 24 hours. The membranes were then exposed to secondary antibodies conjugated with horseradish peroxidase, and visualized using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Rockford, IL, USA). ImageJ, a program for quantification, was used to measure the density of the scanned immunoblot bands. The density of phosphorylated NF-κB, SAPK/JNK, and cleaved caspase-3 was normalized to the levels of NF-κB, SAPK/JNK, and caspase-3, respectively.
Immunofluorescence analysis
Pancreatic tissue samples were fixed with 4% paraformaldehyde for 24 hours and embedded within paraffin. Paraffin sections of 7 µm were dewaxed in xylene, and incubated with 0.5% hydrogen peroxide in methanol for 0.5 hours to block endogenous peroxidase activity. After washing with phosphate-buffered saline, tissue samples were blocked using normal goat serum for 0.5 hours, and then incubated with primary antibodies for insulin (Millipore, Billerica, MA, USA), glucagon, Glut2, monocyte chemoattractant protein-1 (MCP1), ATF6α, and GRP78 (Santa Cruz Biotechnology) for 24 hours. The tissue samples were then incubated with secondary antibodies conjugated with fluorescent dye (Thermo Fisher Scientific) for 1 hour. DAPI (4′,6-diamidino-2-phenylindole) solution (Invitrogen, Carlsbad, CA, USA) was used to visualize the nucleus, and fluorescent images were obtained using an Olympus BX52 microscope (Olympus Optical Co., Tokyo, Japan).
RNA isolation and real-time polymerase chain reaction analysis
At 60% to 70% confluence, total RNA was extracted using TRizol (Life Technologies, Carlsbad, CA, USA). After dose measurement, 2 µg of RNA was used to synthesize the first strand of cDNA using the RT enzyme Mix (Thermo Fisher Scientific), and it was amplified by SYBR green I Master (Roche Diagnostics, Indianapolis, IN, USA) and the following primers: TNFA, sense 5′-GTTCTATGGCCCAGACCCTCAC-3′ and antisense 5′-GGCACCACTAGTTGGTTGTCTTTG-3′ (NM-013693, 144 bp); IL6, sense 5′-CCACTTCACAAGTCGGAGGCTTA-3′ and antisense 5′-GCAAGTGCATCATCGTTGTTCATAC-3′ (NM-031168, 115 bp); IL1B, sense 5′-AGGTCATCACTATTGGCAAC-3′ and antisense 5′-ACTCATCGTACTCCTGCTTG-3′ (NM-008361, 135 bp). Polymerase chain reaction (PCR) was performed in a Light Cycler 480 real-time PCR machine (Roche, Lewis, UK) with a pre-denaturation, denaturation, and annealing step, followed by additional 40 cycles and an extension step. The emission levels of green fluorescence by SYBR were detected and the relative expression levels of RNA were determined using the ΔΔCt method.
Poly (ADP-ribose) polymerase activity assay
Poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) activity was measured using a universal colorimetric PARP assay kit (Trevigen, Gaithersburg, MD, USA). The proteins extracted from MIN6 cells were loaded onto a histone-coated 96-well plate, and incubated with biotinylated poly (ADP-ribose) for 1 hour. After treatment with horseradish peroxidase and colorimetric substrates, absorbance was read at 450 nm in a spectrophotometer.
Statistical analysis
PASW version 17 (SPSS Inc., Chicago, IL, USA), a statistical software program, was used for all statistical analyses in the present study, and one-way analysis of variance was used to evaluate the statistical differences among mean values in different experimental groups. Differences with a P value <0.05 were regarded as statistically significant changes.
RESULTS
Pioglitazone attenuates palmitate-induced β-cell dysfunction and apoptosis
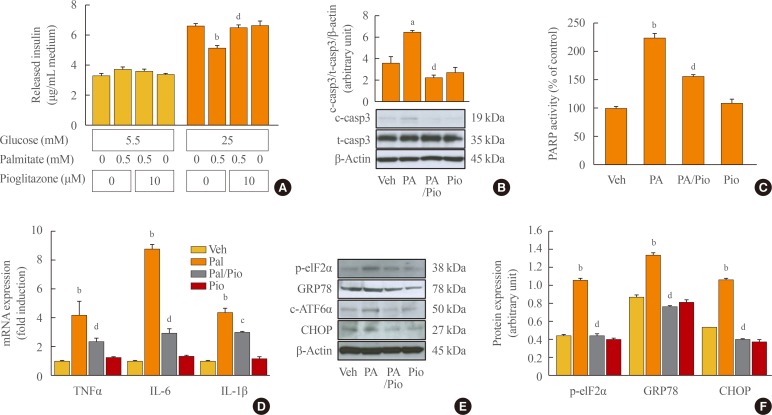
To study the role of pioglitazone in β-cells, MIN6 cells were incubated with palmitate and/or pioglitazone. Treatment with 0.5 mM palmitate significantly inhibited 25 mM glucose-stimulated insulin secretion (1% BSA vs. 500 µM palmitate/1% BSA, P<0.001), while insulin levels were significantly restored by pioglitazone treatment for 24 hours (500 µM palmitate/1% BSA vs. 10 nM pioglitazone/500 µM palmitate/1% BSA, P<0.001) (Fig. 1A). Pioglitazone treatment also attenuated apoptosis, with a significant decrease of cleaved caspase-3 and PARP activity observed in MIN6 cells treated with high glucose and palmitate (Fig. 1B, C).
Fig. 1. Pioglitazone (Pio) improves palmitate (PA)-induced β-cell impairment via repression of the inflammatory response and endoplasmic reticulum (ER) stress. (A) Mouse insulinoma 6 (MIN6) cells were incubated with 0.5 mM PA in the presence or absence of 10 µM Pio for 24 hours, and the glucose-stimulated (1 or 4.5 g/L) insulin secretion of MIN6 cells was evaluated. Secreted insulin was measured by a mouse insulin enzyme-linked immunosorbent assay (ELISA) kit. The values are representative of 6 independent experiments. (B) Expression of cleaved caspase-3 (c-casp3), an apoptotic protein, was measured by Western blot analysis. (C) Poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) activity is represented as the percentage of relative absorbance compared to the vehicle group. (D) Transcription of tumor necrosis factor α (TNFA), interleukin 6 (IL6), and IL1B was measured by real time reverse-transcription polymerase chain reaction, and normalized with β-actin. (E) The ER stress proteins, including phosphor-eukaryotic translation initiation factor 2α (p-eIF2α), glucose-regulated protein 78 (GRP78), cleaved-activating transcription factor 6 (c-ATF6), and C/EBP homologous protein (CHOP), were measured by Western blot analysis and (F) the ratio of p-eIF2α, GRP78, and CHOP to β-actin was described. Each value represents the mean of three experiments. t-casp3, total caspase-3; Veh, vehicle; PA, palmitate. aP<0.01, bP<0.001 compared with the vehicle group; cP<0.05, dP<0.001 compared with the PA group.
Pioglitazone spontaneously improves the palmitate-induced inflammatory response and ER stress
As it has been reported that inflammation and ER stress are major causes of apoptosis and dysfunction in pancreatic β-cells [16,17], we measured the inflammatory response and ER stress. To examine whether the protective effect of pioglitazone against free fatty acid-induced β-cell dysfunction was related to the inflammatory response and ER stress, we measured the expression of cytokines and proteins related to ER stress. The extreme increase in the expression of TNFA, IL6, and IL1B induced by palmitate was significantly ameliorated by co-treatment with pioglitazone (Fig. 1D). Treatment with palmitate induced the expression of specific markers of the UPR pathways responding to ER stress, including p-eIF2α, GRP78, ATF6α, and CHOP, and the expression of these proteins was significantly reduced by pioglitazone treatment (Fig. 1E, F). These results suggest that pioglitazone inhibits palmitate-induced inflammatory response and ER stress signaling, contributing to protection from β-cell dysfunction.
Pioglitazone represses the inflammatory response and ER stress in LPS-treated MIN6 cells
As shown in Fig. 1, pioglitazone simultaneously repressed the palmitate-induced inflammatory response and reduced ER stress. Therefore, we evaluated the role of pioglitazone in MIN6 cells in which an inflammatory response or ER stress was induced. First, MIN6 cells were treated with 10 µg/mL LPS for 48 hours to induce an inflammatory response. In LPS-treated cells, increased phosphorylation of NF-κB and JNK was detected, and the expression of TNFA, IL6, and IL1B was elevated, meaning that LPS induced a major inflammatory response in the MIN6 cells. The induction of the inflammatory response by LPS also increased the expression of ER stress markers, such as p-eIF2α, GRP78, and CHOP. These data suggest that the inflammatory response could induce ER stress in MIN6 cells. Treatment with pioglitazone remarkably repressed the inflammatory response induced by LPS, with diminished phosphorylation of NF-κB and JNK, and reduced expression of the mRNA of inflammatory cytokines (Fig. 2A-C). Consistently, markers of ER stress were also significantly decreased by pioglitazone (Fig. 2D, E). These data suggest that pioglitazone can repress the LPS-induced inflammatory response and ER stress in pancreatic β-cells.
Fig. 2. Pioglitazone (Pio) represses lipopolysaccharide (LPS)-induced inflammatory response and endoplasmic reticulum (ER) stress. The mouse insulinoma 6 (MIN6) cells were incubated with 10 µg/mL LPS in the presence or absence of 10 µM Pio for 8 hours. (A) Phosphorylation of nuclear factor-kappa B (NF-κB) and jun N-terminal kinase (JNK), essential factors of the inflammatory response, was evaluated by Western blot. (B) The density of phosphorylated NF-κB and JNK was normalized to that of the total forms. (C) The inflammatory cytokines were measured by reverse-transcription polymerase chain reaction, and normalized with β-actin. (D) The ER stress proteins, including phosphor-eukaryotic translation initiation factor 2α (p-eIF2α), glucose-regulated protein 78 (GRP78), and C/EBP homologous protein (CHOP), were measured by Western blot analysis and (E) the ratio to β-actin was described. Each value represents the mean of three experiments. t-NF-κB, total nuclear factor kappa-light-chain-enhancer of activated B cells; p-JNK, phosphorylated c-Jun N-terminal kinase; t-JNK, total c-Jun N-terminal kinase; TNFα, tumor necrosis factor α; IL, interleukin. aP<0.05, bP<0.01, cP<0.001 compared with the vehicle (Veh) group; dP<0.05, eP<0.01, fP<0.001 compared with the LPS group.
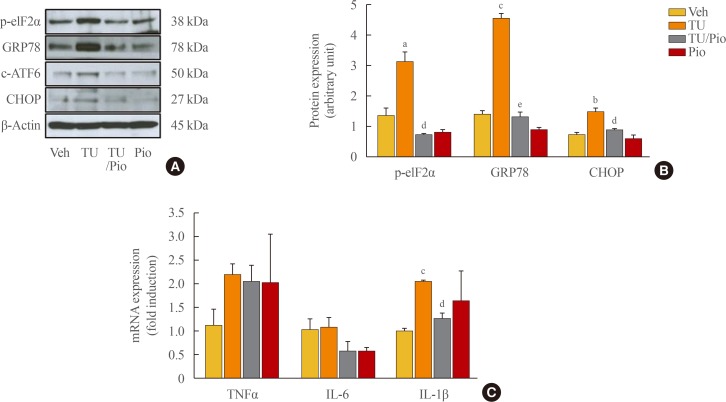
Pioglitazone represses ER stress in tunicamycin-treated MIN6 cells
We next administered tunicamycin, an inducer of ER stress, to evaluate the effects of pioglitazone on the regulation of ER stress. Pioglitazone significantly repressed the expression of ER stress-related proteins, such as p-eIF2α, GRP78, cleaved-ATF6, and CHOP (Fig. 3A, B). Treatment with tunicamycin resulted in a slight increase in the expression of TNFA and IL1B, but not IL6 (Fig. 3C), while pioglitazone repressed only IL1B expression, indicating that the induction of the inflammatory response might be upstream of ER stress in β-cell dysfunction and that pioglitazone represses both the inflammatory response and ER stress in pancreatic β-cells.
Fig. 3. Pioglitazone (Pio) reduces endoplasmic reticulum (ER) stress, but not the inflammatory response in tunicamycin (TU)-challenged mouse insulinoma 6 (MIN6) cells. The MIN6 cells were incubated with 2 µg/mL TU in the presence or absence of 10 µM Pio for 24 hours. (A) The ER stress proteins, including phosphor-eukaryotic translation initiation factor 2α (p-eIF2α), glucose-regulated protein 78 (GRP78), cleaved-activating transcription factor 6 (c-ATF6), and C/EBP homologous protein (CHOP), were measured by Western blot analysis, and (B) the ratio to β-actin was described. (C) The transcription of tumor necrosis factor α (TNFA), interleukin 6 (IL6), and IL1B was measured by real-time reverse-transcription polymerase chain reaction, and normalized with β-actin. Each value represents the mean of three experiments. aP<0.05, bP<0.01, cP<0.001 compared with the vehicle (Veh) group; dP<0.01, eP<0.001 compared with the TU group.
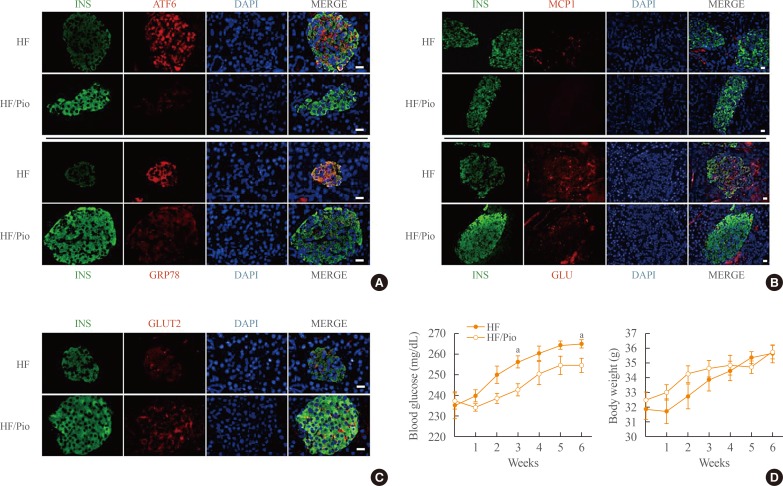
Pioglitazone represses inflammation and ER stress in the pancreatic islet of diabetic mice
We next employed morphological investigations of pancreatic islet samples from KK-Ay mice, which were fed HF diets with or without pioglitazone, to explore the effects of pioglitazone on inflammation and ER stress. In the pancreas of HF-fed mice without pioglitazone, the expression of ATF6 and GRP78 was strongly detected in the islet, showing co-localization with insulin. However, the expression of ATF6 and GRP78 was remarkably decreased by pioglitazone, providing evidence that pioglitazone repressed ER stress in the pancreatic islets (Fig. 4A). Treatment with pioglitazone also repressed the inflammatory response in the pancreatic islets, as a significant decrease of MCP1 was observed. Additionally, α-cell infiltration into the core of the pancreatic islet was decreased by pioglitazone (Fig. 4B). In contrast, GLUT2, the main sensor of glucose in β-cells, was significantly increased by pioglitazone treatment (Fig. 4C). These results indicated that pioglitazone treatment preserved β-cells against ER stress and the inflammatory response. Treatment with pioglitazone also attenuated the rise of blood glucose levels induced by the HF diet without altered body weight. In the 6th week, the blood glucose levels of the pioglitazone-treated mice were significantly lower than those in the HF group (254.50±3.33 mg/dL vs. 264.75±2.10 mg/dL, respectively). These data suggest that the preservation of pancreatic β-cell function by pioglitazone may play a meaningful role in the maintenance of blood glucose levels.
Fig. 4. Pioglitazone (Pio) protects pancreatic β-cells and regulates blood glucose levels in high-fat (HF)-diet-induced diabetic mice. The pancreatic islet from KK-Ay mice, which were fed an HF diet with or without Pio, were examined by double-immunofluorescence for (A) insulin-activating transcription factor 6 and insulin-glucose-regulated protein 78 (GRP78), (B) insulin-monocyt e chemoattractant protein-1 and insulin-glucagon (GLU), (C) insulin-glucose transporter 2 (GLUT2). (A-C) The nucleus was visualized by 4′,6-diamidino-2-phenylindole (DAPI). Bars=20 µm. (D) Blood glucose levels and body weight were measured every week. INS, insulin; ATF6, activating transcription factor 6; MCP1, monocyte chemoattractant protein-1. aP<0.05 compared with the HF group.
DISCUSSION
The use of PPARγ agonists to improve insulin resistance and prevent diabetes onset has been proposed in numerous studies using animal models and type 2 diabetes patients [18,19,20]. It has also been suggested that PPARγ agonist administration preserves islet mass in animal models, but this has been considered to be a systemic effect of PPARγ activation, not a direct effect on pancreatic β-cells. Despite the known importance of PPARγ expression [21], its specific and direct role in β-cells is less well known. PPARγ, a nuclear receptor, is expressed in various tissues, including fat, muscle, and the liver, and is involved in the regulation of various genes contributing to glucose and lipid metabolism and insulin signaling [22]. Interestingly, the specific elimination of PPARG in pancreatic β-cells induced islet hyperplasia with increased replication on a chow diet [23], indicating that PPARγ plays a direct role in β-cells.
In this study, incubation with palmitate resulted in β-cell dysfunction and apoptosis, and simultaneously facilitated the expression of inflammatory cytokines and ER stress. Treatment with pioglitazone blocked all cellular toxicity; moreover, pioglitazone significantly attenuated the expression of inflammatory cytokines in LPS-treated β-cells. Lipotoxicity is known to be powerful inducer of inflammation and apoptosis [24]; briefly, the accumulation of intracellular triglycerides results in increased levels of ceramide and facilitates NF-κB activation, thereby inducing apoptosis [25]. NF-κB is also activated by exposure to high glucose via the production of IL-1β or an increase in intracellular Ca2+ [26,27]. Moreover, the active form of NF-κB is transferred to the nucleus, and strongly stimulates the transcription of genes involved in the inflammatory response [28], indicating that NF-κB is essential regulator of glucolipotoxicity, the inflammatory response, and apoptosis in toxicity-induced β-cells. In the present study, treatment with pioglitazone repressed LPS-induced NF-κB phosphorylation, and significantly and consistently decreased the expression of inflammatory cytokines (Fig. 2A, B), suggesting that NF-κB might be a key regulator of the repression of apoptosis and inflammation by pioglitazone in pancreatic β-cells.
In glucolipotoxicity-induced MIN6 cells and pancreatic islet samples from diabetic mice, pioglitazone significantly suppressed the expression of ER stress markers (Figs. 1, 4). Moreover, the increase of ER stress markers by LPS and tunicamycin was also reversed by pioglitazone (Figs. 2, 3). These data suggest that PPARγ activation may play a direct role in the preservation of ER function and health in pancreatic β-cells. It was recently reported that sarco-ER Ca2+-ATPase (SERCA) 2, a regulator of the ER maintenance and secretion pathway, is also expressed in β-cells and modulated by PPARγ agonists. Moreover, the expression of SERCA2b was remarkably decreased by the inflammatory cytokine IL-1β in pancreatic islets [29]. The reduction of SERCA2b expression by inflammatory cytokines indirectly indicates that induction of the inflammatory response facilitates ER stress, as occurred in LPS induced β-cells (Fig. 2). SERCA2 is also an essential regulator of the interaction between ER stress and insulin secretion, and the improvement of insulin secretion and ER stress by pioglitazone might be mediated by SERCA2 in pancreatic β-cells (Fig. 1).
This study showed that PPARγ activation exerted a protective effect in pancreatic β-cells, mediated via repression of the inflammatory response and ER stress. PPARγ agonists might be used as a means not only to improve insulin resistance, but also to preserve β-cell survival and function.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Research Foundation (NRF), which is funded by the Korea government (NRF-2016R1A6A3A11930792), and Samsung Biomedical Research Institute (SBRI). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS: Conception or design: S.W.H., C.Y.P., W.Y.L. Acquisition, analysis, or interpretation of data: J.H.C., H.K., S.E.P., E.J.R., K.W.O., S.W.P. Drafting the work or revising: S.W.H., J.L. Final approval of the manuscript: S.W.H, W.Y.L.
References
- 1.Poitout V, Amyot J, Semache M, Zarrouki B, Hagman D, Fontes G. Glucolipotoxicity of the pancreatic beta cell. Biochim Biophys Acta. 2010;1801:289–298. doi: 10.1016/j.bbalip.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishida H, Takizawa M, Ozawa S, Nakamichi Y, Yamaguchi S, Katsuta H, et al. Pioglitazone improves insulin secretory capacity and prevents the loss of beta-cell mass in obese diabetic db/db mice: possible protection of beta cells from oxidative stress. Metabolism. 2004;53:488–494. doi: 10.1016/j.metabol.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Lin CY, Gurlo T, Haataja L, Hsueh WA, Butler PC. Activation of peroxisome proliferator-activated receptor-gamma by rosiglitazone protects human islet cells against human islet amyloid polypeptide toxicity by a phosphatidylinositol 3’-kinase-dependent pathway. J Clin Endocrinol Metab. 2005;90:6678–6686. doi: 10.1210/jc.2005-0079. [DOI] [PubMed] [Google Scholar]
- 4.Kim HS, Hwang YC, Koo SH, Park KS, Lee MS, Kim KW, et al. PPAR-γ activation increases insulin secretion through the up-regulation of the free fatty acid receptor GPR40 in pancreatic β-cells. PLoS One. 2013;8:e50128. doi: 10.1371/journal.pone.0050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montane J, Cadavez L, Novials A. Stress and the inflammatory process: a major cause of pancreatic cell death in type 2 diabetes. Diabetes Metab Syndr Obes. 2014;7:25–34. doi: 10.2147/DMSO.S37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 7.Cai K, Qi D, Wang O, Chen J, Liu X, Deng B, et al. TNF-α acutely upregulates amylin expression in murine pancreatic beta cells. Diabetologia. 2011;54:617–626. doi: 10.1007/s00125-010-1972-9. [DOI] [PubMed] [Google Scholar]
- 8.Montane J, Klimek-Abercrombie A, Potter KJ, Westwell-Roper C, Bruce Verchere C. Metabolic stress, IAPP and islet amyloid. Diabetes Obes Metab. 2012;14(Suppl 3):68–77. doi: 10.1111/j.1463-1326.2012.01657.x. [DOI] [PubMed] [Google Scholar]
- 9.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Raalte DH, Diamant M. Glucolipotoxicity and beta cells in type 2 diabetes mellitus: target for durable therapy? Diabetes Res Clin Pract. 2011;93(Suppl 1):S37–S46. doi: 10.1016/S0168-8227(11)70012-2. [DOI] [PubMed] [Google Scholar]
- 11.Igoillo-Esteve M, Marselli L, Cunha DA, Ladriere L, Ortis F, Grieco FA, et al. Palmitate induces a pro-inflammatory response in human pancreatic islets that mimics CCL2 expression by beta cells in type 2 diabetes. Diabetologia. 2010;53:1395–1405. doi: 10.1007/s00125-010-1707-y. [DOI] [PubMed] [Google Scholar]
- 12.Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, et al. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005;280:847–851. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- 13.Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007;50:2486–2494. doi: 10.1007/s00125-007-0816-8. [DOI] [PubMed] [Google Scholar]
- 14.Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, et al. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752–763. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- 15.Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, et al. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H, Cao MM, Wang Y, Li LC, Zhu LB, Xie GY, et al. Endoplasmic reticulum stress is involved in the connection between inflammation and autophagy in type 2 diabetes. Gen Comp Endocrinol. 2015;210:124–129. doi: 10.1016/j.ygcen.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Donath MY, Boni-Schnetzler M, Ellingsgaard H, Ehses JA. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda) 2009;24:325–331. doi: 10.1152/physiol.00032.2009. [DOI] [PubMed] [Google Scholar]
- 18.Finegood DT, McArthur MD, Kojwang D, Thomas MJ, Topp BG, Leonard T, et al. Beta-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes. 2001;50:1021–1029. doi: 10.2337/diabetes.50.5.1021. [DOI] [PubMed] [Google Scholar]
- 19.DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators. Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 20.Higa M, Zhou YT, Ravazzola M, Baetens D, Orci L, Unger RH. Troglitazone prevents mitochondrial alterations, beta cell destruction, and diabetes in obese prediabetic rats. Proc Natl Acad Sci U S A. 1999;96:11513–11518. doi: 10.1073/pnas.96.20.11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubois M, Pattou F, Kerr-Conte J, Gmyr V, Vandewalle B, Desreumaux P, et al. Expression of peroxisome proliferator-activated receptor gamma (PPARgamma) in normal human pancreatic islet cells. Diabetologia. 2000;43:1165–1169. doi: 10.1007/s001250051508. [DOI] [PubMed] [Google Scholar]
- 22.Lazar MA. PPAR gamma, 10 years later. Biochimie. 2005;87:9–13. doi: 10.1016/j.biochi.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Rosen ED, Kulkarni RN, Sarraf P, Ozcan U, Okada T, Hsu CH, et al. Targeted elimination of peroxisome proliferator-activated receptor gamma in beta cells leads to abnormalities in islet mass without compromising glucose homeostasis. Mol Cell Biol. 2003;23:7222–7229. doi: 10.1128/MCB.23.20.7222-7229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unger RH, Zhou YT, Orci L. Regulation of fatty acid homeostasis in cells: novel role of leptin. Proc Natl Acad Sci U S A. 1999;96:2327–2332. doi: 10.1073/pnas.96.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pahan K, Sheikh FG, Khan M, Namboodiri AM, Singh I. Sphingomyelinase and ceramide stimulate the expression of inducible nitric-oxide synthase in rat primary astrocytes. J Biol Chem. 1998;273:2591–2600. doi: 10.1074/jbc.273.5.2591. [DOI] [PubMed] [Google Scholar]
- 26.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernal-Mizrachi E, Wen W, Shornick M, Permutt MA. Activation of nuclear factor-kappaB by depolarization and Ca(2+) influx in MIN6 insulinoma cells. Diabetes. 2002;51(Suppl 3):S484–S488. doi: 10.2337/diabetes.51.2007.s484. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kono T, Ahn G, Moss DR, Gann L, Zarain-Herzberg A, Nishiki Y, et al. PPAR-γ activation restores pancreatic islet SERCA2 levels and prevents β-cell dysfunction under conditions of hyperglycemic and cytokine stress. Mol Endocrinol. 2012;26:257–271. doi: 10.1210/me.2011-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]