Abstract
The pathophysiology of type 2 diabetes is characterized by variable degrees of insulin resistance and impaired insulin secretion. Both genetic and environmental factors serve as etiologic factors. Recent genetic studies have identified at least 83 variants associated with diabetes. A significant number of these loci are thought to be involved in insulin secretion, either through β-cell development or β-cell dysfunction. Environmental factors have changed rapidly during the past half century, and the increased prevalence of obesity and diabetes can be attributed to these changes. Environmental factors may affect epigenetic changes and alter susceptibility to diabetes. A recent epidemiologic study revealed that Korean patients with type 2 diabetes already had impaired insulin secretion and insulin resistance 10 years before the onset of diabetes. Those who developed diabetes showed impaired β-cell compensation with an abrupt decrease in insulin secretion during the last 2 years before diabetes developed. The retrograde trajectory of the disposition index differed according to the baseline subgroups of insulin secretion and insulin sensitivity. We hope that obtaining a more detailed understanding of the perturbations in the major pathophysiologic process of diabetes on the individual level will eventually lead to the implementation of precision medicine and improved patient outcomes.
Keywords: Diabetes mellitus, type 2; Environment; Genetics; Insulin-secreting cells; Insulin resistance; Physiopathology
INTRODUCTION
The pathophysiology of type 2 diabetes is characterized by variable degrees of insulin resistance and impaired insulin secretion. Insulin resistance is a state in which the target tissues, such as skeletal muscle, adipose tissue, and the liver, fail to respond adequately to insulin. This results in decreased glucose utilization in muscle and fat and increased gluconeogenesis in the liver [1]. It has been reported that the β-cell mass was 40% lower in prediabetes subjects compared to body mass index (BMI)-matched controls [2]. Multiple genetic and environmental factors, and the complex interplay thereof, contribute to both insulin resistance and impaired insulin secretion. Understanding the relative contribution of insulin resistance and impaired insulin secretion to the pathogenesis of type 2 diabetes is important for deciphering the clinical presentation of each patient, establishing preventive measures, and determining the optimal treatment approach.
Ethnic and individual variations may be present in the pathophysiology of diabetes. It has been observed that East Asian type 2 diabetes patients have a lower BMI than European patients [3]. In addition, it has been claimed that Korean type 2 diabetes patients are characterized by a decreased insulin secretory capacity [3,4]. Recent advances in epidemiologic and genetic studies have improved our knowledge of the relative contributions of insulin resistance and insulin secretion in Korean diabetes patients. In this article, we briefly review the genetic and environmental factors that contribute to insulin resistance and insulin secretion and compare the roles of insulin resistance and insulin secretion in the development of type 2 diabetes in Koreans.
ETIOLOGIC RISK FACTORS
Genetic risk factors
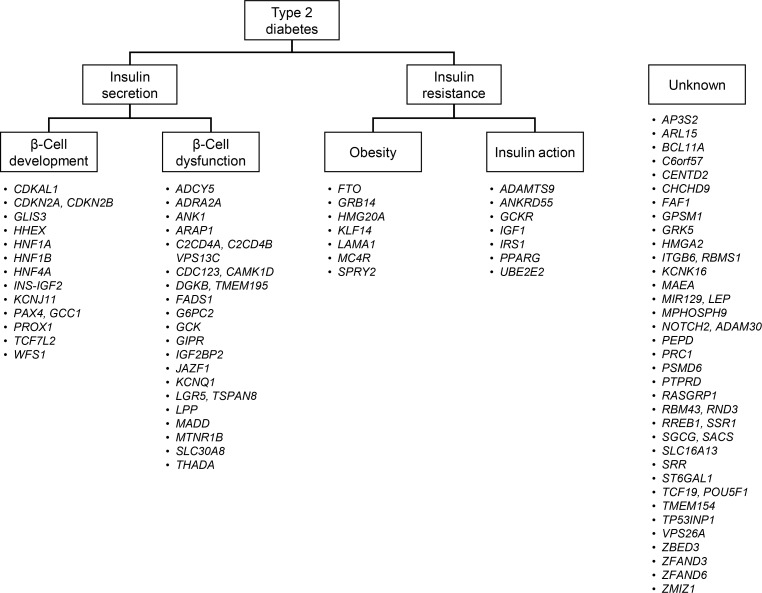
Type 2 diabetes is characterized by a strong genetic predisposition. Having a parent with diabetes increases the risk of developing diabetes by 30% to 40% [5]. Continuing efforts have been made to identify genetic risk factors for type 2 diabetes. However, before the advent of genome-wide association studies (GWASs), only a few genetic variants were strongly associated with diabetes, including variants in CAPN10 (calpain 10), PPARG (peroxisome proliferator activated receptor gamma), KCNJ11 (potassium voltage-gated channel subfamily J member 11), ABCC8 (ATP binding cassette subfamily C member 8), and TCF7L2 (transcription factor 7 like 2) [6]. The initial GWAS and their meta-analyses found variants in CDKAL1 (CDK5 regulatory subunit associated protein 1 like 1), CDKN2A/2B (cyclin dependent kinase inhibitor 2A/2B), SLC30A8 (solute carrier family 30 member 8), HHEX (hematopoietically expressed homeobox), and IGF2BP2 (insulin like growth factor 2 mRNA binding protein 2) to be robustly associated with diabetes [7,8,9,10]. Currently, at least 83 genetic variants have been confirmed to be associated with type 2 diabetes [11]. These findings provided novel insights into the pathogenesis of diabetes. First, many genes that were newly identified had not been previously implicated in the pathophysiology of diabetes. There are ongoing efforts to investigate in detail the role of these genes in the pathophysiology of type 2 diabetes. Second, a significant proportion of these variants are located in genes implicated in insulin secretion [12]. Among the 83 loci, 34 were suggested to be involved in impaired insulin secretion, either through impaired β-cell development or through β-cell dysfunction (Fig. 1). On the contrary, only 14 variants were suggested to be associated with type 2 diabetes, primarily by affecting insulin resistance, via either increased obesity or defects in insulin action. These findings suggest that genetic risk factors mainly contribute to the pathophysiology of type 2 diabetes by impairing insulin secretion. Third, most of the variants had a low effect size (odds ratio <1.4). The cumulative effect of these variants explained less than 20% of type 2 diabetes heritability [13]. Other possibilities for the missing heritability include the existence of an even larger number of low-effect-size common variants, structural variants such as large insertions and deletions, ethnicity-specific variants, and rare high-effect-size functional variants.
Fig. 1. Classification of 83 genetic loci according to their suggested roles in the pathogenesis of type 2 diabetes. The loci were classified as being involved in insulin secretion if a variant was associated with measures of insulin secretion, such as the homeostasis model assessment of β-cell function, insulinogenic index, or the disposition index. Loci were further classified as being involved in β-cell development if the implicated gene played a role as a transcription factor in β-cell development. Genes that played a role in the insulin secretion cascade or β-cell apoptosis were classified as being involved in β-cell dysfunction. Loci were classified as being involved in insulin resistance if a variant was associated with measures of insulin resistance, such as the homeostasis model assessment of insulin resistance or the Matsuda index. Genes known to be associated with the body mass index were classified as being involved in obesity, and genes known to be involved in the insulin signaling pathway were classified as being involved in insulin action. Modified and updated from Kwak et al., with permission from Springer Nature [12].
Some genetic variants were identified in East Asian GWAS studies, and there are ethnicity-specific variants, such as the nonsynonymous variant (rs2233580) in the PAX4 (paired box 4) gene [14]. The strongest genetic associations for type 2 diabetes in Koreans were observed for variants in CDKAL1, CDKN2A/2B, KCNQ1 (potassium voltage-gated channel subfamily Q member 1), and MAEA (macrophage erythroblast attacher) [15]. In Europeans, the most significantly associated genetic variant was located in TCF7L2. This difference is explained by the difference in allele frequency of TCF7L2 variant (rs7903146) between the two ethnicities (30% in Europeans vs. 5% in Koreans) [6]. It is speculated that ethnicity-specific variants can explain the different clinical characteristics and pathophysiology of diabetes in different populations. In a transancestry GWAS metaanalysis, it was shown that certain variants only had an effect in a specific population. For example, a variant in PEPD (peptidase D) (rs3786897) was only associated with diabetes in East Asians, and a variant in KLF14 (Kruppel like factor 14) (rs13233731) was only significant in Europeans [11].
Other genetic risk factors include mitochondrial DNA (mtDNA) variations. It is well known that the mtDNA 3243 A>G variation results in maternally inherited diabetes and deafness. The frequency of this mutation is estimated to be approximately 0.5% to 3% in East Asian diabetes patients [16]. However, the frequency is even lower in Europeans. The mtDNA 16189 T>C variant is a common type 2 diabetes susceptibility risk locus. According to a meta-analysis in East Asians, it was a significant risk factor for type 2 diabetes [17]. It was also modestly associated with diabetes in Europeans [18]. Finally, the mtDNA haplogroup N9a was associated with a decreased risk of diabetes, whereas the F and D5 haplogroups were associated with an increased risk of diabetes in Koreans and Japanese [19].
Genetic research into diabetes has certain limitations. As most of the genetic variants identified are non-coding variants residing in introns or intergenic regions, it is difficult to investigate the direct role of these genes or genetic variants in the pathogenesis of type 2 diabetes. These genetic variants might be just tagging markers of yet unrevealed causal variants. We do not yet have a clear understanding of the role of the majority of the identified variants. In addition, the clinical usefulness of these variants in terms of risk prediction and tailored therapy warrants further research [20,21].
Environmental risk factors
Well known environmental risk factors include physical inactivity, a high-calorie diet, obesity, and certain drugs such as glucocorticoids. During the past 40 to 50 years, the prevalence of diabetes in Korea has rapidly increased. In 1971, the prevalence in Okku-gun, a rural community, was estimated to be 1.5%. It increased by 7- to 8-fold to 12.1% in 2000 to 2001, according to the Ansung-Ansan cohort study [22]. The rapid increase in the prevalence of type 2 diabetes is primarily attributable to environmental factors, as the genetic predisposition of the population has not changed. During this period, Korean society experienced rapid socioeconomic changes. Physical activity decreased in parallel with a 30-fold increase in the number of automobiles [23]. The average time spent watching television was 72% higher in 2000 than in 1983. The average fat intake per individual increased from 23.5 to 41.6 g per day over the same period.
Obesity is a major environmental risk factor for type 2 diabetes. There is still an increasing trend in obesity in men according to the Korean National Health and Nutrition Examination Survey 1998 to 2014 [24]. In women, the overall prevalence of obesity seems to be stabilizing. Nevertheless, the prevalence of grade 2 obesity (BMI ≥30 kg/m2) in women is still increasing. Although Asians have a lower BMI than Europeans, it has been reported that Asians have a higher body fat percent and greater abdominal obesity than Europeans at a similar BMI [25,26]. Excessive fat accumulation in the omentum, liver, muscle, and pancreas plays an important role in insulin resistance and β-cell dysfunction, and it is a predictive factor for cardiovascular disease [27]. It is thought that ectopic fat acts as an active endocrine and paracrine organ. The suggested mechanisms by which ectopic fat induces insulin resistance include increased free fatty acid release, increased adipose tissue inflammation, and the dysregulation of adipokines such as adiponectin, resistin, tumor necrosis factor α, and retinol binding protein 4 [27]. Recently, endocrine-disrupting chemicals have been suggested to be associated with obesity and diabetes [28]. These are chemicals that are found in pesticides, metals, and food containers and interfere with the actions of hormones. Endocrine-disrupting chemicals can induce mitochondrial dysfunction and lead to insulin resistance and β-cell dysfunction [29].
Other suggested environmental factors include intrauterine exposure to diabetes. Exposure to hyperglycemia during pregnancy is associated with later-life obesity and diabetes in one's offspring [30]. It has been reported that the offspring born to mothers with gestational diabetes have as much as an 8-fold elevated risk of diabetes compared to the general population [31]. This could be a result of hyperglycemia-induced epigenetic changes. We have investigated sibling pairs discordant to exposure to maternal gestational diabetes and found several differences in the DNA methylation marking in the offspring [32]. Among them, a CpG site in the HNF4A (hepatocyte nuclear factor 4 alpha) gene was hypermethylated in gestational diabetes- exposed offspring. In addition, the overall DNA methylation of the gene had an inverse correlation with its mRNA expression. We suggest that environmental factors could exert their effects on the pathophysiology of type 2 diabetes through epigenetic alterations. However, the field of epigenetic studies is relatively new, and further advances are anticipated.
NATURAL COURSE OF THE DEVELOPMENT OF DIABETES
To obtain a better understanding of the pathophysiology of type 2 diabetes, it is important to investigate how insulin resistance and insulin secretion change during the development of type 2 diabetes. Prospective cohorts with serial measurements of glucose and insulin during glucose tolerance tests are required. The Ansung-Ansan cohort is a community-based prospective study designed to investigate trends and risk factors of chronic complex diseases, such as type 2 diabetes, hypertension, and dyslipidemia [22]. The ongoing project finished its baseline survey in 2001 to 2002, and each participant is being followed up every 2 years with a detailed survey and clinical investigations, including a 2-hour 75-g oral glucose tolerance test (OGTT). We have investigated a total of 4,106 participants who had normal glucose tolerance at baseline and were followed until 2012. During that 10-year period, 1,093 participants (27%) progressed to prediabetes, and 498 (12%) progressed to diabetes [33]. Insulin sensitivity was estimated using the composite Matsuda insulin sensitivity index [34,35], and insulin secretion was assessed by the 1-hour insulinogenic index, both of which were derived from the 75-g OGTT. Those who had progressed to diabetes already had impaired insulin secretion and increased insulin resistance. In Koreans, it is suggested that impaired insulin secretion is already present in the normal glucose tolerance state of individuals who develop diabetes.
Retrograde trajectory of insulin secretion and insulin sensitivity
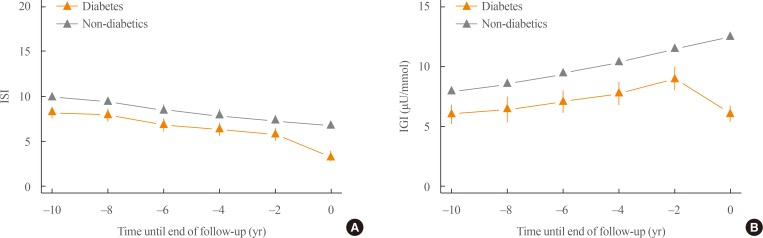
We investigated the retrograde trajectory of insulin secretion and insulin sensitivity by setting the onset of diabetes as time zero and tracing back every 2 years. This retrograde tracing enabled us to understand what key changes occurred at specific time points before the development of diabetes. Fig. 2A shows the changes in insulin sensitivity during the development of type 2 diabetes. Those who developed diabetes had lower insulin sensitivity than those who did not develop diabetes, even 10 years prior to the onset of diabetes. Both groups showed a similar decline in insulin sensitivity during the follow-up period. However, there was a steeper decline in insulin sensitivity during the last 2 years prior to the development of diabetes. Fig. 2B shows changes in insulin secretion during the course of diabetes development. There was a significant decrease in insulin secretion in those who developed diabetes compared to the control group, even 10 years before the onset of diabetes. Those who did not develop diabetes showed a compensatory increase in insulin secretion until the end of follow-up. The most significant difference was the abrupt decrease in insulin secretion during the last 2 years before the onset of diabetes.
Fig. 2. Retrograde trajectory of (A) insulin sensitivity and (B) the insulinogenic index. Diabetes onset or the end of follow-up was set as time zero and each follow-up was traced towards the back. Modified and updated from Ohn et al., with permission from Elsevier [33]. ISI, composite (Matsuda) insulin sensitivity index (unitless); IGI, 1-hour insulinogenic index.
Subgroup analysis according to insulin sensitivity and insulin secretion
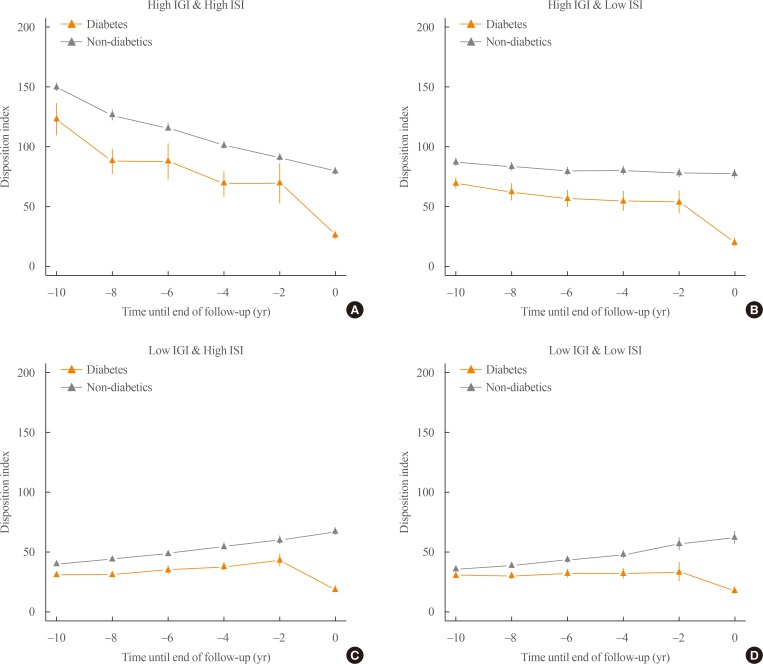
The participants can be subdivided according to their baseline insulin sensitivity and insulin secretion. The subgroup with impaired insulin secretion and low insulin resistance at baseline had about a 3.35-fold increased risk of diabetes compared to those with high insulin secretion and insulin sensitivity [33]. This subgroup had the highest population attributable fraction (38%) [33]. The change in the disposition index for the various subgroups is depicted in Fig. 3. In those with high baseline insulin secretion (Fig. 3A, B), a steady decrease in the disposition index took place during diabetes development. However, those with decreased baseline insulin secretion (Fig. 3C, D) showed a compensatory increase in the disposition index until 2 years before diabetes developed. During the last 2 years, there was a marked decrease in the disposition index in all four subgroups. It would be crucial to investigate the molecular and physiologic perturbations that occurred during the last 2 years before the onset of diabetes.
Fig. 3. Subgroup analysis of the retrograde trajectory of the disposition index. (A) Participants with a high 1-hour insulinogenic index (IGI) and composite (Matsuda) insulin sensitivity index (ISI), (B) participants with a high IGI and a low ISI, (C) participants with a low IGI and a high ISI, (D) participants with a low IGI and a low ISI. Modified and updated from Ohn et al., with permission from Elsevier [33].
Differences between Koreans and Europeans
It has been stated that in Europeans, insulin resistance is the primary defect that triggers type 2 diabetes. In the Whitehall II study, which consisted of 6,538 British participants, those who progressed to diabetes had significantly lower insulin sensitivity at baseline and a steeper decline in insulin sensitivity [36]. Compared to the findings in Europeans, Korean diabetes patients seem to have a decreased insulin secretion capacity and limited β-cell compensation. Similarly, it has been reported in Japanese subjects that impaired insulin secretion had a greater impact on the incidence of diabetes than insulin resistance [37]. This is also evidenced by Starling's curve of the pancreas for insulin secretion [38]. When Europeans, Pima Indians, and Koreans were compared, Koreans showed significantly lower peak insulin levels [4,38,39]. These pathophysiological characteristics could at least partially explain the clinical features of type 2 diabetes in non-obese individuals and relative insulin deficiency in Korean patients.
CONCLUSIONS
In this study, we have reviewed the etiologic factors of diabetes in terms of insulin resistance and insulin secretion and their relative contributions to the pathophysiology of diabetes in Koreans. During the past decade, our understanding of the genetic risk factors of diabetes has significantly improved. The large number of variants identified so far underscores the genetic determination of insulin secretory capacity. Some genetic variants are specifically associated with diabetes in East Asians, and they might explain the clinical characteristics of diabetes in our population. The increase in the prevalence of diabetes is primarily attributable to the rapid socioeconomic development that has occurred during the past half century. An analysis of the retrograde trajectory of insulin secretion and insulin sensitivity showed that those who progressed to diabetes had significantly lower insulin secretion even 10 years before the onset of diabetes. In addition, there was a limited compensatory increase in insulin secretion in those who developed diabetes. An abrupt change occurred in both insulin sensitivity and insulin secretion during the last 2 years prior to the onset of diabetes.
We have focused on insulin sensitivity and insulin secretion. However, the specific pathways involved in insulin sensitivity and insulin secretion are very complex and involve various systems and tissues. Recently, it has been proposed that individuals may have different levels of perturbations in different major pathophysiological processes [40]. This view might be more suitable for understanding the position of the individual in the overall pathophysiology of diabetes. We hope that a more detailed understanding of the pathophysiology of diabetes will provide us with better insights into each of the individual patients we engage with. This will eventually lead to the implementation of precision medicine, with improved risk prediction, prevention, tailored therapy, and better patient outcomes.
ACKNOWLEDGMENTS
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI15C1595, and HI15C3131).
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 3.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 4.Min HK. Non-insulin-dependent diabetes mellitus (NIDDM) in Korea. Diabet Med. 1996;13(9 Suppl 6):S13–S15. [PubMed] [Google Scholar]
- 5.Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes. 2000;49:2201–2207. doi: 10.2337/diabetes.49.12.2201. [DOI] [PubMed] [Google Scholar]
- 6.Kwak SH, Park KS. Recent progress in genetic and epigenetic research on type 2 diabetes. Exp Mol Med. 2016;48:e220. doi: 10.1038/emm.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 8.Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diabetes Genetics Initiative of Broad Institute of Harvard and MIT, Lund University, and Novartis Institutes of Bio-Medical Research. Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 11.DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium; Asian Genetic Epidemiology Network Type 2 Diabetes (AGEN-T2D) Consortium; South Asian Type 2 Diabetes (SAT2D) Consortium; Mexican American Type 2 Diabetes (MAT2D) Consortium; Type 2 Diabetes Genetic Exploration by Nex-generation sequencing in muylti- Ethnic Samples (T2D-GENES) Consortium. Mahajan A, et al. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet. 2014;46:234–244. doi: 10.1038/ng.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwak SH, Park KS. Genetics of type 2 diabetes and potential clinical implications. Arch Pharm Res. 2013;36:167–177. doi: 10.1007/s12272-013-0021-x. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med. 2010;363:2339–2350. doi: 10.1056/NEJMra0906948. [DOI] [PubMed] [Google Scholar]
- 14.Fuchsberger C, Flannick J, Teslovich TM, Mahajan A, Agarwala V, Gaulton KJ, et al. The genetic architecture of type 2 diabetes. Nature. 2016;536:41–47. doi: 10.1038/nature18642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho YS, Chen CH, Hu C, Long J, Ong RT, Sim X, et al. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nat Genet. 2011;44:67–72. doi: 10.1038/ng.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwak SH, Park KS. Role of mitochondrial DNA variation in the pathogenesis of diabetes mellitus. Front Biosci (Landmark Ed) 2016;21:1151–1167. doi: 10.2741/4447. [DOI] [PubMed] [Google Scholar]
- 17.Park KS, Chan JC, Chuang LM, Suzuki S, Araki E, Nanjo K, et al. A mitochondrial DNA variant at position 16189 is associated with type 2 diabetes mellitus in Asians. Diabetologia. 2008;51:602–608. doi: 10.1007/s00125-008-0933-z. [DOI] [PubMed] [Google Scholar]
- 18.Ye Z, Gillson C, Sims M, Khaw KT, Plotka M, Poulton J, et al. The association of the mitochondrial DNA OriB variant (16184-16193 polycytosine tract) with type 2 diabetes in Europid populations. Diabetologia. 2013;56:1907–1913. doi: 10.1007/s00125-013-2945-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuku N, Park KS, Yamada Y, Nishigaki Y, Cho YM, Matsuo H, et al. Mitochondrial haplogroup N9a confers resistance against type 2 diabetes in Asians. Am J Hum Genet. 2007;80:407–415. doi: 10.1086/512202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Florez JC. Pharmacogenetics in type 2 diabetes: precision medicine or discovery tool? Diabetologia. 2017;60:800–807. doi: 10.1007/s00125-017-4227-1. [DOI] [PubMed] [Google Scholar]
- 21.Franks PW, Poveda A. Lifestyle and precision diabetes medicine: will genomics help optimise the prediction, prevention and treatment of type 2 diabetes through lifestyle therapy? Diabetologia. 2017;60:784–792. doi: 10.1007/s00125-017-4207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho NH, Jang HC, Choi SH, Kim HR, Lee HK, Chan JC, et al. Abnormal liver function test predicts type 2 diabetes: a community-based prospective study. Diabetes Care. 2007;30:2566–2568. doi: 10.2337/dc07-0106. [DOI] [PubMed] [Google Scholar]
- 23.Choi YJ, Cho YM, Park CK, Jang HC, Park KS, Kim SY, et al. Rapidly increasing diabetes-related mortality with socioenvironmental changes in South Korea during the last two decades. Diabetes Res Clin Pract. 2006;74:295–300. doi: 10.1016/j.diabres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 24.Shin HY, Kang HT. Recent trends in the prevalence of underweight, overweight, and obesity in Korean adults: the Korean National Health and Nutrition Examination Survey from 1998 to 2014. J Epidemiol. 2017;27:413–419. doi: 10.1016/j.je.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park YW, Allison DB, Heymsfield SB, Gallagher D. Larger amounts of visceral adipose tissue in Asian Americans. Obes Res. 2001;9:381–387. doi: 10.1038/oby.2001.49. [DOI] [PubMed] [Google Scholar]
- 26.He Q, Horlick M, Thornton J, Wang J, Pierson RN, Jr, Heshka S, et al. Sex and race differences in fat distribution among Asian, African-American, and Caucasian prepubertal children. J Clin Endocrinol Metab. 2002;87:2164–2170. doi: 10.1210/jcem.87.5.8452. [DOI] [PubMed] [Google Scholar]
- 27.Lim S, Meigs JB. Links between ectopic fat and vascular disease in humans. Arterioscler Thromb Vasc Biol. 2014;34:1820–1826. doi: 10.1161/ATVBAHA.114.303035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2: the endocrine society's second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36:E1–150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim S, Cho YM, Park KS, Lee HK. Persistent organic pollutants, mitochondrial dysfunction, and metabolic syndrome. Ann N Y Acad Sci. 2010;1201:166–176. doi: 10.1111/j.1749-6632.2010.05622.x. [DOI] [PubMed] [Google Scholar]
- 30.Gillman MW. Developmental origins of health and disease. N Engl J Med. 2005;353:1848–1850. doi: 10.1056/NEJMe058187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care. 2008;31:340–346. doi: 10.2337/dc07-1596. [DOI] [PubMed] [Google Scholar]
- 32.Kim E, Kwak SH, Chung HR, Ohn JH, Bae JH, Choi SH, et al. DNA methylation profiles in sibling pairs discordant for intrauterine exposure to maternal gestational diabetes. Epigenetics. 2017;12:825–832. doi: 10.1080/15592294.2017.1370172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohn JH, Kwak SH, Cho YM, Lim S, Jang HC, Park KS, et al. 10-Year trajectory of β-cell function and insulin sensitivity in the development of type 2 diabetes: a communitybased prospective cohort study. Lancet Diabetes Endocrinol. 2016;4:27–34. doi: 10.1016/S2213-8587(15)00336-8. [DOI] [PubMed] [Google Scholar]
- 34.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 35.Tura A, Kautzky-Willer A, Pacini G. Insulinogenic indices from insulin and C-peptide: comparison of beta-cell function from OGTT and IVGTT. Diabetes Res Clin Pract. 2006;72:298–301. doi: 10.1016/j.diabres.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Tabak AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimaki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373:2215–2221. doi: 10.1016/S0140-6736(09)60619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morimoto A, Tatsumi Y, Deura K, Miyamatsu N, Noda M, Watanabe S. Impact of impaired insulin secretion and insulin resistance on the incidence of diabetes in a Japanese cohort. Reply to Yamauchi K and Aizawa T [letter] Diabetologia. 2013;56:2546–2547. doi: 10.1007/s00125-013-3068-9. [DOI] [PubMed] [Google Scholar]
- 38.DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992;15:318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- 39.Knowler WC, Pettitt DJ, Saad MF, Bennett PH. Diabetes mellitus in the Pima Indians: incidence, risk factors and pathogenesis. Diabetes Metab Rev. 1990;6:1–27. doi: 10.1002/dmr.5610060101. [DOI] [PubMed] [Google Scholar]
- 40.McCarthy MI. Painting a new picture of personalised medicine for diabetes. Diabetologia. 2017;60:793–799. doi: 10.1007/s00125-017-4210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]