Abstract
Evidence has emerged that endocrine-disrupting chemicals (EDCs) can produce adverse effects, even at low doses that are assumed safe. However, systemic reviews and meta-analyses focusing on human studies, especially of EDCs with short half-lives, have demonstrated inconsistent results. Epidemiological studies have insuperable methodological limitations, including the unpredictable net effects of mixtures, non-monotonic dose-response relationships, the non-existence of unexposed groups, and the low reliability of exposure assessment. Thus, despite increases in EDC-linked diseases, traditional epidemiological studies based on individual measurements of EDCs in bio-specimens may fail to provide consistent results. The exposome has been suggested as a promising approach to address the uncertainties surrounding human studies, but it is never free from these methodological issues. Although exposure to EDCs during critical developmental periods is a major concern, continuous exposure to EDCs during non-critical periods is also harmful. Indeed, the evolutionary aspects of epigenetic programming triggered by EDCs during development should be considered because it is a key mechanism for developmental plasticity. Presently, living without EDCs is impossible due to their omnipresence. Importantly, there are lifestyles which can increase the excretion of EDCs or mitigate their harmful effects through the activation of mitohormesis or xenohormesis. Effectiveness of lifestyle interventions should be evaluated as practical ways against EDCs in the real world.
Keywords: Chemical mixtures, Endocrine-disrupting chemicals, Exposure assessment, Epidemiology, Evolution, Metabolism-disrupting chemicals, Non-monotonic dose response relationship, Predictive adaptive response hypothesis, Persistent organic pollutants, Reliability
INTRODUCTION
Endocrine-disrupting chemicals (EDCs) are defined as exogenous compounds that interfere with any aspect of endogenous hormones, including their production, release, transport, metabolism, binding, action, or elimination [1,2]. The chemicals classified as EDCs are highly heterogeneous. They include pesticides, fungicides, plastics, plasticizers, synthetic chemicals used as industrial solvents, heavy metals, and pharmaceutical agents [1,2]. Although most EDCs are man-made, some EDCs are naturally present in the human diet [1,2].
The list of EDCs is rapidly growing. According to the TEDX (The Endocrine Disruption Exchange) database, the number of suspected EDCs was 881 in 2011, increasing to 1,419 in 2017 [3]. As EDCs do not share any structural similarities, it is difficult to predict whether a compound will exert endocrine-disrupting actions [1]. Even nanoparticles are suspected to be EDCs [4].
Humans are continuously exposed to a variety of EDCs via food, air, water, and consumer products. The exposure to EDCs begins in utero as many EDCs are easily transferred across the placenta of pregnant women to the developing fetus. During infancy, breastmilk is the main source of exposure to EDCs.
During the last decade, the number of research articles on EDCs has increased dramatically; however, our knowledge of EDCs is still limited and fragmentary. It is noteworthy that a section in the 2012 World Health Organization/United Nations Environment Programme (WHO/UNEP) summary for decisionmakers was titled "The tip of the iceberg," despite including a very long list of references [5]. Indeed, debates are continuing about the evidence of the possible harm that EDCs may cause in humans.
This review article (1) provides a brief history of research into EDCs, (2) discusses debates and issues surrounding the evidence of the potential harm of EDCs to humans, (3) describes evolutionary aspects of epigenetic programming due to the exposure to EDCs during developmental periods, and (4) suggests practical ways to mitigate the possible harm caused by EDCs.
A BRIEF HISTORY OF RESEARCH INTO EDCs
The term "endocrine disrupter" was first used at the Wingspread meeting in 1991 [6]. However, the hormone-disrupting effects of certain chemicals have been reported since the mid-20th century. Initially, research into EDCs focused on their estrogenic property and their role in decreased fertility, decreased semen quality, and increased birth anomalies [7]. Soon, concerns expanded to more hormone-disrupters, including those that affect androgens or thyroid hormones [8], and other health issues, such as hormone-related cancers [9] and problems with neurodevelopment [10].
Over recent decades, researchers have begun to recognize that environmental chemicals can disturb many other hormonal pathways, as well as sex and thyroid hormones. In particular, evidence is accumulating that environmental chemicals are linked to metabolic diseases, including obesity and diabetes, via diverse mechanisms [11]. As such, the new term "metabolismdisrupting chemicals" (MDCs) was coined [11]; a chemical can be an EDC or MDC, depending on the context. Although in this review, EDCs will be used as a general term encompassing these compounds, it is worthwhile to note that the terms EDC and MDC are not sufficient to cover the extensive disturbing features of these environmental chemicals in living organisms.
Most EDCs identified to date interact with the nuclear receptors of several hormones as agonists or antagonists. However, research has shown that their mechanisms of action are much broader than originally recognized. As well as through nuclear receptors, EDCs act via non-nuclear steroid hormone receptors, non-steroid receptors, orphan receptors, and enzymatic pathways involved in steroid biosynthesis and/or metabolism [12].
Recently, organizations such as the Endocrine Society [1,2] and the WHO/UNEP [5] have issued official reports describing the possible health threats posed by EDCs. They focused on exposure during critical periods of development and concluded that exposure to EDCs is related to a multitude of diseases, including impaired reproduction, neurodevelopment, thyroid function, and metabolism, as well as increases in hormone-sensitive cancers. In particular, EDCs show their effects at low doses within the range of human environmental exposure that are assumed to be safe under the current regulations on chemicals. Thus, these reports called for urgent action to regulate EDCs.
DEBATES ABOUT THE EVIDENCE OF HARM TO HUMANS
After the release of the WHO/UNEP report in 2012, however, debates have continued between researchers who support the conclusions of the report [13,14,15,16,17] and those who oppose it [18,19,20,21]. Although the opponents are often accused of being associated with or funded by chemical industries [15,16], some of their arguments deserve sober discussion from the scientific community. One criticism is the lack of evidence of the harm of EDCs to humans, despite the persuasive evidence from in vitro and in vivo studies.
In fact, in contrast to the conclusion of official reports on EDCs [1,2,5], many recent systemic reviews or meta-analyses focusing on epidemiological studies, especially of short-lived EDCs, seem to support the opponents' position. For example, reviews of the effects of bisphenol A (BPA) in humans reported conflicting results with respect to pubertal development [22] and diabetes [23]. Similar conclusions have been reached on phthalate and obesity [24], triclosan and diverse health effects [25], and developmental exposure to many EDCs and male reproductive disorders [26].
The economic costs from EDC exposure-related disease burden in the EU and USA have recently been estimated to be huge [27,28]. However, the articles describing cost estimations were criticized as pseudoscience by opponents [29,30] because these estimations were reasonable only with the assumption of causality in humans.
WHY IS EVIDENCE OF HARM IN HUMANS STILL AN UNSOLVABLE PUZZLE?
Although consistent findings from epidemiological studies is regarded as one of the critical elements needed to support the establishment of a causal link between an exposure and disease, studies of EDCs in humans may be the exception. In fact, inconsistency may be inevitable due to insuperable methodological issues. Herein, three key issues will be briefly discussed. This topic was thoughtfully discussed in another article in which other issues, such as complicated interactions with established risk factors such as diet and obesity and the difficulty of exposure assessment during critical periods of the lifespan, were also addressed [31].
Issue 1: can we predict the net effect of EDC mixtures in the real world?
Mixtures of EDCs are the most complicated of these three issues. Until now, laboratory studies of EDC mixtures were mainly performed using several similarly-acting compounds, such as mixtures of estrogenic compounds, antiandrogens, and thyroiddisrupting chemicals, and mostly reported additive or synergic interactions [32]. However, studies of mixtures of several EDCs with different endocrine-disrupting properties demonstrated modulating or antagonistic interactions that could not be predicted in terms of additivity or synergistic concepts [33,34,35,36]. In fact, all EDC mixtures used in laboratory studies are very simple, unrealistic mixtures compared to real-world EDC mixtures. As such, the net effect of the mixture of several hundreds of EDCs, all with diverse activities, is unpredictable at the individual level in humans.
Issue 2: can non-monotonic dose-response relationships be validly evaluated in human studies?
The biological responses to EDCs are often non-monotonic dose responses (NMDRs) [37]. NMDRs can arise from numerous mechanisms, such as opposing effects induced by multiple receptors differing in their affinity, receptor desensitization, negative feedback with increasing doses, or dose-dependent metabolism modulation [38]. Hormesis, the overcompensation of various adaptive responses through cellular stresses, is also a mechanism of NMDRs [39].
NMDRs have been also observed in human studies of many environmental chemicals; the risk of diseases does not increase with an increasing dose, but often tends to plateau or even decrease with an increasing dose [40,41,42,43,44,45]. Importantly, the identification of NMDRs in a particular human dataset is difficult when non-exposure groups do not exist (as is the case for many common EDCs) and the exposure range is limited [31]. Depending on the exposure range of the population, positive, null, and even inverse associations are possible [31].
Issue 3: can exposure to EDCs with short half-lives be reliably assessed in humans?
The quality of exposure assessment is a key determinant of the overall quality of environmental epidemiological studies [46]. Serial measurements of many ubiquitous EDCs with short halflives in spot urines have demonstrated large within-subject variability [47,48,49]. Even 24-hour urine samples did not help in the estimation of typical exposure levels due to large day-to-day variability [47,48,49]. Thus, the collection of repeated biospecimens is recommended to estimate typical exposure levels for these EDCs. However, this recommendation is often unrealistic. For example, in a simulation study, the pooling of 35 repeated urine samples from a single individual was estimated as being necessary to reduce the attenuation bias of BPA to 10% [50].
CAN THE EXPOSOME BE A SOLUTION?
The exposome has emerged as a methodology to evaluate the health effects of EDCs in humans [51,52]. Although the concept of the exposome sounds fascinating (for example, the totality of lifetime environmental exposure or the development of omicsbased chemical fingerprints), the study of the exposome is inevitably affected by the fundamental methodological limitations discussed above.
Besides genomics, all omics are dynamic. Epigenomics, proteomics, and metabolomics are continuously affected by every aspect of the continuously changing external and internal environment [53]. Although epigenetic programming during the developmental period is stable, its evolutionary aspects need to be considered, as discussed below. Additionally, although sophisticated statistical methods have been suggested for analyzing huge datasets derived from the exposome [54,55], they ironically presume unrealistic simple conditions, such as linearity.
WHAT CAN BE EVIDENCE OF THE HARM OF EDCs IN HUMANS?
Although we can observe an increasing trend of EDC-linked diseases at the population level, traditional epidemiological studies based on individual measurements of EDCs, especially many short-lived EDCs currently in wide use, may fail to provide consistent results due to methodological limitations. Additionally, pinpointing specific EDCs as the culprit for certain conditions is neither possible nor useful because diseases in the endocrine system may be the result of a mixture of a tremendous number of EDCs, including those we know as well as those we do not currently know.
Meanwhile, chronic exposure to a low dose of persistent organic pollutants (POPs), a mixture of strongly lipophilic chemicals, has been recently associated with obesity-related diseases such as type 2 diabetes in human studies [56,57]. Even though the study of POPs also suffers from some inconsistencies, these findings have at least been more consistent than those of studies of common EDCs with short half-lives [57]. Importantly, human studies on POPs, especially chlorinated POPs, are less affected by methodological issues due to their long half-lives, the presence of a reference group that is closer to a non-exposure group, and the role of several POPs as surrogate markers of lipophilic chemical mixtures, including measured and unmeasured ones. Even though POPs are well-known EDCs, the findings on POPs in humans cannot be attributed to the hormonedisturbing properties of POPs because POPs are a mixture of diverse EDCs. Other mechanisms, such as mitochondrial dysfunction or chronic glutathione depletion, have been suggested as possible mechanisms linking chlorinated POPs to health outcomes [56,58].
PRACTICAL RECOMMENDATIONS FOR CLINICIANS AND THE PUBLIC: WHAT CAN WE DO ABOUT EDCs RIGHT NOW?
If environmental chemicals are really harmful to humans, what is the next step?
Exposure during critical periods and irreversible epigenetic programming
Early-life exposure to EDCs, especially during the fetal and infant stages, is the primary concern in the field of EDCs because exposure during these periods has permanent effects on the exposed individuals and their descendants through epigenetic programming, even in the absence of further exposure. Unfortunately, however, it is almost impossible to provide sensible advice to pregnant women on how to effectively avoid EDCs in order to protect their children [59].
Although the prenatal and early life periods are the most susceptible to toxicity of environmental chemicals, importantly, epigenetic programming during critical periods triggered by various environmental stressors is a key mechanism for developmental plasticity to buffer individuals from environmental changes [60]. After birth, if offspring have to live in conditions similar to the in utero conditions, their overall survival may be enhanced by virtue of the epigenetic programming.
For example, the findings from a cohort of babies from the Dutch famine during World War II, in which malnutrition during pregnancy led to the increased risk of many chronic diseases, are now referred to the exemplary case supporting the developmental origin of health and disease (DOHaD) [61]. However, studies of the famine in Leningrad failed to replicate the findings from the Netherlands [62]. The difference between these two cohorts was the nutritional status of individuals after birth. In the Netherlands, many starved babies were promptly wellnourished postnatally, but the Leningrad cohort experienced similar poor nutritional status even after birth [62]. The degree of mismatch between the prenatal and postnatal environment, rather than prenatal exposure itself, may be a major determinant of DOHaD; this is known as the predictive adaptive response (PAR) hypothesis [63]. It is not only confined to nutrition. The PAR hypothesis warrants investigation in the field of EDCs as well.
Although the obesity-inducing effects of EDCs are a major research topic, this issue also should consider an evolutionary perspective. Although obesity is an important risk factor of many chronic diseases, the expansion of fat mass is not itself pathological, but primarily conveys a protective and adaptive role with regard to maintaining insulin sensitivity [64]. When hypertrophic adipocytes face limits of expansion, dysfunctional adipocytes and/or insulin resistance can develop [65]. Therefore, the expansion of adipose tissue by EDCs itself cannot be considered as harmful, but provide a protective role by securing a relatively safe storage organ for strongly lipophilic EDCs with long half-lives [66].
Exposure during non-critical periods and reversible effects
Exposure to EDCs in adulthood can also alter the physiology of the endocrine system [67], although this topic has not received as much attention as exposure during development. In particular, when exposure is continuous (as we now experience), the risk of EDC-related diseases increases. Importantly, although early-life exposure to EDCs produces irreversible effects, the effects of exposure during a non-critical period can be reversible [1,2].
Clinicians may consider the direct measurement of established or suspected EDCs in biospecimens of patients to provide advice on which EDCs should be avoided or how to avoid EDCs. However, there are insurmountable challenges in ascertaining which specific EDCs contribute to a particular disorder in individual patients, similar to the limitations of epidemiological studies. Instead, it may be wise to simply assume that EDCmixtures play a role in the development of disease in the patient, unless they have other obvious causes for their conditions.
The most important recommendation may be to focus on EDC mixtures, instead of several specific EDCs individually. Although largely unknown, the most important source of EDC mixtures is not outside the patient, but inside the patient. Contemporary human adipose tissue contains the most complex EDC mixtures because humans are at the top of the food chain [66]. It is particularly necessary to re-evaluate obesity-related diseases from the viewpoint of lipophilic chemical mixtures in adipose tissue because they are slowly and continuously released into circulation in an uncontrolled way in obese persons with dysfunctional adipocytes [66]. Therefore, determining how to efficiently eliminate EDC mixtures from circulation may be central to decreasing the burden of the EDC mixtures delivered to critical organs.
Interestingly, healthy behaviors can increase the excretion of chemical mixtures from the body [66] and mitigate the harmful effects of chemical mixtures at the cellular level through the activation of mitohormesis or xenohormesis [68]. Briefly, examples include exercise, a clear feeding-fasting cycle, and the inclusion of fiber and phytochemicals in the diet. Detailed mechanisms can be found elsewhere [66,68]. Additionally, similar to human adipose tissue, fat in animal food such as meat, milk, and fish is also widely contaminated with many EDCs. Thus, if people want to avoid further exposure to exogenous EDCs, avoiding animal fat would be a better choice than avoiding the various individual EDCs that are contained in consumer products.
CONCLUSIONS
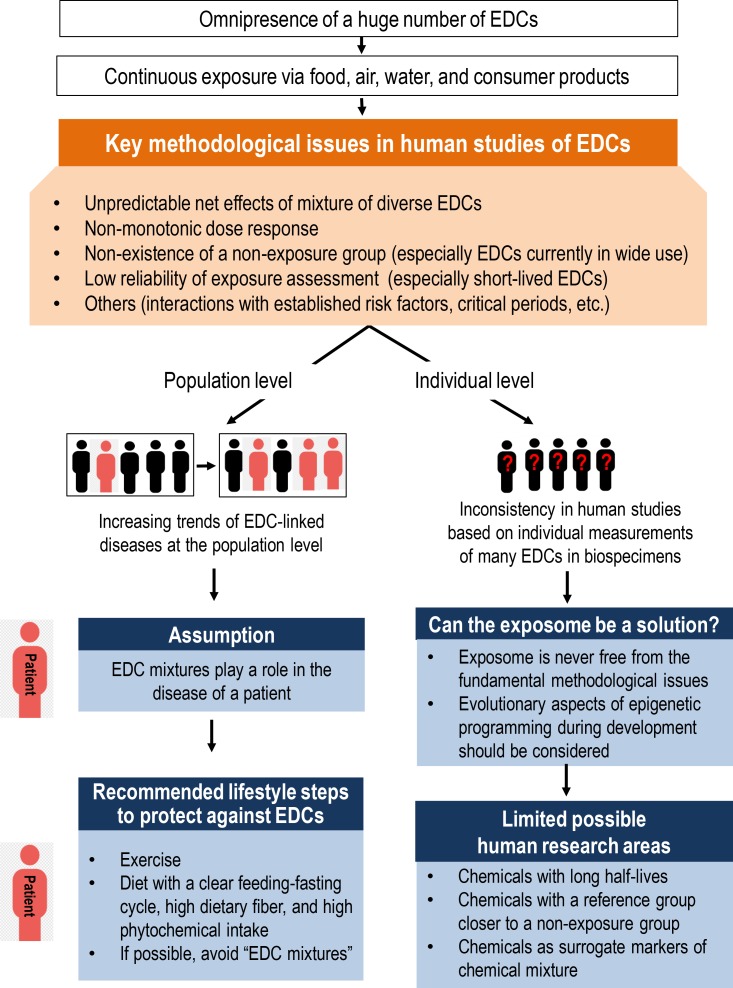
Fig. 1 depicts the contents of this article. Living without exposure to EDCs is impossible. Although certain lifestyle choices, such as living without plastic, can decrease exposure to several EDCs [69], these sources account for only a tiny proportion of possible EDC exposure. Although enhanced regulation via the adoption of precautionary principles is called for, regulations also have limited value, as many EDCs have already contaminated our bodies and our environment. Therefore, alternative ways to reduce the possible harm of EDCs should be explored. Importantly, some lifestyle can guard against EDCs. Examples include exercise, a clear feeding-fasting cycle, a high intake of dietary fiber, and a high intake of phytochemicals. Although this lifestyle is regarded to be healthy even outside of the context of EDCs, adopting these behaviors is especially necessary in light of the omnipresence of EDCs and their deleterious effects.
Fig. 1. Issues in human studies of endocrine-disrupting chemicals (EDCs) and practical recommendations for clinicians and the public. Despite the increasing trend of EDC-linked diseases, it is difficult to find consistent human evidence on the harms of EDCs, especially the short-lived EDCs currently in wide use, due to critical methodological issues. In the case of human studies, possible research areas are limited. Both early-life exposure during critical periods and continuous exposure during non-critical periods can be harmful to humans. However, epigenetic programming induced during critical periods is a key to developmental plasticity and evolutionary aspects should be considered. Harms due to continuous exposure during the non-critical period may be mitigated by adopting lifestyle measures that counteract the harmful effects of EDCs. Effectiveness of lifestyle interventions should be evaluated as practical ways against EDCs in the real world.
ACKNOWLEDGMENTS
This study was supported by the Environmental Health Action Program (2016001370002), funded by the Korea Ministry of Environment of the Republic of Korea.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2: the Endocrine Society's second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36:E1–E150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Endocrine Disruption Exchange. TEDX list of potential endocrine disruptors [Internet] Eckert: TEDX; 2017. [cited 2018 Feb 21]. Available from: https://endocrinedisruption.org/ [Google Scholar]
- 4.Iavicoli I, Fontana L, Leso V, Bergamaschi A. The effects of nanomaterials as endocrine disruptors. Int J Mol Sci. 2013;14:16732–16801. doi: 10.3390/ijms140816732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. The state-of-the-science of endocrine disrupting chemicals 2012 [Internet] Geneva: WHO; c2018. [cited 2018 Feb 21]. Available from: http://www.who.int/ceh/publications/endocrine/en/index.html. [Google Scholar]
- 6.Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fry DM. Reproductive effects in birds exposed to pesticides and industrial chemicals. Environ Health Perspect. 1995;103(Suppl 7):165–171. doi: 10.1289/ehp.95103s7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zoeller TR. Environmental chemicals targeting thyroid. Hormones (Athens) 2010;9:28–40. doi: 10.14310/horm.2002.1250. [DOI] [PubMed] [Google Scholar]
- 9.Scsukova S, Rollerova E, Bujnakova Mlynarcikova A. Impact of endocrine disrupting chemicals on onset and development of female reproductive disorders and hormone-related cancer. Reprod Biol. 2016;16:243–254. doi: 10.1016/j.repbio.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Parent AS, Naveau E, Gerard A, Bourguignon JP, Westbrook GL. Early developmental actions of endocrine disruptors on the hypothalamus, hippocampus, and cerebral cortex. J Toxicol Environ Health B Crit Rev. 2011;14:328–345. doi: 10.1080/10937404.2011.578556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017;68:3–33. doi: 10.1016/j.reprotox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kretschmer XC, Baldwin WS. CAR and PXR: xenosensors of endocrine disrupters? Chem Biol Interact. 2005;155:111–128. doi: 10.1016/j.cbi.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Kortenkamp A, Martin O, Evans R, Orton F, McKinlay R, Rosivatz E, et al. Response to a critique of the European Commission Document, “State of the Art Assessment of Endocrine Disrupters” by Rhomberg and colleagues: letter to the editor. Crit Rev Toxicol. 2012;42:787–789. doi: 10.3109/10408444.2012.712943. [DOI] [PubMed] [Google Scholar]
- 14.Gore AC, Balthazart J, Bikle D, Carpenter DO, Crews D, Czernichow P, et al. Policy decisions on endocrine disruptors should be based on science across disciplines: a response to Dietrich et al. Eur J Endocrinol. 2013;169:E1–E4. doi: 10.1530/EJE-13-0763. [DOI] [PubMed] [Google Scholar]
- 15.Bergman A, Andersson AM, Becher G, van den Berg M, Blumberg B, Bjerregaard P, et al. Science and policy on endocrine disrupters must not be mixed: a reply to a “common sense” intervention by toxicology journal editors. Environ Health. 2013;12:69. doi: 10.1186/1476-069X-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zoeller RT, Bergman A, Becher G, Bjerregaard P, Bornman R, Brandt I, et al. A path forward in the debate over health impacts of endocrine disrupting chemicals. Environ Health. 2014;13:118. doi: 10.1186/1476-069X-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergman A, Becher G, Blumberg B, Bjerregaard P, Bornman R, Brandt I, et al. Manufacturing doubt about endocrine disrupter science: a rebuttal of industry-sponsored critical comments on the UNEP/WHO report “State of the Science of Endocrine Disrupting Chemicals 2012”. Regul Toxicol Pharmacol. 2015;73:1007–1017. doi: 10.1016/j.yrtph.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich DR, von Aulock S, Marquardt H, Blaauboer B, Dekant W, Kehrer J, et al. Scientifically unfounded precaution drives European Commission's recommendations on EDC regulation, while defying common sense, well-established science and risk assessment principles. Toxicol In Vitro. 2013;27:2110–2114. doi: 10.1016/j.tiv.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Rhomberg LR, Goodman JE, Foster WG, Borgert CJ, Van Der Kraak G. A critique of the European Commission document, “State of the Art Assessment of Endocrine Disrupters”. Crit Rev Toxicol. 2012;42:465–473. doi: 10.3109/10408444.2012.690367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamb JC, 4th, Boffetta P, Foster WG, Goodman JE, Hentz KL, Rhomberg LR, et al. Comments on the opinions published by Bergman et al. (2015) on Critical Comments on the WHO-UNEP State of the Science of Endocrine Disrupting Chemicals (Lamb et al., 2014) Regul Toxicol Pharmacol. 2015;73:754–757. doi: 10.1016/j.yrtph.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 21.Lamb JC, 4th, Boffetta P, Foster WG, Goodman JE, Hentz KL, Rhomberg LR, et al. Critical comments on the WHOUNEP State of the Science of Endocrine Disrupting Chemicals: 2012. Regul Toxicol Pharmacol. 2014;69:22–40. doi: 10.1016/j.yrtph.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Leonardi A, Cofini M, Rigante D, Lucchetti L, Cipolla C, Penta L, et al. The effect of bisphenol a on puberty: a critical review of the medical literature. Int J Environ Res Public Health. 2017;14:E1044. doi: 10.3390/ijerph14091044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lakind JS, Goodman M, Mattison DR. Bisphenol A and indicators of obesity, glucose metabolism/type 2 diabetes and cardiovascular disease: a systematic review of epidemiologic research. Crit Rev Toxicol. 2014;44:121–150. doi: 10.3109/10408444.2013.860075. [DOI] [PubMed] [Google Scholar]
- 24.Goodman M, Lakind JS, Mattison DR. Do phthalates act as obesogens in humans? A systematic review of the epidemiological literature. Crit Rev Toxicol. 2014;44:151–175. doi: 10.3109/10408444.2013.860076. [DOI] [PubMed] [Google Scholar]
- 25.Goodman M, Naiman DQ, LaKind JS. Systematic review of the literature on triclosan and health outcomes in humans. Crit Rev Toxicol. 2018;48:1–51. doi: 10.1080/10408444.2017.1350138. [DOI] [PubMed] [Google Scholar]
- 26.Bonde JP, Flachs EM, Rimborg S, Glazer CH, Giwercman A, Ramlau-Hansen CH, et al. The epidemiologic evidence linking prenatal and postnatal exposure to endocrine disrupting chemicals with male reproductive disorders: a systematic review and meta-analysis. Hum Reprod Update. 2016;23:104–125. doi: 10.1093/humupd/dmw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trasande L, Zoeller RT, Hass U, Kortenkamp A, Grandjean P, Myers JP, et al. Burden of disease and costs of exposure to endocrine disrupting chemicals in the European Union: an updated analysis. Andrology. 2016;4:565–572. doi: 10.1111/andr.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Attina TM, Hauser R, Sathyanarayana S, Hunt PA, Bourguignon JP, Myers JP, et al. Exposure to endocrine-disrupting chemicals in the USA: a population-based disease burden and cost analysis. Lancet Diabetes Endocrinol. 2016;4:996–1003. doi: 10.1016/S2213-8587(16)30275-3. [DOI] [PubMed] [Google Scholar]
- 29.Dietrich DR. EU safety regulations: don’t mar legislation with pseudoscience. Nature. 2016;535:355. doi: 10.1038/535355c. [DOI] [PubMed] [Google Scholar]
- 30.Bond GG, Dietrich DR. Human cost burden of exposure to endocrine disrupting chemicals. A critical review. Arch Toxicol. 2017;91:2745–2762. doi: 10.1007/s00204-017-1985-y. [DOI] [PubMed] [Google Scholar]
- 31.Lee DH, Jacobs DR., Jr Methodological issues in human studies of endocrine disrupting chemicals. Rev Endocr Metab Disord. 2015;16:289–297. doi: 10.1007/s11154-016-9340-9. [DOI] [PubMed] [Google Scholar]
- 32.Kortenkamp A. Ten years of mixing cocktails: a review of combination effects of endocrine-disrupting chemicals. Environ Health Perspect. 2007;115(Suppl 1):98–105. doi: 10.1289/ehp.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carnevali O, Notarstefano V, Olivotto I, Graziano M, Gallo P, Di Marco Pisciottano I, et al. Dietary administration of EDC mixtures: a focus on fish lipid metabolism. Aquat Toxicol. 2017;185:95–104. doi: 10.1016/j.aquatox.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Sarria MP, Santos MM, Reis-Henriques MA, Vieira NM, Monteiro NM. The unpredictable effects of mixtures of androgenic and estrogenic chemicals on fish early life. Environ Int. 2011;37:418–424. doi: 10.1016/j.envint.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Ribeiro E, Ladeira C, Viegas S. EDCs mixtures: a stealthy hazard for human health? Toxics. 2017;5:E5. doi: 10.3390/toxics5010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu Y, Wang R, Xiang Z, Qian W, Han X, Li D. Antagonistic effects of a mixture of low-dose nonylphenol and di-n-butyl phthalate (monobutyl phthalate) on the Sertoli cells and serum reproductive hormones in prepubertal male rats in vitro and in vivo. PLoS One. 2014;9:e93425. doi: 10.1371/journal.pone.0093425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lagarde F, Beausoleil C, Belcher SM, Belzunces LP, Emond C, Guerbet M, et al. Non-monotonic dose-response relationships and endocrine disruptors: a qualitative method of assessment. Environ Health. 2015;14:13. doi: 10.1186/1476-069X-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calabrese EJ, Mattson MP. How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech Dis. 2017;3:13. doi: 10.1038/s41514-017-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pastor-Barriuso R, Fernandez MF, Castano-Vinyals G, Whelan D, Perez-Gomez B, Llorca J, et al. Total effective xenoestrogen burden in serum samples and risk for breast cancer in a population-based multicase-control study in Spain. Environ Health Perspect. 2016;124:1575–1582. doi: 10.1289/EHP157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee DH, Steffes MW, Sjodin A, Jones RS, Needham LL, Jacobs DR., Jr Low dose of some persistent organic pollutants predicts type 2 diabetes: a nested case-control study. Environ Health Perspect. 2010;118:1235–1242. doi: 10.1289/ehp.0901480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee DH, Steffes MW, Sjodin A, Jones RS, Needham LL, Jacobs DR., Jr Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS One. 2011;6:e15977. doi: 10.1371/journal.pone.0015977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vlaanderen J, Portengen L, Rothman N, Lan Q, Kromhout H, Vermeulen R. Flexible meta-regression to assess the shape of the benzene-leukemia exposure-response curve. Environ Health Perspect. 2010;118:526–532. doi: 10.1289/ehp.0901127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinault L, Tjepkema M, Crouse DL, Weichenthal S, van Donkelaar A, Martin RV, et al. Risk estimates of mortality attributed to low concentrations of ambient fine particulate matter in the Canadian community health survey cohort. Environ Health. 2016;15:18. doi: 10.1186/s12940-016-0111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LaKind JS, Sobus JR, Goodman M, Barr DB, Furst P, Albertini RJ, et al. A proposal for assessing study quality: Biomonitoring, Environmental Epidemiology, and Short-lived Chemicals (BEES-C) instrument. Environ Int. 2014;73:195–207. doi: 10.1016/j.envint.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z, Romanoff LC, Lewin MD, Porter EN, Trinidad DA, Needham LL, et al. Variability of urinary concentrations of polycyclic aromatic hydrocarbon metabolite in general population and comparison of spot, first-morning, and 24-h void sampling. J Expo Sci Environ Epidemiol. 2010;20:526–535. doi: 10.1038/jes.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Preau JL, Jr, Wong LY, Silva MJ, Needham LL, Calafat AM. Variability over 1 week in the urinary concentrations of metabolites of diethyl phthalate and di(2-ethylhexyl) phthalate among eight adults: an observational study. Environ Health Perspect. 2010;118:1748–1754. doi: 10.1289/ehp.1002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye X, Wong LY, Bishop AM, Calafat AM. Variability of urinary concentrations of bisphenol A in spot samples, first morning voids, and 24-hour collections. Environ Health Perspect. 2011;119:983–988. doi: 10.1289/ehp.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perrier F, Giorgis-Allemand L, Slama R, Philippat C. Within-subject pooling of biological samples to reduce exposure misclassification in biomarker-based studies. Epidemiology. 2016;27:378–388. doi: 10.1097/EDE.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Messerlian C, Martinez RM, Hauser R, Baccarelli AA. ‘Omics’ and endocrine-disrupting chemicals: new paths forward. Nat Rev Endocrinol. 2017;13:740–748. doi: 10.1038/nrendo.2017.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vrijheid M, Slama R, Robinson O, Chatzi L, Coen M, van den, et al. The human early-life exposome (HELIX): project rationale and design. Environ Health Perspect. 2014;122:535–544. doi: 10.1289/ehp.1307204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet. 2012;13:97–109. doi: 10.1038/nrg3142. [DOI] [PubMed] [Google Scholar]
- 54.Taylor KW, Joubert BR, Braun JM, Dilworth C, Gennings C, Hauser R, et al. Statistical approaches for assessing health effects of environmental chemical mixtures in epidemiology: lessons from an innovative workshop. Environ Health Perspect. 2016;124:A227–A229. doi: 10.1289/EHP547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agier L, Portengen L, Chadeau-Hyam M, Basagana X, Giorgis-Allemand L, Siroux V, et al. A systematic comparison of linear regression-based statistical methods to assess exposome-health associations. Environ Health Perspect. 2016;124:1848–1856. doi: 10.1289/EHP172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee DH, Porta M, Jacobs DR, Jr, Vandenberg LN. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr Rev. 2014;35:557–601. doi: 10.1210/er.2013-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor KW, Novak RF, Anderson HA, Birnbaum LS, Blystone C, Devito M, et al. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a national toxicology program workshop review. Environ Health Perspect. 2013;121:774–783. doi: 10.1289/ehp.1205502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lim S, Cho YM, Park KS, Lee HK. Persistent organic pollutants, mitochondrial dysfunction, and metabolic syndrome. Ann N Y Acad Sci. 2010;1201:166–176. doi: 10.1111/j.1749-6632.2010.05622.x. [DOI] [PubMed] [Google Scholar]
- 59.Kortenkamp A. Endocrine disruptors: the burden of endocrine-disrupting chemicals in the USA. Nat Rev Endocrinol. 2016;13:6–7. doi: 10.1038/nrendo.2016.198. [DOI] [PubMed] [Google Scholar]
- 60.Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev. 2014;94:1027–1076. doi: 10.1152/physrev.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ravelli AC, van der, Michels RP, Osmond C, Barker DJ, Hales CN, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 62.Stanner SA, Yudkin JS. Fetal programming and the Leningrad Siege study. Twin Res. 2001;4:287–292. doi: 10.1375/1369052012498. [DOI] [PubMed] [Google Scholar]
- 63.Bateson P, Gluckman P, Hanson M. The biology of developmental plasticity and the predictive adaptive response hypothesis. J Physiol. 2014;592:2357–2368. doi: 10.1113/jphysiol.2014.271460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med. 2012;18:1539–1549. doi: 10.1038/nm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee YM, Kim KS, Jacobs DR, Jr, Lee DH. Persistent organic pollutants in adipose tissue should be considered in obesity research. Obes Rev. 2017;18:129–139. doi: 10.1111/obr.12481. [DOI] [PubMed] [Google Scholar]
- 67.Frye CA, Bo E, Calamandrei G, Calza L, Dessi-Fulgheri F, Fernandez M, et al. Endocrine disrupters: a review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J Neuroendocrinol. 2012;24:144–159. doi: 10.1111/j.1365-2826.2011.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim SA, Lee YM, Choi JY, Jacobs DR, Jr, Lee DH. Evolutionarily adapted hormesis-inducing stressors can be a practical solution to mitigate harmful effects of chronic exposure to low dose chemical mixtures. Environ Pollut. 2018;233:725–734. doi: 10.1016/j.envpol.2017.10.124. [DOI] [PubMed] [Google Scholar]
- 69.Hutter HP, Kundi M, Hohenblum P, Scharf S, Shelton JF, Piegler K, et al. Life without plastic: a family experiment and biomonitoring study. Environ Res. 2016;150:639–644. doi: 10.1016/j.envres.2016.05.028. [DOI] [PubMed] [Google Scholar]